Abstract
Background and Objectives:
Gastrointestinal cancers are the most frequently occurring cancers worldwide. Diagnosis and removal of polyps during screening endoscopy decreases the prevalence of colon cancer and cancer-related mortality, and it is considered to be the gold standard in gastrointestinal system cancer prevention. Technological innovations in endoscopy have led to revolutionary developments in many areas. Flexible spectral imaging color enhancement (FICE) and narrow-band imaging (NBI) are forms of digital chromoendoscopy and enhance the endoscopic images without the need for a dye. This study seeks to evaluate the efficacy of FICE and NBI on polyp screening and real-time histologic diagnosis with endoscopy and to compare them.
Methods:
A total of 134 patients (male/female = 72/62) and 161 polyps were evaluated with FICE or NBI, and real-time histologic diagnosis predictions were classified as neoplastic or nonneoplastic, according to Kudo's pit pattern classification. Pathological results and real-time endoscopic diagnoses were statistically interpreted for both FICE and NBI. Positive predictive value, negative predictive value, sensitivity, specificity, and accuracy rates were calculated and compared for both modalities.
Results:
When both systems were compared, the negative predictive value of NBI was found to be higher than that of FICE statistically (P < .001). Specificity and positive predictive value in the FICE group were higher than in the NBI group, but the difference was not statistically significant (P = .082 and P = .153, respectively).
Conclusions:
Aside from being safe in polyp detection, digital chromoendoscopy also helps the endoscopist in selecting the type of simultaneous intervention (eg, polypectomy, endomucosal resection, or submucosal dissection) by enabling endoscopic histologic diagnosis.
Keywords: Chromoendoscopy, Endoscopy, Image enhancement, Polyp
INTRODUCTION
Gastrointestinal system (GIS) cancers are second in the cancer-related mortality rate.1 Most GIS cancers can be prevented by a good screening program. Endoscopy is the best way to screen cancers and adenomatous polyps.2 A significant portion of GIS cancers are sporadic. The most widely accepted hypothesis for the development of sporadic cancers is known as adenoma–carcinoma sequence. According to this hypothesis, polyps are the precursors of cancer. Diagnosis and removal of polyps during screening endoscopy decreases the frequency of colon cancer and cancer-related mortality.3 In a randomized controlled study, it has been reported that endoscopic polypectomies can prevent cancers by 80%.4
Technological innovations in endoscopy have led to revolutionary developments in the diagnosis and treatment of early-stage GIS cancers, high-grade dysplasias, and adenomas. Thanks to the development of resolution and high-contrast ratios in endoscopic optic systems, the surface pattern and microvascular architecture of the lesions can be visualized. Thus, it is possible to make real-time histopathologic predictions about the lesions during endoscopy. Aside from identifying the lesions, it also makes removal of the lesion possible, with negative margins. Together with endoscopic innovations, good bowel preparation, and optimal time allocation for endoscopic practices, improvements in the identification of the lesions have been made.
Although large polyps can be identified in conventional white-light endoscopy, some studies have reported that small polyps or flat lesions may be missed. It is stated in many studies that the polyp miss rate is ∼5–24%, even when the procedure is conducted by an experienced endoscopist,5,6 and 25% of missed polyps are neoplastic lesions.3 Reasons for missing adenomas in colonoscopy are inadequate bowel preparation, the presence of flat polyps that look like normal mucosa at a glance, and insufficient visualization because of technical limitations, especially behind the right colonic folds.6
After it became possible to obtain more histologic details about polyps and to identify them by means of chromoendoscopy developed by Kudo et al,7 chromoendoscopy techniques using different dyes have begun to be widely used, especially in Japan. Major disadvantages of chromoendoscopy include that it requires experience, it is time-consuming, and its cost is high. These disadvantages have led to recent technological developments, such as electronic chromoendoscopy, digital chromoendoscopy, and chromoendoscopy without dyes; these approaches are related to improvements in image resolution, software processing, and optical filter technology. Narrow-band imaging (NBI; Olympus Inc, Hauppauge, New York, USA), flexible spectral imaging color enhancement (FICE; Fujinon Inc, Saitama, Japan) and I-Scan (Pentax Inc, Tokyo, Japan) are the most widely used and commercially available methods among these technological developments. NBI is a technology that uses short-wave blue and green light sources instead of red light, whereas FICE and I-Scan use the same light source as conventional endoscopy, but they are based on a software technology that makes arithmetic changes in the endoscopy processing system and virtual filter changes.
The purpose of this study was to evaluate the adaptation of the endoscopists for FICE and NBI use for identification of GIS polyps and to evaluate the real-time polyp histology estimation capabilities of these new endoscopic techniques.
METHODS
The study was conducted on patients who came to our endoscopy unit from February 2013 through February 2016 to have an upper GIS endoscopy or a colonoscopy and who agreed to participate in the study. The patients were divided into 2 groups: FICE and NBI. The study was conducted by 7 endoscopists who perform more than 200 endoscopies annually. Institutional ethics committee approval was obtained for the study, and all procedures performed involving human participants were in accordance with the 1964 Helsinki Declaration.
Procedures were performed with conscious sedation (intravenous midazolam + meperidine). The endoscopies were performed by 1 of the 7 experienced endoscopists, using different Olympus and Fujinon endoscopes. The cecum and duodenum were reached aided by white light. After cecal or duodenal intubation, the localization, size, and morphology of each polyp were documented during the withdrawal phase. Once a polyp was detected, FICE or the NBI optical system was switched on by the use of a button on the head of the endoscope. All polyps were classified according to Kudo's pit pattern classification.7 During the study, withdrawal times had to take a mean of at least 6 minutes and to be equal in the 2 arms. Polyps noted during insertion were relocated and removed during withdrawal.
The Modified Kudo Pit Pattern Classification
Characteristic pit patterns of mucosa (Kudo classification) distinguished nonneoplastic from neoplastic colonic mucosal lesions. Pit pattern 1 (round pits) and 2 (stellar or papillary pits) were associated with nonneoplastic lesions, whereas 3 (tubular pits), 4 (branchlike or gyruslike pits), and 5 (nonstructural pits) predicted neoplastic lesions, including intramucosal cancer7 (Table 1).
Table 1.
The Modified Kudo Pit Classification
| Type | Description |
|---|---|
| 1 | Normal round |
| 2 | Stella or papillary |
| 3 S | Tubular or round; smaller than pit type I |
| 3 L | Tubular/large |
| 4 | Sulcus/gyrus |
| 5 | Irregular arrangement, with size equal to grade III L, III S, or IV |
Flexible Spectral Imaging Color Enhancement
FICE, also known as Fujinon intelligent chromoendoscopy, enhances the visualization of mucosal structures and microcirculation by the selection of spectral transmission with a dedicated wavelength. It emits and captures the entire white-light spectrum without the use of any optical filters. After the light capture, digital software-based computer algorithms modify the captured images. Certain combinations of wavelengths are selectively enhanced, which results in improved visualization of subtle mucosal surface changes, especially of mucosal vessels and pit patterns.8 The FICE systems come with 10 presets that can be customized and configured from a large number of wavelength permutations. Endoscopists can select spectral images at visible wavelengths between 400 and 695 nm that can be activated by a switch on the head of the endoscope.9
Narrow-Band Imaging
NBI enables, via the application of narrow band width filters to standard white-light endoscopy, clear definition of the contrast between the epithelial surface and the adjacent vascular structures. NBI allows blue and green wavelengths from the white-light spectrum to pass through, but blocks red wavelengths. Green and especially blue-light wavelengths fall into the peak absorption of hemoglobin. As a consequence, blue and green light are absorbed by superficial and deep mucosal vessels, respectively, but are reflected by the remaining mucosa. This reflection improves visualization of mucosal vessels, which are frequently altered in form, density, and size in neoplastic colorectal lesions.8,10
NBI improves the definition of the epithelial surface and emphasizes the contrast of mucosal microvessels, which appear as dark brownish structures (virtual chromoscopy). In conjunction with zoom endoscopy technology, enables analysis of pit pattern subtypes and associated vascular abnormalities.11
Polyp Description
Flat lesions were grouped into size categories based on their endoscopic measurement performed by comparison with the known diameter of open forceps or the diameter of the snare in use. Lesions less than 2,5 mm in height were accepted as flat lesions and classified morphologically according to the Paris Classification. Each flat lesion was retrieved separately for pathologic examination. All detected flat lesions were removed. Flat lesions were classified as neoplastic (Kudo Pit Pattern Type III, IV, V) or nonneoplastic (Kudo Pit Pattern Type II) (Figure 1–6).
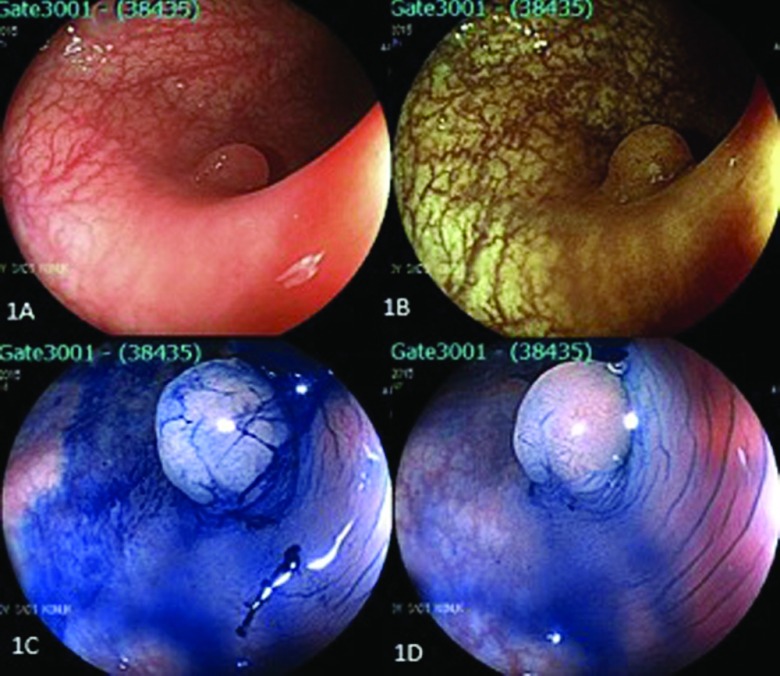
Figure 1.
Left-side colon polyp (pit pattern 3). (A) Conventional endoscopic view of the lesion. (B) Endoscopic view of the same polyp with FICE. (C, D) Endoscopic view after administration of N-acetyl cysteine and methylene blue dye.
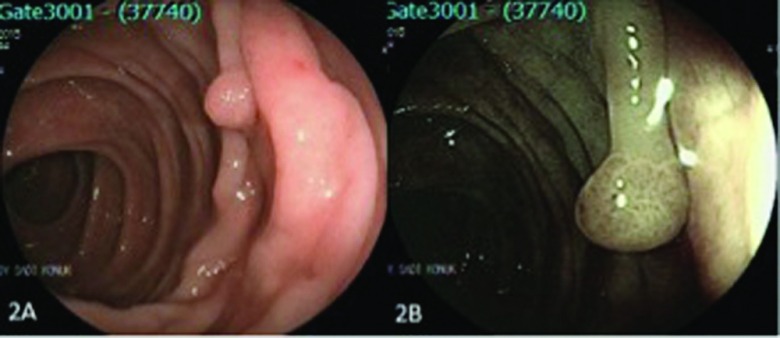
Figure 2.
A polyp in the transverse colon (pit pattern III). (A) Conventional endoscopic view of the lesion and (B) endoscopic view with FICE.
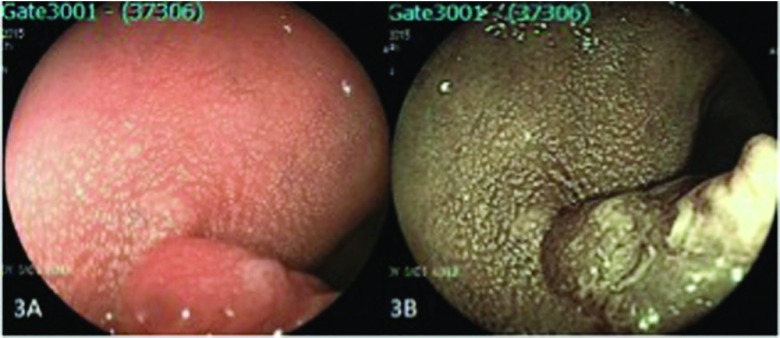
Figure 3.
A polyp in the proximal rectum (pit pattern 4). (A) Conventional endoscopic view of the lesion and (B) endoscopic view with FICE.
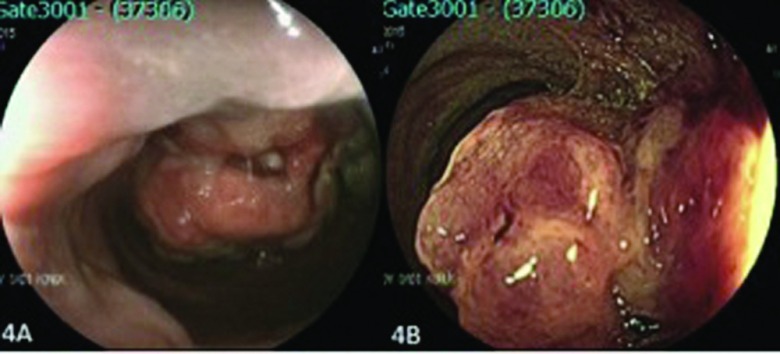
Figure 4.
A polyp in sigmoid colon (pit pattern 5). (A) Conventional endoscopic view of the lesion and (B) endoscopic view with FICE.
Figure 5.
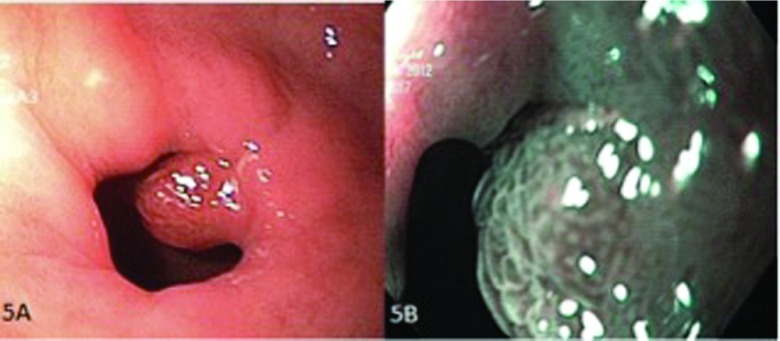
A polyp in the pylorus (pit pattern 4). (A) Conventional endoscopic view of the lesion and (B) endoscopic view with NBI.
Figure 6.
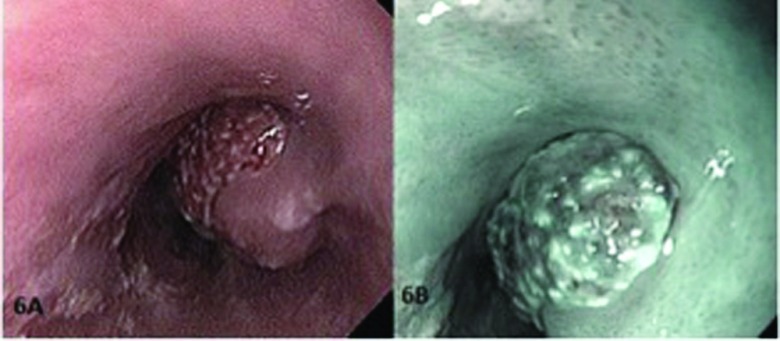
A polyp in the distal esophagus (pit pattern 5). (A) Conventional endoscopic view of the lesion and (B) endoscopic view with NBI.
Bowel preparation was evaluated and graded as previously described.11,12 There were 4 categories of bowel preparation: excellent (>90% of mucosa seen, mostly liquid colonic contents, minimal suctioning needed for adequate visualization), good (>90% of mucosa seen, mostly liquid colonic contents, significant suctioning needed for adequate visualization), fair (>90% of mucosa seen, mixture of liquid and semisolid colonic contents that could be suctioned and/or washed), and inadequate (<90% of mucosa seen, mixture of semisolid and solid colonic contents that could not be suctioned or washed).
Exclusion criteria were previous surgical resection of any part of the gastrointestinal tract, a history of gastrointestinal tract cancer, a history of inflammatory bowel disease, use of antiplatelet agents or anticoagulants that precluded the removal of the gastrointestinal tract polyps, poor general condition, or any other reason to avoid prolonged procedure time, history of polyposis syndrome or hereditary nonpolyposis colon cancer, or the inability to give informed consent. Patients in whom the cecum and duodenum could not be intubated or bowel prep was inadequate were excluded as well.
Statistical Analysis
Statistical analysis was performed with JMP software version 10.0.0 (SAS Institute, Inc, Cary, North Carolina, USA). Patient characteristics were analyzed via descriptive statistics. For continuous variables, the mean and standard derivation or median and range were calculated. For categorical variables, the numbers and percentages in each category were recorded. Differences between parameters were compared with Student's t test. Frequency distributions were compared with the χ2 test. The number of true positives (TPs), true negatives (TNs), false positives (FPs), and false negatives (FNs) for these endoscopic imaging modalities were determined with 2 × 2 tables. The diagnostic value of thee modalities was also assessed for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy, using relevant formulas. Differences reaching P < .05 were considered statistically significant, and all of the performed tests were 2-sided.
RESULTS
The study included 134 patients. Demographic data of the patients is given in Table 2. The FICE group had 71 patients, and the NBI group had 63 patients. Gastroscopic and colonoscopic polyp diagnosis rates in both groups are also given in Table 2.
Table 2.
Demographic Data
| Parameter | FICE |
NBI |
P |
|---|---|---|---|
| (n = 71) | (n = 63) | ||
| Age (years, mean ± SD) | 59.9 ± 13.2 | 58.6 ± 14.9 | .60 |
| Sex (F/M) | 32/39 | 30/33 | .77 |
| Gastroscopy/colonoscopy (n) | 27/44 | 32/31 | .13 |
In total, 161 polyps were found in 134 of the patients included in the study. A total of 34.7% of the polyps were nonneoplastic (Pit pattern type 2), whereas 63.3% of them were neoplastic (Pit pattern types 3+4+5) (Table 3).
Table 3.
Lesions According to the Pit Pattern
| Pit Type | FICE |
NBI |
||
|---|---|---|---|---|
| (n = 81)* |
(n = 80)* |
|||
| Compatible | Incompatible | Compatible | Incompatible | |
| 2 | 20 | 5 | 26 | 1 |
| 3 | 31 | 2 | 23 | 2 |
| 4 | 14 | – | 17 | 2 |
| 5 | 9 | – | 8 | 1 |
Number of lesions evaluated.
According to the pathologic examination results of the polyps, 15.5% of the polyps were hyperplastic, 13.6% were inflammatory, 3.1% were hamartomas, 36% were adenomatous, 10.5% were low-grade dysplasia, 9.9% high-grade dysplasia, and 11.1% were cancer. The distribution of these polyps in both groups during endoscopy according to Kudo's pit pattern classification and the correlation of these endoscopic diagnoses with pathological results are shown in Table 3.
In both groups, the results of endoscopic diagnosis and pathologic examination are evaluated statistically. PPV, NPV, sensitivity, specificity, and accuracy rates are shown in Table 4. NPV in the NBI group was found to be significantly higher than that in the FICE group (P < .001). Specificity in the FICE group was higher than in the NBI group, but the difference was not statistically significant (P = .082).
Table 4.
Predictive Values in Groups
| Diagnostic Value | FICE (%) | NBI (%) | P |
|---|---|---|---|
| PPV | 96.4 | 90.5 | .153 |
| NPV | 80.0 | 96.3 | <.001 |
| Sensitivity | 91.5 | 97.9 | .089 |
| Specificity | 90.9 | 83.8 | .082 |
| Accuracy | 91.3 | 92.5 | .999 |
DISCUSSION
This study was conducted to evaluate the efficacy of FICE and NBI use on polyp screening and their importance in real-time histologic diagnosis with endoscopy and to compare the 2 approaches. The data of the patients were recorded prospectively. The procedures were conducted by experienced endoscopists who used the FICE and NBI endoscopic systems for the first time. Although there are some studies in the literature that compare FICE and NBI separately in terms of polyp diagnosis and treatment, the number of studies comparing these 2 methods is very limited.
The polyps are precancerous lesions (adenoma–carcinoma sequence). Endoscopy is the best method for screening cancers and adenomatous polyps.2,13
Endoscopic polypectomies are protective against cancer and decrease cancer-related mortality.3,14 However, despite endoscopy, 2–6% of the patients develop interval cancers.15 Most of these interval cancers result, not from newly developed foci but from neoplastic lesions missed in the endoscopy.16 The rate of polyp detection depends on careful examination of the mucosal surface and on allocating sufficient time.17 According to The American Society for Gastrointestinal Endoscopy (ASGE), the miss rate of adenomas larger than 10 mm is 2%, the miss rate of adenomas between 5–10 mm is 13% and the miss rate of adenomas smaller than 5 mm is 26%.18
Corley et al19 and Kaminski et al20 reported in their separate studies that low adenoma detection rate is an independent risk factor for interval colorectal cancers. It is shown that 3.8% more polyps can be diagnosed with high-definition (HD) endoscopes compared to standard endoscopy.21 The biggest reason for low adenoma detection is diminutive polyps. Diminutive polyps (<1.5 cm) constitute 10% of colorectal tumors.22,23 According to ASGE, 26% of the diminutive adenomas are missed.18 Enabling high-quality and real-time imaging for detection of diminutive polyps and adenomas is the most important point for detection and resection of these adenomas.24 According to ASGE, endoscopic innovations that have more than 90% NPV in determining adenomatous histology play a significant role in specifying the postpolypectomy surveillance intervals.18
Chromoendoscopy is the technique of developing endoscopic images by using dye or optical techniques. Although, the term, chromoendoscopy has been limited to the use of a dye, recently it has begun to be used for endoscopies which use optic image development methods, and it is called dyeless or digital chromoendoscopy. Digital chromoendoscopy enhances the image quality by using advanced optical systems instead of a dye (Figure 1). With the help of a button on the endoscope, transition between these imaging systems can easily be made (optical enhancement mode can be simply switched on or off with a button). FICE, NBI, and I-SCAN technologies are called digital chromoendoscopy. Chromoendoscopy techniques help visualize the mucosal structure, and hence, they increase the possibility of detecting lesions compared to standard endoscopy (standard white-light colonoscopy) and allow endoscopists to obtain more detail about vascular architecture, surface, and edge properties.
The first study about predicting polyp histology by chromoendoscopy was reported by Kudo et al.7 First conducted for chromoendoscopy, this study was also adapted to digital chromoendoscopy.25 In our study, the polyps are classified according to the pit pattern classification of Kudo, and histologic diagnosis predictions are made (Table 1). According to the Kudo classification both FICE and NBI technologies assess polyps by comparing their surface patterns and vascular architecture to the adjacent mucosa. In our study, 36 polyps in FICE group and 25 polyps in NBI group [in total, 61 polyps (37.8%)] are classified as type 3 (adenomatous). Although dysplasia was detected in pathology in all of the polyps diagnosed to have dysplasia in the FICE group, the dysplasia detection capability of the NBI group was 89.4%. Nine polyps in each group [in total, 18 (11.1%)] were considered to be cancerous. Although all the polyps evaluated to be type 5 in the FICE group were cancerous according to pathology results, a polyp that was considered to be benign in NBI was cancerous according to pathology; this mistake is thought to be related to the presence of a hematoma over the polyp.
Through FICE, it is possible to develop the surface and vascular images of the polyps by means of virtual optic filter adjustments. Each image can be transformed into 10 different appearances. The FICE system uses both HD endoscopes and magnification technology (Figures 2–4). Although it has been reported that there is no difference between FICE and HD endoscopy in terms of missed adenomas,26,27 according to Longcroft-Wheaton et al,28 while the prediction of in vivo polyp histology is 83% in white-light endoscopy, this rate can increase up to 97% with the use of FICE. Aside from the increase in polyp detection rate, enhancing the histologic diagnostic capability helps determine the endoscopic surveillance time and hence decrease the cost. In this study, the rates of PPV, NPV, sensitivity, specificity, and accuracy with the use of FICE for polyp histology prediction are 96.4, 80.0, 91.5, 90.9, and 91.3%, respectively.
In the NBI endoscopy system, thanks to the filter used, the emitted blue light is absorbed by hemoglobin within the vascular bed, and the vascular pattern can be imaged in detail (Figure 5). The chaotic vascularity of the neoplastic lesions is important for histologic diagnosis, and it increases the value of real-time endoscopic diagnosis. It is very important that the vascular be distinct, to determine the borders of the lesion with healthy mucosa (Figure 6). Together with developing the vascular pattern, NBI also helps develop the surface pattern through its magnification and high resolution. This ability facilitates removal of lesions with clear margins. Although NBI does not have notable superiority over white-light endoscopy in detecting and removing large polyps, it is better than white-light endoscopy in histologic diagnosis of diminutive polyps and flat lesions.29 Aside from histologic prediction, NBI is beneficial for detecting the invasion depth of cancer.30 In our study, with NBI, 6 polyps were considered type 5 according to the Kudo classification and diagnosed as cancerous. In this study, the rate of PPV, NPV, sensitivity, specificity, and accuracy rates of NBI for polyp histology prediction were 90.5, 96.3, 97.9, 83.8, and 92.5%, respectively.
In accordance with the literature, our study did not find any superiority of FICE or NBI for detecting large polyps. However, we observed that the use of FICE and NBI has some advantages and disadvantages for detecting diminutive polyps and the exact borders of polypectomies. NBI and FICE showed polyp borders, surface patterns, and vascularity better than conventional white-light endoscopy. These technologies are more expensive and time-consuming, and they require experience. Comparison of FICE and NBI showed that NBI revealed microvascular architecture and surface pattern better, whereas FICE showed brighter and more images than NBI, and its use was more practical. The NPV of NBI was higher than that of FICE (P < .001). The PPV and specificity in the FICE group were higher than those in the NBI group but the differences were not statistically significant (P = .153 and .082, respectively). It has been demonstrated that both methods are successful and reliable for polyp histology assessment. In addition, it was concluded by all of the researchers that the images obtained by NBI were darker than those of FICE and their interpretation required experience.
The limitations of the study include the inclusion of patients with nonspecific symptoms, together with screening endoscopy. Another limitation is that not all the pathologists use the same criteria for polyp diagnosis. Yet another one is that the study is the first performed by the clinic based on results of FICE and NBI use—the reason being that there is a learning curve for adaptation to the technical properties of the new devices and to the Kudo pit pattern classification used for endoscopic diagnosis. Some polyps may have been classified wrongly at first. However, that the endoscopists were experienced may have minimized the margin of error.
Removal of all the lesions detected in endoscopy and sending them for pathologic evaluation is very expensive. One of the recently discussed topics is whether technological advances in endoscopy field will enable in vivo histologic diagnosis of the lesions during endoscopy. Throughout the world, the endoscopic surveillance time detection and determination of lesion treatment process still depend on diagnosis by pathology. The importance of FICE and NBI optical systems result from their contribution to detection of polyp histologies from polyp detection. Endoscopic histologic diagnosis predictions can decrease costs by preventing unnecessary polypectomies and screening endoscopies. Furthermore, digital chromoendoscopy may decrease the development of cancer by increasing the detection and removal of neoplastic diminutive polyps and flat adenomas.
Contributor Information
Cevher Akarsu, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
Nuri Alper Sahbaz, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
Ahmet Cem Dural, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
Mustafa Gokhan Unsal, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
Osman Kones, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
Ali Kocatas, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
Ilkay Halicioglu, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
Halil Alis, General Surgery Department, University of Medical Sciences Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Istanbul, Turkey..
References:
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2. Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 3. Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy: The National Polyp Study Work group. N Engl J Med. 1993;329:1977–1981. [DOI] [PubMed] [Google Scholar]
- 4. Thiis E, Hoff G, Sauar J, Langmark F, Majak B, Vatn M. Population based surveillance by colonoscopy: Effect on the incidence of colorectal cancer. Telemark Polyp Study I Scand J Gastroenterol. 1999;34:414–420. [DOI] [PubMed] [Google Scholar]
- 5. Paggi S, Radaelli F, Amato A, et al. The impact of narrow band imaging in screening colonoscopy: a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2009;7:1049–1054. [DOI] [PubMed] [Google Scholar]
- 6. Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–290. [DOI] [PubMed] [Google Scholar]
- 7. Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. [DOI] [PubMed] [Google Scholar]
- 8. Bartel MJ, Picco MF, Wallace MB. Chromocolonosc Gastrointest Endoscopy Clin N Am. 2015;25:243–260. [DOI] [PubMed] [Google Scholar]
- 9. Subramanian V, Ragunath K. Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol. 2014;12:368–376. [DOI] [PubMed] [Google Scholar]
- 10. Hurlstone DP. The detection of flat and depressed colorectal lesions: which endoscopic imaging approach? Gastroenterology. 2008;135:338–343. [DOI] [PubMed] [Google Scholar]
- 11. Naravadi V, Gupta N, Early D, et al. Prevalence of advanced histological features and synchronous neoplasia in patients with flat adenomas. Gastrointest Endosc. 2016;83:795–799. [DOI] [PubMed] [Google Scholar]
- 12. Rex DK, Schwartz H, Goldstein M, et al. Safety and colon cleansing efficacy of a new residue free formulation of sodium phosphate tablets. Am J Gastroenterol. 2006;101:2594–2604. [DOI] [PubMed] [Google Scholar]
- 13. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. [DOI] [PubMed] [Google Scholar]
- 14. Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population based analysis. Gastroenterology. 2007;132:96–102. [DOI] [PubMed] [Google Scholar]
- 16. Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858–864. [DOI] [PubMed] [Google Scholar]
- 17. Lee TJ, Blanks RG, Rees CJ, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: Evidence from the Bowel Cancer Screening Programme in England. Endoscopy. 2013;45:20–26. [DOI] [PubMed] [Google Scholar]
- 18. ASGE (American Society of Gastrointestinal Endoscopy). Technology Status Evaluation Report. Gastrointest Endosc. 2015;81:1122–1129.25746978 [Google Scholar]
- 19. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. [DOI] [PubMed] [Google Scholar]
- 21. Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499–505. [DOI] [PubMed] [Google Scholar]
- 22. East JE, Stavrindis M, Gibson S, Guenther T, Tekkis PP, Saunders BP. A comparative study of standard vs. high definition colonoscopy for adenoma and hyperplastic polyp detection with optimized withdrawal technique. Aliment Pharmacol Ther. 2008;28:768–776. [DOI] [PubMed] [Google Scholar]
- 23. Stolte M, Bethke B. Colorectal mini-de novo carcinoma: a reality in Germany too. Endoscopy. 1995;27:286–290. [DOI] [PubMed] [Google Scholar]
- 24. Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpectCharacterise Resect and Discard; DISCARD trial) : a prospective cohort study. Lancet Oncol. 2009;10:1171–1178. [DOI] [PubMed] [Google Scholar]
- 25. Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–607. [DOI] [PubMed] [Google Scholar]
- 26. Aminalai A, Rosch T, Aschenbeck J, et al. Live image processing does not increase adenoma detection rate during colonoscopy: a randomized comparison between FICE and conventional imaging (Berlin Colonoscopy Project 5, BECOP-5). Am J Gastroenterol. 2010;105:2383–2388. [DOI] [PubMed] [Google Scholar]
- 27. Chung SJ, Kim D, Song JH, et al. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc. 2010;72:136–142. [DOI] [PubMed] [Google Scholar]
- 28. Longcroft-Wheaton GR, Bhandari P. In-vivo diagnosis of diminutive colonic polyps using FICE and indigocarmine chromoendoscopy: results from a large Western prospective series. Gastrointest Endosc. 2011;73:377. [Google Scholar]
- 29. Tischendorf JJ, Wasmuth HE, Koch A, et al. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092–1096. [DOI] [PubMed] [Google Scholar]
- 30. Hirata M, Tanaka S, Oka S, et al. Evaluation of microvessels in colorectal tumors by narrow band imaging magnification. Gastrointest Endosc. 2007;66:945–952. [DOI] [PubMed] [Google Scholar]