Abstract
Background: An understudied component of the diet, branched-chain fatty acids (BCFAs) are distinctive saturated fatty acids that may have an important influence on health. Human-milk fatty acid composition is known to differ worldwide, but comparative data are lacking on BCFAs.
Objective: We tested the hypotheses that concentrations of BCFAs in human milk differ between populations and are associated with maternal diet.
Design: We surveyed the BCFA composition of samples collected as part of a standardized, prospective study of human-milk composition. Mothers were enrolled from 3 urban populations with differing diets: Cincinnati, Ohio; Shanghai, China; and Mexico City, Mexico. Enrollment was limited to healthy mothers of term singleton infants. We undertook a cross-sectional analysis of milk from all women with samples at postpartum week 4 (n = 359; ∼120 women/site). Fatty acids were extracted from milk by using a modified Bligh-Dyer technique and analyzed by gas chromatography. Statistical analysis was performed by ANOVA and Tobit regression. For Cincinnati mothers, 24-h diet recalls were analyzed in relation to the individual BCFA concentrations measured in milk samples.
Results: Total BCFAs in milk differed by site, with the highest concentration in Cincinnati followed by Mexico City and Shanghai (mean ± SE: 7.90 ± 0.41, 6.10 ± 0.36, and 4.27 ± 0.25 mg/100 mL, respectively; P < 0.001). Site differences persisted after delivery mode, maternal age, and body mass index were controlled for. The individual concentrations of iso-14:0, iso-16:0, iso-18:0, anteiso-15:0, and anteiso-17:0 also differed between sites. Milk concentrations of iso-14:0 and anteiso-15:0 were associated with maternal intake of dairy; iso-16:0 was associated with maternal intakes of dairy and beef.
Conclusions: BCFA concentrations in milk at 4 wk postpartum differed between mothers from Cincinnati, Shanghai, and Mexico City. Variations in human-milk BCFAs are influenced by diet. The impact of BCFAs on infant health warrants investigation.
Keywords: human milk, fatty acids, branched-chain fatty acids, lactation, maternal diet
INTRODUCTION
Mother’s own milk is recommended as the sole source of infant nutrition for the first 6 mo of life by the American Academy of Pediatrics (1) and the WHO (2). Human milk, however, varies in its fatty acid composition as the result of differences in maternal diet and body stores (3). Among the least-studied fatty acids in human milk are the branched-chain fatty acids (BCFAs)10, which are also rarely studied in human nutrition in general. BCFAs are synthesized in sebaceous and meibomian glands of human skin and are prominent components of many bacterial membranes (4–6).
BCFAs are primarily SFAs and include mono-, di-, or poly-methyl BCFAs with ≥1 methyl branching point on the carbon chain; the predominant branching is near the terminal end of the carbon chain (7, 8). BCFAs are labeled iso or anteiso depending on the terminating isopropyl or isobutyl group, respectively (7, 9) (Figure 1). BCFAs are first introduced to the fetal gastrointestinal tract in utero through the ingestion of amniotic fluid containing suspended vernix caseosa particles (8, 10–13). After delivery, BCFAs are provided to the infant through human-milk consumption. BCFAs have been reported to reduce the occurrence of necrotizing enterocolitis in a mouse model and shift microbiota toward organisms that use BCFAs in their membranes (14).
FIGURE 1.
The BCFA with a terminal isopropyl group represents an iso-BCFA (A) and that with a terminal isobutyl group represents an anteiso-BCFA (B). MarvinSketch (MarvinSketch 16.6.20, 2016; ChemAxon; http://www.chemaxon.com) was used for drawing, displaying, and characterizing chemical structures, substructures, and reactions. BCFA, branched-chain fatty acid.
The BCFA composition of foods is not routinely reported due to their low concentrations—1–3% of total lipid content (15)—and because their quantitation is obscured by more abundant straight-chain SFA and MUFA peaks in gas chromatography analyses (16). However, previous studies reported that BCFA concentrations in the diet derive predominantly from dairy products and beef, with small amounts detected in canned tuna and fermented foods such as miso and sauerkraut (8, 14). There are 7 major BCFAs in food products, which include iso-14:0, iso-15:0, anteiso-15:0, iso-16:0, iso-17:0, anteiso-17:0, and iso-18:0 (15).The American diet delivers ∼317 and 170 mg BCFAs/d consumed from dairy and beef products, respectively (8); for the sake of comparison, intakes of the well-studied n–3 fatty acids EPA and DHA in reproductive-age women are generally <300 mg/d (17).
Very few data are available on the range and quantity of BCFAs present in human milk or the extent to which maternal diet influences milk BCFA concentrations. Global comparative data on milk BCFA composition are also lacking. To pursue the relations to the microbiota, initial work is needed to understand the human diet and milk-composition relations of BCFAs. We tested the hypotheses that maternal-milk BCFAs would differ across urban populations with distinct cultures and dietary patterns and that human-milk BCFA concentrations are influenced by maternal consumption of dairy and beef products.
METHODS
Subjects
The prospective Global Exploration of Human Milk (GEHM) Study is a longitudinal cohort of mother-infant dyads residing in Cincinnati, Ohio; Shanghai, China; and Mexico City, Mexico. The primary aim of the GEHM study was to enable a comprehensive characterization of human-milk composition, comparing diverse urban populations selected for their differing genetic and dietary influences, and to determine maternal and dietary influences on human-milk composition. Samples were obtained from mothers who were identified and enrolled within 2 wk of delivery. Subject enrollment was from January 2007 to December 2008 and limited to healthy singleton term infants (≥37 wk) whose mothers planned to provide ≥75% of feedings as breast milk for ≥3 mo. Target enrollment was 120 mothers/site. The specific aims for the current analysis were to characterize the variation in fatty acid concentrations of human milk between populations and between mothers within populations and to examine the impact of maternal diet on milk fatty acid composition. Power calculations determined that 120 subjects/site provided ≥80% power (given α = 0.05 and a 2-sided test) to detect differences in the distribution of human-milk components within and between populations and to detect a correlation as low as 0.25 between maternal factors with a continuous distribution (e.g., maternal diet and fatty acids of human milk). For a balanced 1-factor ANOVA with 3 sites, n = 120/site was considered to be sufficient to detect differences between the largest and smallest group means in pooled SD units, given f = 0.17 (a small to medium Cohen’s f, in which f = square root[summation of (ni/N) × (mui – mu) ˄2/pooled variance]) where ni is the sample size of group i, N the total samples size, mui the mean of group i, and mu the grand mean. Subject enrollment methods and characteristics have been previously detailed (18).
Data and samples
Data on maternal and infant demographic factors, diet, health history, and anthropometric measures were collected by using standardized questionnaires and data collection procedures and transmitted to the Cincinnati data coordinating center electronically. Milk samples were collected between 0900 and 1300 at 2, 4, 13, 26, and 52 wk postpartum. One entire breast was emptied at the collection by using the Medela Symphony electric breast pump (Medela Corporation). Samples were separated into aliquots in 2-mL cryogenic vials with barcoded labels and stored at −80°C until analysis. Specifically for this cross-sectional analysis of the GEHM prospective cohort, week 4 human-milk samples and Cincinnati dietary data were analyzed to investigate human-milk BCFA concentrations and dietary associations. All 365 mothers enrolled in the study had a milk sample at 4 wk (n = 120 each from Cincinnati and Shanghai, n = 125 from Mexico); 359 (98%) samples were successfully analyzed.
Fatty acid analysis
The fatty acid content in breast milk was analyzed by gas chromatography by a fatty acid research laboratory (CJV and KAD) at Cincinnati Children’s Hospital. Sample results were compared with those of a fatty acid reference laboratory (JTB and RRR-R) to ensure the accuracy of peaks and concentrations.
Total lipids were extracted from 100-μL aliquots of breast milk according to a modified Bligh and Dyer method (19). A methyl heptadecanoate (10 μg/μL; Nu-Chek Prep, Inc.) internal standard was added before lipid extraction. The organic phase was collected and dried under a nitrogen stream and reconstituted in benzene. A 1-step methylation and esterification procedure was then performed with methanolic sulfuric acid (10% vol:vol) at 65°C for 1 h. The sample was neutralized with a saturated solution of sodium bicarbonate, then re-extracted with hexane. The organic phase was once again collected and dried under a nitrogen stream, then reconstituted in n-heptane for analysis.
Analysis was performed on an Agilent 7890 gas chromatograph with flame-ionization detection equipped with a 7693A autosampler. Samples were analyzed on an Agilent Durabond DB-23 30 m × 0.25 mm internal diameter × 0.25 μm film thickness. Helium was used as the carrier gas with a linear velocity of 30 cm/s. The injection port was maintained at 250°C and the detector at 260°C. The fatty acid methyl esters were eluted by using linear column temperature ramps as follows: 80°C hold for 2 min, 10°C/min to 174°C hold for 2 min, 10°C/min to 240°C hold for 10 min, and 5°C/min to 245°C hold for 5 min for a total run time of 36 min. The samples were injected at 1 μL with a split determined by the estimated amount of fatty acid present (range from 1:10 to 1:100). Fatty acid methyl esters were quantified by using experimentally derived standard curves. Six BCFAs were used as authentic reference standards (iso-14:0, anteiso-15:0, iso-16:0, anteiso-17:0, iso-18:0, and iso-20:0; Larodan Lipids).
Dietary assessment
The dietary intake of Cincinnati participants was assessed via 3 random 24-h dietary recall interviews conducted in person or by phone between weeks 4 and 13 postpartum. Trained interviewers from the Bionutrition Core of the Clinical Translational Research Center at Cincinnati Children’s Hospital Medical Center used the USDA multiple-pass method to ensure accurate collection of data with regard to food items and amounts consumed. Participants were also provided with instructions and handouts to assist them with estimating portion sizes during the interview.
Nutrition Data Systems for Research (NDSR; Nutrition Coordinating Center, University of Minnesota) was used to assess total energy and macronutrient consumption, as well as intakes from food groups, including meat and dairy groups and subgroups. Results from the 3 d of intake were averaged to obtain an estimation of usual daily intake. The NDSR software and foods database provide the highest quality analysis of nutrients for research purposes, with a complete profile of >150 nutrients and other food components for every item in the database. To reflect the marketplace throughout the study, dietary intake data were collected by using NDSR software versions 2006, 2007, and 2008; and final calculations were completed by using NDSR 2009. The NDSR time-related database updates analytic data while maintaining nutrient profiles true to the version used for data collection (20).
Ethics
Approval was obtained from the Institutional Review Board of Christ Hospital for hospital recruitment of subjects for the Cincinnati site. Additional institutional review board approval was obtained from both the Mexico (Instituto Nacional de Ciencias Médicas y Nutrición) and China (Fudan University) sites. All of the mothers provided written informed consent.
Statistical analysis
Differences across sites in infant and maternal demographic and health characteristics were tested by ANOVA for continuous variables and chi-square tests or Fisher’s exact tests for categorical variables. Tobit regression was used to obtain geometric means ± SEs for BCFAs (mg/100 mL), accounting for concentrations below the limit of detection (i.e., left censored) (21, 22). Tobit regression is a generalized linear model for censored data that uses a latent variable approach to model observed data by maximum likelihood estimation and assumes that censored data arise from the same distribution producing the measured values. In the absence of censored values, Tobit regression is the Gaussian generalized linear model (23). BCFAs were natural log transformed before regression to improve model fit and to limit the influence of extreme values. The limit of detection was considered to be the lowest observed value across the 3 sites. Differences in BCFA concentrations between sites were tested by a likelihood ratio test that compared the inclusion of site to the null model. Additional analyses were performed to test whether differences in BCFA concentrations between sites persisted after adjustment for delivery mode, maternal age, and BMI. Tobit regression was also used to examine associations between dietary intake, defined as the number of serving sizes of beef and dairy on the basis of the 2000 Dietary Guidelines for Americans, and BCFA concentrations in the milk of Cincinnati mothers. Beef and dairy servings were adjusted for total energy intake by using the multivariate nutrient density method (24). An indicator variable denoting no beef intake was included as a covariate in models for beef due to the nonnegligible number of participants who reported no consumption (n = 29). The inclusion of both the continuous and indicator variables provides a parameter estimate for the association of beef intake among those who reported beef consumption, the association for those not eating beef, and the retention of all subjects in the model. Regressions were performed on the log-log scale to better meet model assumptions. Due to the large number of observations below the limit of detection for iso-18:0 (n = 74), logistic regression was performed to assess whether higher consumption of beef or dairy was associated with the ability to detect this BCFA in breast milk. All of the analyses were performed by using SAS version 9.3 statistical software (2011; SAS Institute, Inc.) or Stata Statistical Software version 12 (2011; StataCorp).
RESULTS
Maternal demographic and health characteristics differed across the 3 sites (Table 1). Cincinnati, Shanghai, and Mexico City mothers, respectively, had mean prepregnancy BMIs (in kg/m2) of 27.6, 20.6, and 23.9 (P < 0.001); mean ages at delivery of 31.4, 29.3, and 24.4 y (P < 0.001); and a mean previous number of children of 1.54, 0.04, and 1.01 (P < 0.001). The proportion of cesarean deliveries for Cincinnati, Shanghai, and Mexico City were 23%, 70%, and 43%, respectively (P < 0.001).
TABLE 1.
Demographic characteristics of study mothers and their infants in Cincinnati, Shanghai, and Mexico City at baseline1
| Cincinnati | Mexico City | Shanghai | Omnibus P2 | |
| n | 115 | 125 | 119 | |
| Infant characteristics | ||||
| Male, n (%) | 51 (44.4) | 64 (51.2) | 63 (52.9) | 0.38 |
| Birth weight, kg | 3.54 ± 0.453 | 3.11 ± 0.37 | 3.43 ± 0.47 | <0.01 |
| Maternal delivery characteristics | ||||
| Age at delivery, y | 31.4 ± 5.20 | 24.4 ± 5.60 | 29.3 ± 3.70 | <0.01 |
| Parity, n | 1.54 ± 1.56 | 1.01 ± 1.28 | 0.04 ± 0.02 | <0.01 |
| Prepregnancy BMI, kg/m2 | 27.6 ± 6.8 | 23.9 ± 3.20 | 20.6 ± 2.3 | <0.01 |
| Prepregnancy BMI category, n (%) | ||||
| Underweight (<18) | 0 (0.00) | 1 (0.80) | 11 (9.24) | <0.01 |
| Normal (18–24.9) | 51 (44.4) | 76 (60.8) | 102 (85.7) | |
| Overweight (25–29.9) | 29 (25.2) | 30 (24.0) | 4 (3.36) | |
| Obese (≥30) | 33 (28.7) | 5 (4.00) | 1 (0.84) | |
| Gestational weight gain, kg | 13.6 ± 5.0 | 11.2 ± 5.3 | 16.1 ± 5.3 | <0.01 |
| Delivery type (vaginal), n (%) | 89 (77.4) | 72 (56.6) | 36 (30.3) | <0.01 |
n = 358. Baseline data were available for only 118 mothers in Mexico City.
Omnibus P values for comparisons across cohorts were derived by using ANOVA or chi-square tests.
Mean ± SD (all such values).
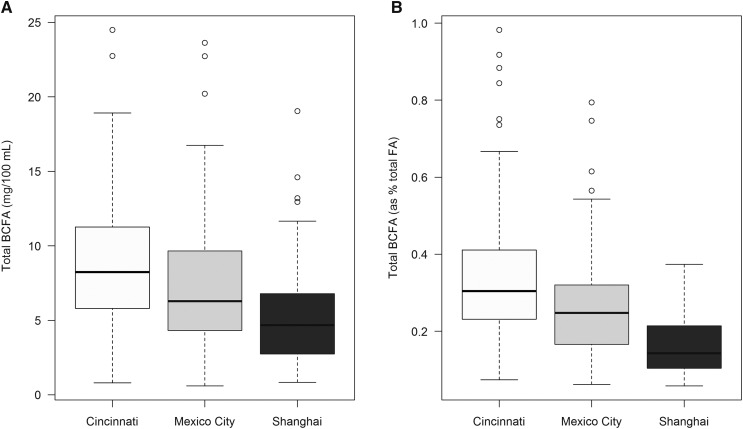
Total and specific BCFAs according to site are presented in Figures 2 and 3. The sum of total BCFAs in milk samples differed by site, with the highest total milk BCFA concentration in Cincinnati followed by Mexico and Shanghai (mean ± SE: 7.90 ± 0.41, 6.10 ± 0.36, and 4.27 ± 0.25 mg/100 mL, respectively; P < 0.001). The BCFAs iso-14:0, anteiso-15:0, iso-16:0, anteiso-17:0, and iso-18:0 differed in milk samples between the 3 sites (P < 0.001; Table 2). Differences in BCFA concentrations across sites persisted after controlling for delivery mode and maternal age and BMI (data not shown). BCFA concentrations across sites were not different on the basis of infant sex (data not shown).
FIGURE 2.
Box plots showing medians and IQRs of total BCFAs by site. Panel A presents total BCFA concentrations (mg/100 mL) by site. Panel B presents BCFAs as a percentage of total FAs by site (BCFAs divided by the sum total of FAs determined included 10:0–22:6n–3). The top and bottom of each box plot represents the 25th and 75th percentiles, and whiskers indicate 1.5 times the IQR. Data points set outside these limits are plotted. BCFA, branched-chain fatty acid; FA, fatty acid.
FIGURE 3.
Box plots showing medians and IQRs of individual BCFAs by site. Panel A presents BCFA concentrations (mg/100 mL) by site. Panel B presents individual BCFAs as a percentage of total BCFAs by site. Plots include imputed data for values below the limit of detection. Single imputation was performed from a truncated log-normal distribution with means ± SDs obtained from Tobit regression. The top and bottom of each box plot represents the 25th and 75th percentiles, and whiskers indicate 1.5 times the IQR. Data points set outside these limits are plotted. BCFA, branched-chain fatty acid.
TABLE 2.
BCFA concentrations in human milk by site1
| Cincinnati (n = 115) |
Mexico City (n = 125) |
Shanghai (n = 119) |
|||||
| BCFA, mg/100 mL | Censored, n | Mean ± SE | Censored, n | Mean ± SE | Censored, n | Mean ± SE | P2 |
| iso-14:0 | 5 | 0.48 ± 0.04 | 11 | 0.36 ± 0.04 | 36 | 0.13 ± 0.02 | <0.01 |
| anteiso-15:0 | 0 | 1.91 ± 0.11 | 1 | 1.34 ± 0.09 | 14 | 0.56 ± 0.06 | <0.01 |
| iso-16:0 | 0 | 1.49 ± 0.08 | 1 | 1.09 ± 0.06 | 2 | 0.73 ± 0.05 | <0.01 |
| anteiso-17:0 | 4 | 3.61 ± 0.23 | 0 | 2.87 ± 0.17 | 0 | 2.24 ± 0.12 | <0.01 |
| iso-18:0 | 74 | — | 36 | 0.16 ± 0.02 | 29 | 0.18 ± 0.02 | 0.673 |
| Total BCFAs | 0 | 7.90 ± 0.41 | 0 | 6.10 ± 0.36 | 0 | 4.27 ± 0.25 | <0.01 |
Unless otherwise indicated, values are geometric means ± SEs obtained from Tobit regression. BCFA, branched-chain fatty acid; —, estimates are not provided where >50% of observations were censored.
P values for likelihood-ratio test assessing differences in log means across sites unless otherwise indicated.
P value for the comparison of Shanghai and Mexico City only.
The geometric means ± SEs of milk BCFAs, obtained from Tobit regression, according to site are provided in Table 2. Estimates of geometric means were not provided for iso-18:0 in Cincinnati, for which >50% of observations were censored. BCFA concentrations were typically lower in Shanghai than in Cincinnati and Mexico City, except for iso-18:0, for which concentrations were low across all 3 sites.
Dietary data were available for the Cincinnati site, and adjusted total energy models were used to compare dietary consumption of BCFAs and human-milk BCFA concentrations (Table 3). The median servings (IQR) of beef were 0.71 servings/d (1.68) for all subjects and 1.20 servings/d (1.75) for those who reported beef consumption. The median serving of dairy was 2.05 servings/d (2.02). Beef consumption was positively associated with the BCFA iso-16:0 in breast milk. Dairy consumption was positively associated with the BCFAs iso-14:0, anteiso-15:0, and iso-16:0 in breast milk. A 10% increase in servings of beef was associated with a 4.7% (P = 0.03) increase in iso-16:0 concentrations in breast milk, and in dairy this was associated with a 4.1% (P < 0.001) increase in iso-14:0, a 1.8% (P = 0.01) increase in anteiso-15:0, and a 1.6% (P = 0.02) increase in iso-16:0 concentrations (Table 3). No associations were detected between dairy or beef consumption and anteiso-17:0 or iso-18:0 BCFAs examined. The intake of beef associated with anteiso-15:0 and total BCFAs from human milk approached significance (P = 0.06). Neither dairy nor beef intake was associated with the ability to detect iso-18:0 in human milk (data not shown). In addition, total BCFA concentrations were not significantly associated with beef or dairy consumption (Table 3).
TABLE 3.
Dietary beef and dairy consumption in relation to milk BCFA concentrations in mothers in Cincinnati, Ohio1
| Diet and BCFA | n | Censored, n | β ± SE | <95% CI | >95% CI | P |
| Beef | ||||||
| iso-14:0 | 103 | 5 | 0.42 ± 0.36 | −0.29 | 1.13 | 0.24 |
| anteiso-15.0 | 103 | 0 | 0.41 ± 0.22 | −0.02 | 0.85 | 0.06 |
| iso-16:0 | 103 | 0 | 0.47 ± 0.21 | 0.05 | 0.89 | 0.03 |
| anteiso-17:0 | 103 | 4 | 0.26 ± 0.27 | −0.26 | 0.78 | 0.33 |
| Total BCFAs | 103 | 0 | 0.39 ± 0.21 | −0.02 | 0.81 | 0.06 |
| Dairy | ||||||
| iso-14:0 | 103 | 5 | 0.41 ± 0.10 | 0.21 | 0.62 | 0.01 |
| anteiso-15.0 | 103 | 0 | 0.18 ± 0.07 | 0.05 | 0.31 | 0.01 |
| iso-16:0 | 103 | 0 | 0.16 ± 0.06 | 0.06 | 0.28 | 0.05 |
| anteiso-17:0 | 103 | 4 | 0.06 ± 0.08 | −0.10 | 0.21 | 0.49 |
| Total BCFAs | 103 | 0 | 0.11 ± 0.06 | −0.02 | 0.23 | 0.09 |
Associations are shown for natural log-log models. Tobit regression was used to account for values below the limit of detection, indicated by number censored. Parameter estimates for beef intake reflect associations in women who reported beef consumption. Median servings (IQRs) of beef intake were 0.71 (1.68) for all subjects and 1.20 (1.75) for those who reported beef consumption. The median (IQR) serving of dairy was 2.05 (2.02). The sample includes all mothers who completed at least one 24-h recall. The β-coefficients represent the percentage increase in milk BCFAs (in mg/100 mL) for a 1% increase in dietary servings. One serving size is defined by the recommendations of the 2000 Dietary Guidelines for Americans (https://health.gov/dietaryguidelines/dga2000/document/frontcover.htm). BCFA, branched-chain fatty acid.
DISCUSSION
The concentrations of BCFAs in human milk differed significantly between our global study sites in Cincinnati, Ohio; Shanghai, China; and Mexico City, Mexico. Within Cincinnati, human milk iso-14:0, anteiso-15:0, and iso-16:0 concentrations were significantly associated with maternal dairy intake, whereas beef consumption was associated only with iso-16:0 concentration. International differences in milk BCFA concentrations persisted after adjustment for prepregnancy BMI, maternal age at delivery, and delivery mode. In addition, BCFA concentrations in human milk were not associated with infant sex.
Ran-Ressler et al. (8) estimated that the daily intake of BCFAs from the diet in the United States is ∼500 mg/d, derived mainly from intakes of dairy and beef. Concentrations of BCFAs in beef fat are reportedly lower than those from dairy fat, which is consistent with our finding that higher BCFA concentrations in mother’s milk were significantly associated with dairy compared with beef. Additional dairy products to consider for associations of BCFAs are cheese from sheep and goats, which have 2.7% and 2.2% BCFAs, respectively (8), and BCFAs can reach 6.1% (wt:wt) in cheddar cheese made from the milk of Nepalese yaks (25). However, because these are not commonly consumed food items in our Cincinnati site, they were not used in the current dietary analysis. They could be of potential interest in future analyses of dietary assessments with the Shanghai and Mexico City sites. An animal-based food product to consider for future analysis includes fish, especially for the Shanghai site. A recent study showed that wild domestic freshwater fish contain concentrations of BCFAs in total lipids similar to those in dairy, although fish likely contribute ∼2.5–24 mg/70-g serving, depending on species (26). The BCFA concentration in human milk in the United States was estimated to be 19 mg/100 mL on the basis of concentrations previously measured in human milk (27) and estimated from fat (16). Our data indicate that this previous estimate is higher than our measured concentrations in human milk, because the total BCFA concentration in the milk of Cincinnati mothers was 7.9 mg/100 mL (Figure 3). This difference could be due to the assumptions of the estimate or our ability to measure only 5 BCFAs, whereas previous studies (8, 15) reported measurements of 7 BCFAs from foods. However, our data are similar to those in previous studies insofar as anteiso-15:0 and anteiso-17:0 comprise half of the total BCFA concentrations in cow milk–based dairy products and iso-18:0 contributes very little to total BCFA concentrations (8, 15).
Endogenous human BCFA synthesis occurs in surface glands, mainly the sebaceous and meibomian glands, from chain elongation of deaminated branched-chain amino acids. Very little is known about de novo BCFA synthesis in internal tissues (e.g., the mammary gland). Similarly, nothing is known about human pathways of BCFA interconversion on ingestion—for instance, chain shortening or elongation. BCFAs are seldom reported in fatty acid profiles of nonruminant tissues.
Historically, SFAs, especially those from animal products, have been associated with increased health risks; however, current evidence has shown that not all SFAs are the same, especially in relation to chronic disease (28, 29). Recent studies have shown that fat intakes from dairy products may have beneficial health effects and are not associated with an increased risk of type 2 diabetes (30, 31), cardiovascular disease (30), or obesity (31). One study showed that yogurt intake was inversely associated with the risk of type 2 diabetes (30). In addition, recent studies indicated that animal-based diets have a greater effect on the microbiota than do plant-based diets. In a diet study in 6 men and 4 women, changes in gut microbial communities were assessed on the basis of differing dietary groups as an animal- or plant-based diet, with similar caloric intake. Changes in the microbiota, assessed by 16S ribosomal RNA gene sequencing, in individuals who consumed the animal-based diet were significantly different from baseline microbial communities within 1 d of consuming the diet; however, this was not observed for individuals who consumed the plant-based diet (32). In a randomized trial in 45 exclusively breastfed infants, complementary feeding with a puréed meat diet resulted in a butyrate-producing microbial pattern different that in from the cereal-based group (33). Food products containing BCFAs may help to promote gut health because BCFAs have active roles in reducing inflammation and altering the microbiota of the gut in an animal model (34).
Many bacterial species synthesize BCFAs, which are essential to their membrane function (16), and modulating properties similarly to cis MUFAs (35); however, BCFAs are not vulnerable to attack by oxygen because they are saturated. For example, 81% of the fatty acids of Staphylococcus aureus and >90% of Brevibacterium species incorporated are BCFAs (4, 36). As integral components of bacteria, the dietary BCFAs could modify the microbial community, favoring bacteria that use BCFAs in their membranes (16).
This study included lactating women in 3 diverse global populations. Although the women enrolled were not randomly selected from all of the eligible women in their respective populations, they reasonably represent healthy women with term deliveries. The data show the variability in BCFAs in human milk of different mothers and diverse populations, which also raises the question of the source or derivation of BCFAs in human milk. We found that some of the variability in BCFA composition of human milk is attributable to the maternal diet. Not all of the BCFAs in human milk are due to dairy, which is not commonly consumed by women in Shanghai. Another source of BCFAs may be maternal intestinal bacteria. BCFAs synthesized by bacteria may be transported into human milk via endogenous pathways (37–41). A further understanding of specific SFAs and their dietary source may enable clinicians to guide patients in their choice of foods to target a healthier microbiota and outcome. Our work provides an initial landscape on which future work can define the source or derivation of BCFAs found in human milk and how this relates to infant gut health. Historically, animal fats have been removed from infant formula and replaced with vegetable oils (42). On the basis of BCFA abundance in human milk, and their potential health benefits, opportunities may exist for future innovations in infant formula.
In conclusion, human milk is a source of diverse bioactive and dietary components that contribute to an infant’s health and immunity (41). The BCFA concentration of human milk differs across populations and between mothers. A higher concentration of shorter-chain BCFAs in mother’s milk is significantly associated with dietary intakes of dairy and beef, but the intake data do not fully account for the range of BCFAs found. The maternal microbiota is a likely source of de novo BCFA synthesis. The transmission of maternal BCFAs to the nursing infant may plausibly support commensal bacteria for the infant gut, which are known to originate, in part, from the mother’s microbiota. This global examination provides a foundation to investigate health relations of dairy and beef intakes in the maternal diet, BCFAs in mother’s milk, and their potential effects on the infant microbiota and health.
Acknowledgments
We acknowledge the expert assistance of Donna Wuest in manuscript preparation.
The authors’ responsibilities were as follows—CJV, YMP, MLG, GMR-P, RJM, JTB, and ALM: designed the research; KAD, BSD, and SS: conducted the research; RRR-R and JTB: provided essential reagents; KAD, NJO, JGW, and ALM: analyzed the data; KAD, NJO, and ALM: interpreted results for this manuscript; KAD, CJV, NJO, and ALM: wrote the manuscript; KAD, CJV, RJM, and ALM: had primary responsibility for final content; and all authors: read and approved the final manuscript. ALM and CJV received funding from Mead Johnson Nutrition, Inc., as a scientific research grant; RJM was an employee of the Mead Johnson Pediatric Nutrition Institute. The remaining authors had no conflicts of interest.
Footnotes
Abbreviations used: BCFA, branched-chain fatty acid; GEHM, Global Exploration of Human Milk; NDSR, Nutrition Data Systems for Research.
REFERENCES
- 1.American Academy of Pedicatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–41. [DOI] [PubMed] [Google Scholar]
- 2.WHO/UNICEF. Protecting, promoting and supporting breastfeeding: the special role of maternity services—a joint WHO/UNICEF statement. Int J Gynaecol Obstet 1990;31(Suppl 1):171–83. [PubMed] [Google Scholar]
- 3.Brenna JT, Carlson SE. Docosahexaenoic acid and human brain development: evidence that a dietary supply is needed for optimal development. J Hum Evol 2014;77:99–106. [DOI] [PubMed] [Google Scholar]
- 4.Kaneda T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol Rev 1991;55:288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang HY, Huang SY, Chen PY, King VA, Lin YP, Tsen JH. Basic characteristics of Sporolactobacillus inulinus BCRC 14647 for potential probiotic properties. Curr Microbiol 2007;54:396–404. [DOI] [PubMed] [Google Scholar]
- 6.Veerkamp JH. Fatty acid composition of Bifidobacterium and Lactobacillus strains. J Bacteriol 1971;108:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie WW. The Lipid Library. Analysis of trans fatty acids by chromatography and spectroscopy 2012 [cited 2016 Oct 28]; Available from: http://lipidlibrary.aocs.org/History/content.cfm?ItemNumber=40367.
- 8.Ran-Ressler RR, Bae S, Lawrence P, Wang DH, Brenna JT. Branched-chain fatty acid content of foods and estimated intake in the USA. Br J Nutr 2014;112:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egge H, Murawski U, Ryhage R, Gyorgy P, Chatranon W, Zilliken F. Minor constituents of human milk. IV. Analysis of the branched chain fatty acids. Chem Phys Lipids 1972;8:42–55. [DOI] [PubMed] [Google Scholar]
- 10.Nicolaides N, Ray T. Skin lipids. 3. Fatty chains in skin lipids: the use of vernix caseosa to differentiate between endogenous and exogenous components in human skin surface lipid. J Am Oil Chem Soc 1965;42:702–7. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen TA, Luukkainen T. Gas-liquid chromatographic and mass spectrometric studies on sterols in vernix caseosa, amniotic fluid and meconium. Acta Chem Scand 1968;22:2603–12. [DOI] [PubMed] [Google Scholar]
- 12.Sherman DJ, Ross MG, Day L, Ervin MG. Fetal swallowing: correlation of electromyography and esophageal fluid flow. Am J Physiol 1990;258:R1386–94. [DOI] [PubMed] [Google Scholar]
- 13.Ran-Ressler RR, Devapatla S, Lawrence P, Brenna JT. Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr Res 2008;64:605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ran-Ressler RR, Khailova L, Arganbright KM, Adkins-Rieck CK, Jouni ZE, Koren O, Ley RE, Brenna JT, Dvorak B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS One 2011;6:e29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauff S, Vetter W. Quantification of branched chain fatty acids in polar and neutral lipids of cheese and fish samples. J Agric Food Chem 2010;58:707–12. [DOI] [PubMed] [Google Scholar]
- 16.Ran-Ressler RR, Glahn RP, Bae S, Brenna JT. Branched-chain fatty acids in the neonatal gut and estimated dietary intake in infancy and adulthood. Nestle Nutr Inst Workshop Ser 2013;77:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fats and fatty acids in human nutrition: report of an expert consultation. FAO Food Nutr Pap 2010;91:1–166. [PubMed] [Google Scholar]
- 18.Woo JG, Guerrero ML, Ruiz-Palacios GM, Peng YM, Herbers PM, Yao W, Ortega H, Davidson BS, McMahon RJ, Morrow AL. Specific infant feeding practices do not consistently explain variation in anthropometry at age 1 year in urban United States, Mexico, and China cohorts. J Nutr 2013;143:166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7. [DOI] [PubMed] [Google Scholar]
- 20.Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products—a research perspective. J Food Compos Anal 2001;14:315–22. [Google Scholar]
- 21.Tobin J. Estimation of relationships for limited dependent variables. Econometrica 1958;26:24–36. [Google Scholar]
- 22.Amemiya T. Tobit models: a survey. J Econom 1984;24:3–61. [Google Scholar]
- 23.Smithson M, Merkle EC. Generalized linear models for categorical and continuous limited dependent variables. New York: Chapman and Hall/CRC; 2013. [Google Scholar]
- 24.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 Suppl):1220S–8S; discussion: 9S–31S. [DOI] [PubMed] [Google Scholar]
- 25.Or-Rashid MM, Odongo NE, Subedi B, Karki P, McBride BW. Fatty acid composition of yak (Bos grunniens) cheese including conjugated linoleic acid and trans-18:1 fatty acids. J Agric Food Chem 2008;56:1654–60. [DOI] [PubMed] [Google Scholar]
- 26.Wang DH, Jackson JR, Twining C, Rudstam LG, Zollweg-Horan E, Kraft CE, Lawrence P, Kothapalli K, Wang Z, Brenna JT. Saturated branched chain, normal odd-carbon-numbered, and n-3 (omega-3) polyunsaturated fatty acids in freshwater fish in the northeastern United States. J Agric Food Chem 2016. Oct 4 (Epub ahead of print; DOI: 10.1021/acs.jafc.6b03491). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aitchison JM, Dunkley WL, Canolty NL, Smith LM. Influence of diet on trans fatty acids in human milk. Am J Clin Nutr 1977;30:2006–15. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D. Saturated fatty acids and type 2 diabetes: more evidence to re-invent dietary guidelines. Lancet Diabetes Endocrinol 2014;2:770–2. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein AH. Dietary trans fatty acids and cardiovascular disease risk: past and present. Curr Atheroscler Rep 2014;16:433. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med 2014;12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aune D, Norat T, Romundstad P, Vatten LJ. Dairy products and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Am J Clin Nutr 2013;98:1066–83. [DOI] [PubMed] [Google Scholar]
- 32.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, Robertson CE, Frank DN. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J Pediatr 2013;163:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ran-Ressler RR, Sim D, O’Donnell-Megaro AM, Bauman DE, Barbano DM, Brenna JT. Branched chain fatty acid content of United States retail cow’s milk and implications for dietary intake. Lipids 2011;46:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silbert DF, Ladenson RC, Honegger JL. The unsaturated fatty acid requirement in Escherichia coli: temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta 1973;311:349–61. [DOI] [PubMed] [Google Scholar]
- 36.Selvam R, Maheswari P, Kavitha P, Ravichandran M, Sas B, Ramchand CN. Effect of Bacillus subtilis PB6, a natural probiotic on colon mucosal inflammation and plasma cytokines levels in inflammatory bowel disease. Indian J Biochem Biophys 2009;46:79–85. [PubMed] [Google Scholar]
- 37.Martin V, Maldonado-Barragan A, Moles L, Rodriguez-Banos M, Campo RD, Fernandez L, Rodriguez JM, Jimenez E. Sharing of bacterial strains between breast milk and infant feces. J Hum Lact 2012;28:36–44. [DOI] [PubMed] [Google Scholar]
- 38.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–7. [DOI] [PubMed] [Google Scholar]
- 39.Roitt IM, Delves PJ, Martin SJ, Burton DR. Roitt’s essential immunology. 12th ed. Hoboken (NJ): Wiley Blackwell; 2011. [Google Scholar]
- 40.Jeurink PV, van Bergenhenegouwen J, Jimenez E, Knippels LM, Fernandez L, Garssen J, Knol J, Rodriguez JM, Martin R. Human milk: a source of more life than we imagine. Benef Microbes 2013;4:17–30. [DOI] [PubMed] [Google Scholar]
- 41.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Committee on the Evaluation of the Addition of Ingredients New to Infant Formula; Food and Nutrition Board, Institute of Medicine. Comparing infant formulas with human milk. In: Carroll S, editor. Infant formula: evaluating the safety of new ingredients. Washington (DC): National Academies Press; 2004. p. 41–54. [Google Scholar]