Abstract
At a population level, there is growing evidence of the beneficial effects of dietary flavonoids on health. However, there is extensive heterogeneity in the response to increased intake, which is likely mediated via wide interindividual variability in flavonoid absorption and metabolism. Flavonoids are extensively metabolized by phase I and phase II metabolism (which occur predominantly in the gastrointestinal tract and liver) and colonic microbial metabolism. A number of factors, including age, sex, and genotype, may affect these metabolic processes. In addition, food composition and flavonoid source are likely to affect bioavailability, and emerging data suggest a critical role for the microbiome. This review will focus on the current knowledge for the main subclasses of flavonoids, including anthocyanins, flavonols, flavan-3-ols, and flavanones, for which there is growing evidence from prospective studies of beneficial effects on health. The identification of key factors that govern metabolism and an understanding of how the differential capacity to metabolize these bioactive compounds affect health outcomes will help establish how to optimize intakes of flavonoids for health benefits and in specific subgroups. We identify research areas that need to be addressed to further understand important determinants of flavonoid bioavailability and metabolism and to advance the knowledge base that is required to move toward the development of dietary guidelines and recommendations for flavonoids and flavonoid-rich foods.
Keywords: absorption, ADME, flavonoids, genotype, health, metabolism, microbiome
INTRODUCTION
Dietary flavonoids represent a diverse range of polyphenolic compounds that are present in many commonly consumed fruits, vegetables, grains, herbs, and beverages (1). Growing evidence from both population-based studies and randomized controlled trials (RCTs)2 suggests that several flavonoid subclasses may be important for cardiometabolic health with substantial interest in other outcomes, including cognitive function, Parkinson disease, and specific cancers, also developing (2–6). In this article, rather than conducting an exhaustive review of the current literature, we set out to summarize the current state of the art in the field by drawing on examples from recent studies on specific subclasses to highlight gaps in our understanding that may explain discrepancies in findings across the translational research pathway. We also identify research areas that need to be addressed to further understand how to optimize intake of flavonoids for different health benefits and in specific subgroups and to advance the knowledge base that is required to move toward the development of dietary guidelines and recommendations for flavonoids and flavonoid-rich foods.
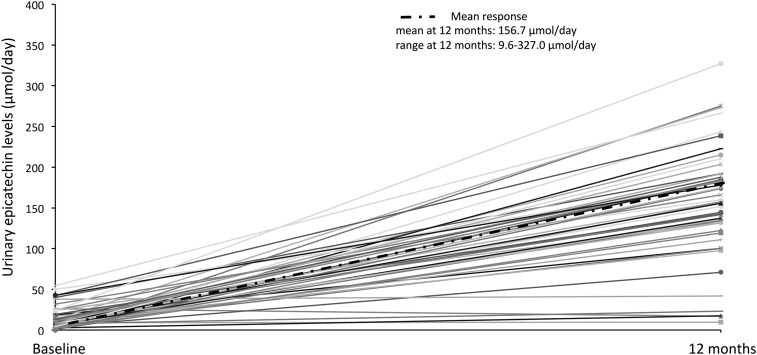
The structural complexity of flavonoids has led to their subclassification as flavonols, flavones, flavanones, flavan-3-ols (including their oligomeric and polymeric forms, proanthocyanidins), isoflavones, and anthocyanins (7–9). The diversity of flavonoid structures undoubtedly contributes to differences in biological efficacy with subtle differences affecting both bioavailability and bioactivity. It is clear that the bioavailability of dietary flavonoids is highly variable between individuals. After ingestion, flavonoids undergo extensive metabolization with absorption occurring in both the small and large intestines with a substantial fraction of intake reaching the colon, where the flavonoids are exposed to colonic microbiota. The resident microbiome operates as a metabolic reactor, thereby playing a key role in catabolizing unabsorbed flavonoids into smaller molecules such as phenolic and aromatic acids, which may become bioavailable (10). Data from available interventions provide evidence to suggest that there is extensive variability in the amount of metabolites that have been measured in biological samples, with 15–99% of the original flavonoid dose recovered as a wide range of flavonoid metabolites (7, 11). The heterogeneity has been highlighted in data from a 1-y flavonoid intervention in 93 participants; mean 24-h urinary epicatechin (flavan-3-ol) excretion rates were 156.7 μmol/d with wide interindividual variability that ranged from 9.6 to 327.0 μmol/d across study participants (Figure 1) (12). This metabolic variability has likely been a factor that has contributed to the wide CIs in the physiologic responsiveness observed in both intervention and observational studies.
FIGURE 1.
Mean interindividual variability in urinary epicatechin excretion in 93 participants after intake of 85 mg epicatechin/d for 1 y (12).
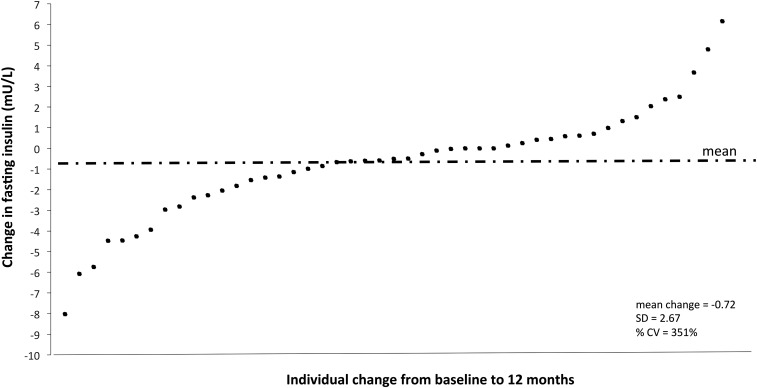
Although a large variability in the physiologic response to flavonoid intake has also been observed (Figure 2 shows the insulin response to the previously mentioned 1-y intervention) (12), RCTs have seldom concurrently addressed metabolism and health outcomes. The few studies that have included plasma or urinary measures of the flavonoid under study did so predominantly as a measure of compliance to the intervention (7, 9, 12). At a population level, the heterogeneity in responsiveness and a poor response to flavonoid intake in certain individuals may, therefore, obscure beneficial associations between intakes and health outcomes in responsive subgroups.
FIGURE 2.
Variability in changes in fasting insulin concentrations (mU/L) in 93 participants after a 1-y flavonoid intervention (12).
In some prospective studies, strong associations between low intakes of anthocyanins (median intakes of 15 mg/d with a range up to 1 g/d) and beneficial health effects have been observed (2, 3), whereas in other studies, no effects have been observed for anthocyanins, but benefits have been reported for other subclasses including flavonols (13, 14). This variability in the strength of the association between flavonoid intake, the wide CIs observed, and the responsiveness both within and between populations were likely, in large part, attributable to differences in the absorption, distribution, metabolism, and elimination (ADME) of flavonoids as will be discussed. This review will focus mainly on the current knowledge and research gaps for the main subclasses of flavonoids, including anthocyanins, flavonols, flavan-3-ols, and flavanones, for which there is growing evidence from prospective studies for beneficial effects on health. The isoflavone-related literature will not be included because isoflavone metabolism and biofficacy have been extensively reviewed previously (15, 16), and intakes of this flavonoid subgroup are low (<3 mg/d) in individuals who have followed a Western-style diet in which soy products are not commonly consumed (17).
OVERVIEW OF FLAVONOID METABOLISM
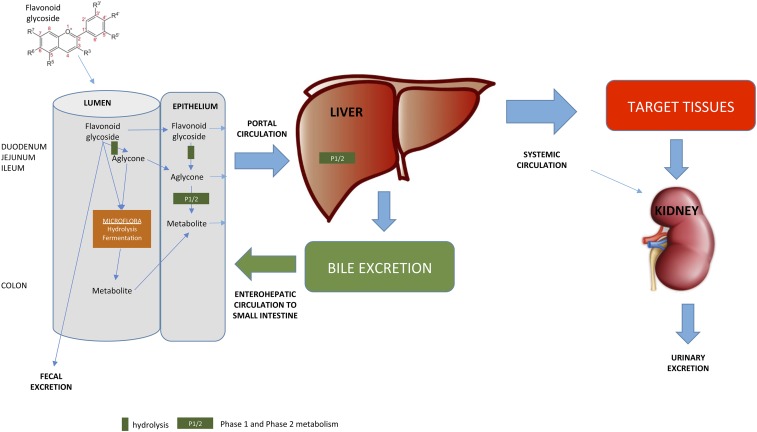
An overview of flavonoid ADME is given in Figure 3 (18–20). Flavonoids are generally consumed as glycosides with a proportion of the aglycone released either in the epithelium or lumen of the small intestine. Unlike dietary macronutrients and micronutrients, a large proportion of ingested flavonoids are unabsorbed in the proximal intestine and reach the colon where they are exposed to microbiome-mediated hydrolysis and fermentation. Within the epithelium, flavonoids undergo phase I metabolism with the resultant metabolites transported to the liver via the portal vein. In the liver, they undergo further phase I and phase II metabolism that result in more-polar compounds, which mediate an array of biological effects in target tissues. The efflux of flavonoids from the body is via the kidney, from the intestinal epithelium, and via bile excretion. Flavonoids secreted via the biliary route into the duodenum are subjected to the action of microbial enzymes and may be reabsorbed and undergo enterohepatic recycling (Figure 3).
FIGURE 3.
Overview of flavonoid absorption and postabsorptive metabolism.
Absorption
In the lumen of the small intestine, lactase phlorizin hydrolase (LPH, lactase) hydrolyses flavonoid glycosides into their respective aglycones (21). LPH is a transmembrane protein with broad substrate specificity for a range of flavonoid-O-β-d-glucosides. Aglycones may enter the epithelial cells by passive diffusion as a result of increased lipophilicity. Alternatively, the glycosides can be directly transported into the epithelium via epithelial transporters such as sodium-dependent glucose transporter (21–24) with the glycosides subsequently hydrolyzed by intracellular β-glucosidases such as cytosolic β-glucosidases (21, 25, 26). Therefore, as a general rule, flavonoid glycosides are cleaved either in the intestinal lumen or epithelium before absorption. However, anthocyanins are an exception and are present in plasma and urine as glycosides (27). The impact of lactase deficiency (which has a 60–100% prevalence in many Latin American, African, and Asian countries) on flavonoid bioavailability is currently unknown.
Membrane-bound ATP-binding cassette (ABC) transporter proteins are involved in the epithelial transcellular passage of many compounds including dietary flavonoids (28). This protein group is involved in the efflux of bioactive compounds either through the basolateral membrane into the portal bloodstream, which facilitates absorption, or transported back into the intestinal lumen thereby reducing bioavailability. The main ABC-group members include P-glycoprotein, multidrug resistance proteins, and breast cancer–resistance protein (28). In addition to transcellular absorption, transport via the paracellular route has also been identified (29, 30) with its relative contribution to the overall bioavailability likely to be dose and isoform dependent.
Postabsorptive metabolism
After absorption, flavonoids may undergo phase I metabolism in the liver (oxidation or O-demethylated) by cytochrome P450 monooxygenases. In humans, there are 57 cytochrome genes that are divided in 18 families with isoforms such as cytochrome 1A1, cytochrome 1A2, cytochrome 1B1, cytochrome 3A4 (the predominant human intestinal and hepatic cytochrome 450s), and cytochrome 2C9 that are involved in flavonoid metabolism (31, 32). However, phase I metabolism–derived oxidation products tend to be minor metabolites of most flavonoids, which is probably due to the rapid glucuronidation, sulphation, or methylation of potential phase I substrates in the intestine and the liver (18, 33) by phase II conjugating enzymes including urine-5′-diphosphate glucuronosyltransferases (UGTs), sulphotransferases, and catechol-O-methyltransferases (COMTs). The glucuronide sulfate and methyl conjugates are more-polar metabolites and may be excreted via the kidneys in urine or via bile or transported by ABC-mediated efflux back into the intestinal lumen. In general, the majority of conjugates in the plasma and urine are glucuronides (34). The conjugation mechanisms are highly efficient, and aglycones are generally either absent in the circulation or present in low concentrations after physiologic intakes.
UGTs catalyze the transfer of a glucuronic acid from UDP-glucuronic acid to polyphenols (including flavonoids) and other xenobiotics. The UGT gene superfamily gives rise to >22 UGT isoforms that belong to the UGT1A, UGT2A, UGT2B, UGT3, or UGT8 families (35). The glucuronidation of flavonoids is regiospecific and isoform dependent (36, 37). Sulphotransferases add a sulfate moiety to the flavonoids. They also belong to a gene superfamily with >10 different sulphotransferase isoforms in humans (38, 39). Sulphotransferases 1A1–4 and 1E1 have been specifically shown to be involved in the metabolism of flavonoids (37, 40–44). COMTs are involved in the O-methylation of catecholic polyphenols including catechins, epicatechins, and epigallocatechins from the flavan-3-ol subclass (45). Methylation decreases the hydrophilicity of compounds, and after methylation, subsequent glucuronidation and sulphation are often needed for the effective elimination from the body.
Tissue uptake
Although likely to be important determinants of flavonoid ADME and their effects on cell and tissue functions, little is known about the tissue uptake and subsequent partitioning of flavonoid metabolites. A limited number of studies that have focused mainly on anthocyanins have indicated that bilitranslocase may be involved in vascular epithelial and hepatic flavonoid uptakes and anthocyanin absorption from the stomach (46–49). However, the relative importance of bilitranslocase and the identity of other transporters remain to be established.
Elimination
Breast cancer–resistance protein, organic anion transporting polypeptide, and, in particular, organic anion transporters 1 and 3 that are expressed in basolateral membranes of renal tubules and couple with phase I and phase II metabolism are thought to be important facets of the elimination process (28, 50–52). The overexpression of organic anion transporter is associated with more-efficient renal uptake and elimination into the urine (51). In addition to urine, a significant proportion of bioavailable flavonoid metabolites may be excreted via bile into the feces although enterohepatic recirculation results in some recycling back to the small intestine through bile excretion (53, 54).
ETIOLOGY OF THE HETEROGENEITY IN FLAVONOID ADME
As detailed above, ∼200 proteins have been identified as having the potential to affect flavonoid ADME. Modulators of the expression and activity of these proteins, such as age, sex, and genotype, are likely, to varying degrees, to influence the circulating concentrations, elimination and tissue exposure to flavonoids (Figure 1) and, ultimately, to dose-response relations (Figure 2). Although little relevant published literature is currently available, lessons may be learned from traditional xenobiotic and, in particular, drug metabolism because many phase I and II metabolic pathways are common to both drug and flavonoid groups. While there is likely to be considerable redundancy in oxidation, glucuronidation, sulphation, and methylation pathways, there exists some evidence that flavonoid intake may influence drug metabolism with the reciprocal relation also likely to exist, with habitual drug use potentially influencing flavonoid ADME in an individual. Although currently largely unknown, this effect may be a particular issue in cases in whom the metabolism of the drug or flavonoid is reliant on one or a limited number of cytochrome, UGT, sulphotransferase, or COMT isoforms or in a situation of compromised phase I and II metabolic capacities, which are perhaps associated with disease or aging. Furthermore, it is also unclear what the physiologic consequences of altered ADME are likely to be. Reduced absorption would be predicted to reduce biopotency, but reduced phase I and phase II metabolism, although potentially reducing excretion rates and increasing the dose and length of tissue exposure, may result in a lower formation of more bioactive metabolites (relative to their parent compounds) and also potentially result in toxicity in susceptible individuals.
Impacts of age and sex on xenobiotic and flavonoid ADME
The aging process is associated with reduced hepatic perfusion and morphology including reduced hepatocyte density, which has been suggested to reduce the phase I and phase II metabolism of xenobiotics (55, 56) and therefore potentially flavonoid metabolism. However, the impact of aging on the activity of oxidation and conjugation enzymes per se is controversial. In isolated perfused livers from 3- to 6- or 22- to 24-mo-old rats exposed to p-nitrophenol, there was evidence of reduced oxidation and glucuronidation with aging, which was speculated to be partly attributed to reduced cofactor availability (57). In contrast, no obvious effect of aging was reported in human (58) or rat (59) microsomal UGT activities in response to commonly prescribed medications. In more-recent publications, the individual or interactive effects of aging and sex on a wider range on xenobiotic metabolizing enzymes were examined. Fu et al. (60) included a gene-expression analysis of 101 xenobiotic-processing genes including cell transporters, phase I and II enzymes, efflux transporters, and transcription factors that were quantified at 10 time points in the livers of male and female mice across the life span (60). Complex impacts of both age and sex emerged, whereby the messenger RNA concentrations for 44% of the genes changed in male mice, and 63% of the genes changed in female mice, according to age. Significant upregulation and downregulation were evident, but overall, 40% of the xenobiotic-processing genes were lower in aged male mice and 43% in aged female mice. Kawase et al. (61) noted higher breast cancer–resistance protein, organic anion transporting polypeptide 1a1, and UGT1A1 and lower cytochrome 3A1 and cytochrome 32 in young female rats than in male rats, with an age-related downregulation of gene expression that was evident only in female rats (61). Two recent publications have specifically focused on flavonoids as model compounds. The glucuronidation characteristics of the flavonoid glucoside tilianin and its aglycone acacetin (flavones) were characterized with the use of human UGT isoforms, liver microsomes, and intestinal microsomes that were obtained from different animal species. Overall, and consistent with Kawase et al. (61), higher glucuronidation rates attributed to higher UGT1A1 activities were evident in females across several species (62). The impact of aging on the glucuronidation of quercetin and genistein in male rat hepatic microsomes was also studied (63). Overall, although some age-related changes were evident, they were modest in their magnitudes. The glucuronidation of genistein decreased with age. The quercetin total glucuronidation capacity was constant with age, but young and old rats had different metabolite profiles.
Therefore, although there is limited evidence to suggest that there is possibly higher UGT1A-mediated glucuronidation in females than in males and a possible overall age-related decline in phase I and phase II metabolism, available data are wholly inadequate to make any definitive conclusion regarding the likely impact of these variables on flavonoid metabolism in humans.
Impact of genotype on xenobiotic and potentially flavonoid metabolism
As with the majority of phenotypes, it is likely that ≥50% of the interindividual heterogeneity in flavonoid ADME is attributable to genetic variability. The most recent output from the 1000 Genome Consortium, which was published in October 2015 (64), indicated that there are typically 88 million variants in a human genome, and with knowledge that the penetrance of individual variants is influenced by a range of behavioral, physiologic, and epistatic (gene × gene interactions) factors, the identification of which factors influence flavonoid ADME represents a major challenge. To date, the limited investigations have taken a candidate-gene approach and have focused on one or a small number of variants in a gene encoding for key phase I or II proteins.
Although currently completely unknown, because of the key role of LPH and β-glucosidases (see Absorption) in the initial hydrolysis of flavonoid glycosides, it is likely that variants in these loci may be important determinants of the bioavailability of the majority of flavonoid subclasses from the small intestine.
Perhaps the most extensively studied genotype that is relevant to flavonoid ADME is the COMT missense mutation (rs4680) with a G-to-A base change that results in a valine-to-methionine amino acid substitution at position 158 of the protein. This polymorphism is thought to produce a less stable protein, which in vitro studies have proposed can result in a 40% decrease in enzyme activity (65) and can influence the metabolism of a number of exogenous and endogenous compounds including catecholamines and a range of drugs (66). In a case-control study of Asian-American women, the consumption of green tea, which is rich in flavan-3-ols, was associated with reduced breast cancer risk with the strongest association evident in subjects with a low-activity COMT A allele (67). In a cross-sectional analysis of a subset of the Shanghai Cohort, the AA genotype had significantly lower urinary total polyphenols and concentrations of 3 of the 5 specific tea polyphenol metabolites [(−)-epigallocatechin, 4′-methylepigallocatechin and 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone] relative to the GG and GA groups with a trend for genotype-associated differences in epicatechin and 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (45). Consistent with these findings, in participants who were prospectively recruited according to genotype, urinary methylated epigallocatechin concentrations were significantly higher in the GG COMT group than in AA homozygotes after acute consumption of green-tea extract (68). In the Minnesota Green Tea Trial, overweight and obese postmenopausal women underwent a 12-mo intervention that examined the impact of green-tea extract on adiposity and measures of cardiometabolic health (69). A response to the intervention was established according to COMT genotype status with no overall impact of the intervention and no genotype × treatment interactions observed. However, no data on plasma or urinary catechin concentrations were reported, which would have allowed for the examination of the impact of the interindividual variability in metabolism on physiologic responses.
UGTs glucuronidate bilirubin, estrogens, and exogenous compounds, including dietary carcinogens and prescribed medications. The effects of UGT genotypes on the endogenous concentrations of these compounds, incidences of associated cancers, and responses to select drugs have been reported (70–81). An et al. (70) examined the impact of 6 single-nucleotide polymorphisms (SNPs), including 3 SNPs in the UGT1A1 gene, on the daily warfarin dosage. One UGT1A1 SNP (rs887829) exhibited significant association with warfarin use with T-allele carriers requiring higher doses than for individuals with the CC genotype (6.3 compared with 5.2 mg/d, respectively). In a study of 1600 colorectal cancer patients and 2500 unaffected siblings, the variation in 4 UGT genes (UGT1A3, UGT1A6, UGT2B4, and UGT2B15) modified risk of colorectal cancer either independently of interactively with nonsteroidal anti-inflammatory use (79). Variants in the promoter of the UGT1A1 gene (UGT1A1*28, rs8175347), which result in 5, 7, or 8 repeats instead of 6 thymine-adenine repeats, were associated with decreased UGT1A1 transcription and higher serum bilirubin with increased numbers of thymine-adenine repeats (75, 82). Several dietary phytochemicals, including flavonoids, have been shown to induce UGT1A1 activity (83, 84), with Lampe and coworkers reporting that the impact of the UGT1A1*28 genotype on bilirubin metabolism was modified by increased intakes of cruciferous vegetables, citrus fruit, and soy (72, 76, 78). Although intuitively, because of the role of UGT1A1 in flavonoid glucuronidation, the UGT1A1*28 variant is also likely to be an important modulator of flavonoid metabolism, its impact is currently unknown but worthy of investigation.
Because ≤10% of flavonoids are sulfated, variants in sulphotransferase genes may also affect flavonoid plasma and urinary profiles. Genetic variants in sulphotransferases with associated functional consequences have been identified with SNPs in sulphotransferases 1A1 and 2A1, which are associated with altered drugs responses and sex-steroid concentrations (18, 43). Cytochrome 3A4 is the most abundant isoform of cytochrome P450 in the adult human liver, with common CYTOCHROME-3A4 variants that have been shown to influence testosterone metabolism (85). However, as with UGTs, the effects of SULPHOTRANSFERASES and CYTOCHROME and cytochrome genotypes on flavonoid metabolism remain to be tested.
Overall, there is a dearth of information on the genetic determinants of flavonoid metabolism. The previously discussed literature on variants of phase I and II genes that influence the metabolism of an array of endogenous and exogenous compounds may help inform future research in the flavonoid field. However, a justification for the selected gene-variant targets has been rarely provided with the functional consequences of genotype often unknown. Future studies should adopt a more genome-wide approach or targeted genotyping that is focused on the key enzymes specific to flavonoid (rather than drug) metabolism. The selection of which individual variants to assess is a challenge with an intuitive focus on exon variants and, in particular, nonsynonymous SNPs or those in gene-promoter regions that have the potential to exclude potentially highly functional variants in intron regions. Although relatively expensive, genome-wide association studies or whole-gene or -genome sequencings represent a more efficient approach to identifying genotypes that are important in flavonoid ADME and, therefore, potentially bioefficacy. Once a potentially functional genotype has been identified by untargeted approaches, its role should be subsequently confirmed with the use of a prospective recruitment according to the genotype approach in human volunteers along with the use of rodent or cell models in which the native gene has been replaced by human variants to establish the effects of the genotype on enzyme activity and flavonoid ADME.
Potential impact of prescribed medication use on flavonoid metabolism
Because flavonoids and many prescribed medications share phase I and II metabolic processes, the effects of dietary flavonoid (and other bioactive) intake on drug ADME and dosing amounts have been of research and clinical interest for several decades (86–89). The impact of grapefruit consumption on cytochrome-3A4 activity and the metabolism of a large number of drug groups, such as calcium channel antagonists β-hydroxy-β-methylglutaryl–CoA reductase inhibitors and antihistamines, represents a widely cited example (90, 91). Although unknown, it is likely that drug use affects flavonoid metabolism and, ultimately, tissue total and metabolite flavonoid exposure and dose-response relations. The multiplicity of transferases with overlapping substrate specificity and likely considerable redundancy in phase I and II metabolic capacities may mean that there is little impact of single drug use on flavonoid metabolism in the majority of the population. However, in select subgroups, such as older adults who commonly consume a drug cocktail and may experience age-related declines in metabolic capacity, or in individuals with gene variants that are associated with the reduced expression or compromised function of key enzymes, habitual drug use may be important.
Impact of habitual diet composition on flavonoid ADME
The impact of habitual dietary intake on flavonoid bioavailability has not been extensively investigated. However, the effects of alcohol, fiber, and dietary fat composition have been studied to a limited degree. In a recent study, differences in microbial metabolite concentrations in feces were measured after intake of either red wine or dealcoholized red wine (92) with the suggestion that the alcohol content may increase the solubility of the polar flavonoid compounds. However, no significant differences in total metabolite amounts were observed. In other studies, although no difference in plasma catechin concentrations were observed after intake of either red wine or dealcoholized red wine, the urinary excretion of catechins was more rapid after red wine intake (93, 94).
The impact of fiber intake on flavonoid bioavailability is not clearly understood and, to our knowledge, has not been investigated in human studies. It has been speculated that a high fiber content may decrease the availability and bioaccessibility of flavonoids from the foods because of factors such as physical entrapment, increased viscosity, and increased bulk (95). However, because of the impact of the microbiome on flavonoid metabolism, the potential bidirectional relation (see Impact of the gut microbiome on flavonoid metabolism) together with the established effects of fiber intake on intestinal transit time and short-chain fatty acid (SCFA) production, there is the potential to enhance flavonoid bioavailability and metabolism in the large intestine. In mice, pectin enhanced quercetin absorption, which was likely the result of an alteration in the metabolic activity of the microbiome (96). Although, to our knowledge, no systematic studies exist for flavonoids, a reduced gastrointestinal transit time has been shown to decrease the bioavailability of various drugs (97). To date, few studies have investigated the effect of dietary fat intakes on flavonoid absorption. Because most polyphenols are water soluble and transported via the portal vein, dietary lipids are likely to have little influence on the more-hydrophilic flavonoids. However, there may be important interactions with the more hydrophobic (lower number of hydroxyl groups) flavonoids (98). Dietary fats also alter the gastrointestinal transit time, and this variation has the potential to alter flavonoid kinetics and absorption. In an acute human study, strawberries, when consumed with cream, delayed the excretion of anthocyanin metabolites in the first 2 h but did not alter the total bioavailability (as measured by the AUC in plasma) of anthocyanins (99). In an in vitro digestion model, the higher fractional bioaccessibility of procyanidins, but not of phenolic acids and flavones, was observed from lipid-rich cacao liquor (45% fat) than from cacao powder (15% fat) (100), thereby supporting the notion that polar flavonoids are, at least in part, micellularized and that lipids from the food matrix may help in stabilizing the mixed micelles or in making them more soluble. Although a clear dose-response effect was not evident, an improved bioavailability of quercetin was observed in pigs fed quercetin together with test meals that differed in fat contents (3, 17, or 32 g fat/100-g diet). The AUC after the 17% fat diet was ∼57% higher than that of the 3% fat diet, with no further increase shown when a 32% fat diet was fed (101). In a small human study (n = 9), the AUC in plasma quercetin concentrations was 45% higher in subjects who consumed a fat-rich breakfast than in subjects who consumed a fat-free breakfast (102). In mice, Giunta et al. (103) showed that fish oil and green tea–derived (−)-epigallocatechin-3-gallate (flavan-3-ols) had a significantly greater antiamyloidogenic effect than that of either component fed separately. The inclusion of fish oils (rich in n–3 fatty acids) in the rodent diet increased both blood and brain (−)-epigallocatechin-3-gallate concentrations.
As well as the potential impact of a habitual diet on metabolism, note that other sources of variability include the wide variation in the flavonoid contents of foods. In epidemiologic studies, food-composition databases have been used to assess intake, which have not accounted for the variability in content that occurs as a result of different growing conditions and processing and cooking techniques. However, despite these sources of variation, observational data have allowed us to rank order intakes, thereby allowing comparisons between high and low intakes in large population groups. Until validated biomarkers that integrate intake with in vivo metabolism are available, current data can only be derived from dietary intake information. In many trials, the wide variability in the flavonoid contents of intervention foods is often not considered, and an independent verification of the amounts that are present in intervention foods and supplements would allow for a more accurate assessment of the dose-response effects in the future.
Impact of the physiochemical properties of food on flavonoid metabolism
In relation to the impact of food composition, Brett et al. (104) observed no differences in the absorption and excretion of flavanones after feeding a whole-fruit matrix compared with an orange-juice matrix. However, the solubility of flavanones are thought to be a key factor for bioavailability, and in juices that contained different flavanone concentrations, higher urinary excretion and plasma concentrations were associated with soluble flavanone concentrations in the juice (105). Although there was little intraindividual variability, the interindividual variability was large, which supported the notion that specific microbiota are required to cleave the glycosides (rutinosides) in the juice, thereby resulting in aglycones that are available for absorption (105). However, more-recent data have suggested that, although food-processing methods to improve solubility can enhance bioavailability, the stratification of volunteers relative to their excretion capabilities was more important (105, 106).
The flavonoid sugar moiety has been suggested to be an important determinant for both the absorption site and overall bioavailability in humans (107, 108). One of the most predominant forms is the attachment to a β-glucoside, which can only be absorbed to a very limited extent, and needs to be hydrolyzed before absorption in the small intestine (see Absorption) (7). For flavonoids with other additional attachments, including rhamnose (the flavonol quercetin), the microflora are required to cleave off the sugar moieties before absorption (109). After enzymatic treatment with rhamnosidase, the rutinoside moiety can be hydrolyzed to produce the glucoside moiety, and after enzyme treatment, the bioavailability of flavanones from juice has increased 4-fold in humans (110). Moreover, it is not only the chemical structure but also their isomeric configuration that can affect absorption. For the metabolism of (R/S) hesperidin, hesperitin-7-glucoside was shown found to have an R:S ratio of 39:69 in human plasma and urine samples, thereby suggesting that the S configuration could be more bioavailable (111). Specifically for flavan-3-ols, the bioavailability may be influenced by the differing proportions of the various enantiomeric forms of the monomeric flavan-3-ols. Unlike other flavonoids, flavan-3-ols exist in plants as aglycones rather than as the glycoside form. In one study in which comparable concentrations of the individual enantiomers were separately consumed, the bioavailability of the different stereoisomers differed widely (112), but in most trials, the individual profile of flavan-3-ol stereoisomers has been rarely characterized but may be one factor that may explain differences in the bioavailability and bioactivity across published human studies.
Impact of the gut microbiome on flavonoid metabolism
Colonic metabolism has long been speculated to be a major contributor to the overall metabolism of not only dietary flavonoids but also of phase I and II metabolites that have been excreted back into the intestine via enterohepatic circulation (10, 113). The microbial metabolism of flavonoids is thought to follow a general pattern whereby a diverse range of compounds are funneled to a reduced number of metabolites. The bacterial enzymes deglycosylate the compounds, but the microbes can also perform a range of other transformations including oxidation, demethylation, and the catabolism to smaller fragments including small phenolic acids and aromatic catabolites (7, 8, 114–116). However, it remains unclear how well these metabolites are absorbed. The colonic bioconversion of flavonoids is thought to be highly variable although the etiology of the heterogeneity is currently unclear. There is wide interindividual variability in the bioconversion of specific flavonoids (115, 117, 118) that has been attributed in part to specific enterotypes and has resulted in the suggestion that individuals may be either low- or high-flavonoid convertors (106, 119). The interindividual variability may also be related to the fact that small differences in the chemical compositions of flavonoids (substitution patterns) can result in major changes in colonic bioconversion (119) or the modulation of the flavonoid-microbiota interaction by the background habitual diet, which varies dramatically across population groups (120). Many of these microbiome-mediated chemical transformations can result in the production of metabolites with increased biological activity, with the most-notable example being the isoflavones, which are a subclass of flavonoids that are derived predominately from soy. In the 1980s, evidence that the microbiome was key for metabolism to the specific microbial-derived metabolite equol emerged (121), and wide interindividual variability in the ability to produce this microbial-derived metabolite has been established, ranging from 25–30% equol producers in Western populations to 50–70% in Asian counties (122–124). Equol has been shown to be more bioactive than its food precursor daidzein in vitro and in trials (predominantly with the equol-producer phenotype assessed retrospectively), the magnitude of the biological effect was greatly enhanced in participants who produced equol after isoflavone ingestion, which suggested that there is a critical role of the microbiome for health effects (125–127). In general terms, there is emerging literature that describes the diverse and significant impact of flavonoid phenolics and other small molecules that are produced in the large intestine on physiologic processes such as SCFA production and bioavailability, bile acid metabolism, redox and inflammatory status, and associated intestinal, hepatic, and overall systemic functions (128). The production of SCFA is of interest in colonic health, dietary energy extraction, and body-weight regulation (128, 129). Although flavonoid-induced effects on the main SCFAs acetic (C2), propionic (C3), and butyric (C4) acids have been repeatedly shown in in vitro fermentation systems and in rodent models (128), data from human interventions have been limited and nonconclusive. For example, in healthy humans, red-wine grape-juice extract, but not grape-juice extract, fed for 4 wk reduced fecal isobutyric acid concentrations (130), whereas Mosele et al. (131) reported no impact of 4 wk of pomegranate-juice consumption on total or individual fecal SCFA concentrations.
The large observed heterogeneity in the bioactivity and bioavailability of different flavonoid metabolites that are formed after ingestion, including the extensive range of the microbial-derived metabolites identified particularly for anthocyanins (11, 116), supports a strong interplay between flavonoids and the microbiome. Although it is likely that flavonoid intake alters the composition and function of the gut microbiome, and, conversely, microflora enhances the metabolism of flavonoids, this bidirectional relation has not yet been addressed in flavonoid research to our knowledge. An examination of this bidirectional relation has been limited to a few small cross-sectional or short-term feeding studies. A cross-sectional study, which included 178 elderly subjects, observed that habitual diet-driven microbiota alterations were associated with health status, including measures of frailty and inflammation (132), whereas in a study that was limited to 15 women, a 2-mo dietary intervention was associated with changes in Gammaproteobacteria and Erysipelotrichi microbial communities (133). In a recent small RCT (n = 9), high amounts of Bifidobacteria were associated with increased amounts of flavonoid microbial metabolites after polyphenol-rich wine intake (134). Several recent animal studies observed profound effects in the gut microbial community structure after intake of flavonoid-rich foods although it is possible that there are differences in the permeability of microbiome-derived metabolites between rodents and humans. In one animal study, a reduction in the ratio of Firmicutes to Bacteroidetes and an increase in Akkermansia muciniphila were observed after intake of grape extract; these changes conferred protection against the negative consequences of a high-fat diet, which resulted in a reduction in inflammation and an improvement in insulin sensitivity (135). An additional study in mice showed that the microbial composition (specifically Akkermansia spp.) played a decisive role in the observed protective effects of a cranberry extract from diet-induced obesity and insulin resistance (136). Other flavonoid-rich foods, including green and black teas, have also been shown to increase the proportion of Akkermansia (137, 138). These animal data provide the first convincing data that the gut microbiome may play a substantial role in mediating the health effects of flavonoids, thereby leading to a reduction in inflammation and improved metabolic function (135, 136). It remains to be determined in humans if the interaction between flavonoids and the gut microbiota is a direct effect or an indirect effect (mediated through altered host physiology), but these animal data provide clear evidence of significant interactions. The similarity in animal responses to different sources of flavonoids (grape and cranberry) also suggest that perhaps diverse sources of flavonoids may have similar effects on the gut microbiome, but it is only through human intervention trials that the significance to human physiology can be established.
In humans, we know that in the subclass anthocyanins, after feeding stable-isotope labeled anthocyanins, they are extensively degraded, which is swiftly followed by further transformation (11, 116). These data provide some support from human data that anthocyanin bioactivity is likely mediated by the high concentrations and longer half-lives of its microbial-derived phenolic metabolites (116). Recent research has suggested that, in vitro, nutritionally relevant amounts of these colonic metabolites exert greater vascular and anti-inflammatory activity than do the metabolites that are formed and absorbed in the small intestine (139–141), thereby providing additional evidence that the bioactivity of anthocyanins is highly likely attributed to their microbial-derived metabolites. Clinical studies to determine whether these effects are also observed in humans are urgently needed because the identities of the main microbiota phyla and species that modulate anthocyanin metabolism in humans are unknown, and data from adequately powered acute studies and long-term human RCTs investigating the potential of microbiota diversity to explain associations between anthocyanin intake and CVD risk are completely lacking.
Potential impact of gut-immune homeostasis and intestinal permeability on flavonoid ADME
The intestinal epithelium, together with the colonic bacteria, is the first site of interactions between food intake and the host immune system, and this interaction can affect the microbiota composition, which, in turn, can directly affect gut-immune homeostasis and intestinal permeability and potentially flavonoid ADME. If flavonoids are acting primarily at the level of intestinal absorption, an understanding of how different dietary flavonoids influence and regulate the intestinal barrier and intestinal permeability is key. In 2 pivotal animal studies, profound effects of flavonoid intake on the microbial community structure were observed with resulting effects on intestinal and systemic inflammation and the metabolic response (135, 136). However, overall, the data suggested that these effects were the result of a direct trophic influence of the flavonoids on Akkermansia rather than an effect on mucin production. In one study, mucus production was increased, but the authors suggest that this increased production may have followed the direct effects of flavonoids on Akkermansia (136), whereas in another study, no differences in mucin gene expression in jejunum or colon samples were observed (135). A direct effect on increasing the abundance of Akkermansia fits with other in vitro data (138).
The relative increase in Akkermansia after cranberry intake was also associated with the prevention of a high fat– and high sugar–induced rise in liposaccharide and a decrease in intestinal inflammation (136). These observations suggest that, by increasing Akkermansia, flavonoids may reduce intestinal permeability and liposaccharide leakage, thereby ameliorating insulin resistance in diet-induced obese mice (136). The understanding of such interactions in humans is a key next step.
Because large proportions of ingested flavonoids reach the colon and undergo extensive bioconversion, it is likely that the resultant metabolites exert local intestinal effects while in the colon and systemic effects after absorption. In vitro, the flavonol quercetin was shown to enhance barrier function in rat small and large intestines and exerted protective effects on cytokine-induced barrier damage. In caco-2 cell monolayers, several flavonoids, including flavanols and flavanones, exerted beneficial effects on intestinal barrier function (increased epithelial resistance and claudin-4 expression in epithelial cells) (142–144). The impact of these localized effects of flavonoids in the colon on flavonoid bioavailability remains to be established.
Concluding remarks
In summary, although there is growing evidence from prospective cohort studies and clinical trials of the potential health benefits of dietary flavonoids, this review highlights the research gaps in the current knowledge base (Text Boxes 1 and 2).
Text Box 1. Research challenges and future studies required in flavonoid research.
Conduct adequately powered clinical studies to determine the impact of age, sex, habitual diet, genotype, drug interactions, and the microbiome on flavonoid metabolism.
Conduct trials to understand the bidirectional relation between flavonoid metabolism and the microbiome.
Prospectively recruit participants to clinical trials on the basis of the extent of absorption and metabolism to establish dose-response relations.
Identify and validate a panel of robust biomarkers of flavonoid intake and subsequent metabolism that can be used to examine associations of bioavailable flavonoids with health outcomes in future prospective cohort studies.
Further develop metabolomic data sets to assist in the development of biomarkers.
Conduct hypothesis-driven research to investigate the impact of specific genotypes on flavonoid metabolism with a particular focus on variants in LPH, β-glucosidases, phase I metabolism, and phase II metabolism with prospective recruitment by genotype for associations established with the use of the retrospective genotype approaches.
Conduct intervention studies to determine how food composition and flavonoid source affect bioavailability.
Conduct trials in which metabolism and health outcomes are addressed simultaneously.
Text Box 2. Study design and research considerations.
Conduct longer-term intervention trials (≥6 mo) and consider long-term trials with clinical outcomes.
Conduct head-to-head comparisons of flavonoid extracts and pure compounds compared with flavonoid-rich foods in clinical trials.
In animal-model experiments, give consideration to the dose fed to ensure it is applicable to human intake.
In examining mechanistic insights in vitro, consider the use of physiologically relevant doses and focus on metabolites.
Develop high-throughput assays for assessing metabolites and develop appropriate standards for mass spectrometry to quantify the range of metabolites produced in vivo.
Develop optimal placebo products for clinical trials.
Select the genotyping approach with close consultation with a genetics expert for a limited targeted genotyping of specific genes of interest, nonsynonymous variants, or variants in the promoter region of the gene that are most likely to be functional.
Once genotype-metabolism associations have been established with the use of retrospective genotyping approaches, confirm the impact of the genotype 1) in an independent human study with the use of prospective recruitment on the basis of genotype and 2) in rodent transgenic models expressing the human variant versions of the gene.
At a population level, the heterogeneity in responsiveness to habitual flavonoid intake obscures beneficial associations between intakes and health outcomes in responsive population subgroups and creates a difficulty in establishing the physiologic and molecular mechanisms that underlie the health benefits of different flavonoid subclasses. Identifying key factors governing metabolism and understanding if a differential capacity to metabolize these bioactive compounds affects health outcomes will greatly enhance the ability to optimize intakes of flavonoids for health benefits. The large observed heterogeneity in the bioactivity and bioavailability of different flavonoid metabolites that are formed after ingestion, including the extensive range of microbial-derived metabolites identified, supports a strong interplay between flavonoids and the microbiome. Although it is likely that flavonoid intake alters the composition and function of the gut microbiome, and conversely, microflora enhances the metabolism of flavonoids, this bidirectional relation has not been addressed in clinical trials to our knowledge. Furthermore, we have identified a dearth of data on genetic determinants of flavonoid metabolism. Although it is thought that ≥50% of the interindividual variability in ADME may be attributed to genetic variability, little research focus has investigated which gene variants may alter flavonoid ADME. An understanding of the impact of compromised phase I and phase II metabolism that are mediated by genotype or variables such as age, sex, or habitual prescription drug intake on flavonoid bioavailability and metabolism is almost completely lacking. Addressing these research gaps (Text Boxes 1 and 2) would provide the basis for the development of targeted dietary advice for subgroups who are likely to be most responsive and help us work toward the development of specific dietary guidelines for several dietary flavonoid subclasses. These research gaps build on the guidance and key considerations for the design and reporting in flavonoid research that were outlined by Balentine et al. (145) in 2015. In prospective studies, an understanding of interindividual variation after flavonoid intake would allow the establishment of validated biomarkers that are indicative of both flavonoid intake and subsequent metabolism to further establish relations between bioavailable flavonoids and health outcomes.
Acknowledgments
The authors’ responsibilities were as follows—AC: formulated the focus of the review; and both authors: conducted the literature searches, wrote the manuscript, were responsible for the final content of the manuscript, and read and approved the final manuscript. AC and A-MM receive funding from the US Highbush Blueberry Council and Abbott Healthcare to conduct flavonoid interventions and are members of the COST POSITIVe consortium funded by the EU Framework Programme Horizon 2020.
Footnotes
Abbreviations used: ABC, ATP-binding cassette; ADME, absorption, distribution, metabolism, and elimination; COMT, catechol-O-methyltransferase; LPH, lactase phlorizin hydrolase; RCT, randomized controlled trial; SCFA, short-chain fatty acid; SNP, single-nucleotide polymorphism; UGT, urine-5′-diphosphate glucuronosyltransferase.
REFERENCES
- 1.Erdman JW Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, Harnly J, Hollman P, Keen CL, Mazza G, et al. . Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. J Nutr 2007;137(3 Suppl 1):718S–37S. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy A, O’Reilly EJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 2011;93:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013;127:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Cassidy A, Schwarzschild MA, Rimm EB, Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012;78:1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett W, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol 2012;72:135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81(1 Suppl):230S–42S. [DOI] [PubMed] [Google Scholar]
- 8.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 2005;81(1 Suppl):243S–55S. [DOI] [PubMed] [Google Scholar]
- 9.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:38–50. [DOI] [PubMed] [Google Scholar]
- 10.Williamson G, Clifford MN. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br J Nutr 2010;104(Suppl 3):S48–66. [DOI] [PubMed] [Google Scholar]
- 11.Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr 2013;97:995–1003. [DOI] [PubMed] [Google Scholar]
- 12.Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012;35:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr 2013;143:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. . Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr 2014;144:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setchell KD, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr 2010;140:1363S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaheer K, Akhtar MH. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr 2015;0. [DOI] [PubMed] [Google Scholar]
- 17.de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study(1-4). J Nutr 2001;131:1826–32. [DOI] [PubMed] [Google Scholar]
- 18.Lampe JW, Chang JL. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol 2007;17:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res 2004;38:771–85. [DOI] [PubMed] [Google Scholar]
- 20.Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med 2004;36:829–37. [DOI] [PubMed] [Google Scholar]
- 21.Day AJ, Canada FJ, Diaz JC, Kroon PA, McLauchlan R, Faulds CB, Plumb GW, Morgan MR, Williamson G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett 2000;468:166–70. [DOI] [PubMed] [Google Scholar]
- 22.Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr 1995;62:1276–82. [DOI] [PubMed] [Google Scholar]
- 23.Wolffram S, Block M, Ader P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J Nutr 2002;132:630–5. [DOI] [PubMed] [Google Scholar]
- 24.Hollman PC, Bijsman MN, van Gameren Y, Cnossen EP, de Vries JH, Katan MB. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic Res 1999;31:569–73. [DOI] [PubMed] [Google Scholar]
- 25.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett 1998;436:71–5. [DOI] [PubMed] [Google Scholar]
- 26.McCann MC, Rogan GJ, Fitzpatrick S, Trujillo WA, Sorbet R, Hartnell GF, Riodan SG, Nemeth MA. Glyphosate-tolerant alfalfa is compositionally equivalent to conventional alfalfa (Medicago sativa L.). J Agric Food Chem 2006;54:7187–92. [DOI] [PubMed] [Google Scholar]
- 27.Cao G, Muccitelli HU, Sanchez-Moreno C, Prior RL. Anthocyanins are absorbed in glycated forms in elderly women: a pharmacokinetic study. Am J Clin Nutr 2001;73:920–6. [DOI] [PubMed] [Google Scholar]
- 28.Brand W, Schutte ME, Williamson G, van Zanden JJ, Cnubben NH, Groten JP, van Bladeren PJ, Rietjens IM. Flavonoid-mediated inhibition of intestinal ABC transporters may affect the oral bioavailability of drugs, food-borne toxic compounds and bioactive ingredients. Biomed Pharmacother 2006;60:508–19. [DOI] [PubMed] [Google Scholar]
- 29.Konishi Y. Transepithelial transport of microbial metabolites of quercetin in intestinal Caco-2 cell monolayers. J Agric Food Chem 2005;53:601–7. [DOI] [PubMed] [Google Scholar]
- 30.Kosińska A, Andlauer W. Cocoa polyphenols are absorbed in Caco-2 cell model of intestinal epithelium. Food Chem 2012;135:999–1005. [DOI] [PubMed] [Google Scholar]
- 31.Hodek P, Trefil P, Stiborova M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact 2002;139:1–21. [DOI] [PubMed] [Google Scholar]
- 32.Otake Y, Walle T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab Dispos 2002;30:103–5. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Zheng S, Li L, Jiang H. Metabolism of flavonoids in human: a comprehensive review. Curr Drug Metab 2014;15:48–61. [DOI] [PubMed] [Google Scholar]
- 34.Schär MY, Curtis PJ, Hazim S, Ostertag LM, Kay CD, Potter JF, Cassidy A. Orange juice-derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: a randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. Am J Clin Nutr 2015;101:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 2005;15:677–85. [DOI] [PubMed] [Google Scholar]
- 36.Tang L, Ye L, Singh R, Wu B, Lv C, Zhao J, Liu Z, Hu M. Use of glucuronidation fingerprinting to describe and predict mono- and dihydroxyflavone metabolism by recombinant UGT isoforms and human intestinal and liver microsomes. Mol Pharm 2010;7:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brand W, Boersma MG, Bik H, Hoek-van den Hil EF, Vervoort J, Barron D, Meinl W, Glatt H, Williamson G, van Bladeren PJ, et al. . Phase II metabolism of hesperetin by individual UDP-glucuronosyltransferases and sulfotransferases and rat and human tissue samples. Drug Metab Dispos 2010;38:617–25. [DOI] [PubMed] [Google Scholar]
- 38.Riches Z, Stanley EL, Bloomer JC, Coughtrie MW. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie”. Drug Metab Dispos 2009;37:2255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H. Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem J 2007;404:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos 2002;30:576–81. [DOI] [PubMed] [Google Scholar]
- 41.Pai TG, Suiko M, Sakakibara Y, Liu MC. Sulfation of flavonoids and other phenolic dietary compounds by the human cytosolic sulfotransferases. Biochem Biophys Res Commun 2001;285:1175–9. [DOI] [PubMed] [Google Scholar]
- 42.Nakano H, Ogura K, Takahashi E, Harada T, Nishiyama T, Muro K, Hiratsuka A, Kadota S, Watabe T. Regioselective monosulfation and disulfation of the phytoestrogens daidzein and genistein by human liver sulfotransferases. Drug Metab Pharmacokinet 2004;19:216–26. [DOI] [PubMed] [Google Scholar]
- 43.Nowell S, Falany CN. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 2006;25:1673–8. [DOI] [PubMed] [Google Scholar]
- 44.Ung D, Nagar S. Variable sulfation of dietary polyphenols by recombinant human sulfotransferase (SULT) 1A1 genetic variants and SULT1E1. Drug Metab Dispos 2007;35:740–6. [DOI] [PubMed] [Google Scholar]
- 45.Inoue-Choi M, Yuan JM, Yang CS, Van Den Berg DJ, Lee MJ, Gao YT, Yu MC. Genetic association between the COMT genotype and urinary levels of tea polyphenols and their metabolites among daily green tea drinkers. Int J Mol Epidemiol Genet 2010;1:114–23. [PMC free article] [PubMed] [Google Scholar]
- 46.Maestro A, Terdoslavich M, Vanzo A, Kuku A, Tramer F, Nicolin V, Micali F, Decorti G, Passamonti S. Expression of bilitranslocase in the vascular endothelium and its function as a flavonoid transporter. Cardiovasc Res 2010;85:175–83. [DOI] [PubMed] [Google Scholar]
- 47.Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. The stomach as a site for anthocyanins absorption from food. FEBS Lett 2003;544:210–3. [DOI] [PubMed] [Google Scholar]
- 48.Ziberna L, Kim JH, Auger C, Passamonti S, Schini-Kerth V. Role of endothelial cell membrane transport in red wine polyphenols-induced coronary vasorelaxation: involvement of bilitranslocase. Food Funct 2013;4:1452–6. [DOI] [PubMed] [Google Scholar]
- 49.Ziberna L, Tramer F, Moze S, Vrhovsek U, Mattivi F, Passamonti S. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic Biol Med 2012;52:1750–9. [DOI] [PubMed] [Google Scholar]
- 50.Tian Y, Bian Y, Jiang Y, Qian S, Yu A, Zeng S. Interplay of breast cancer resistance protein (BCRP) and Metabolizing Enzymes. Curr Drug Metab 2015;16:877–93. [DOI] [PubMed] [Google Scholar]
- 51.Nigam SK, Bush KT, Martovetsky G, Ahn SY, Liu HC, Richard E, Bhatnagar V, Wu W. The organic anion transporter (OAT) family: a systems biology perspective. Physiol Rev 2015;95:83–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong CC, Botting NP, Orfila C, Al-Maharik N, Williamson G. Flavonoid conjugates interact with organic anion transporters (OATs) and attenuate cytotoxicity of adefovir mediated by organic anion transporter 1 (OAT1/SLC22A6). Biochem Pharmacol 2011;81:942–9. [DOI] [PubMed] [Google Scholar]
- 53.Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med 2010;31:446–67. [DOI] [PubMed] [Google Scholar]
- 54.Ma LY, Liu RH, Xu XD, Yu MQ, Zhang Q, Liu HL. The pharmacokinetics of C-glycosyl flavones of Hawthorn leaf flavonoids in rat after single dose oral administration. Phytomedicine 2010;17:640–5. [DOI] [PubMed] [Google Scholar]
- 55.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev 2004;56:163–84. [DOI] [PubMed] [Google Scholar]
- 56.Schmucker DL. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging 2001;18:837–51. [DOI] [PubMed] [Google Scholar]
- 57.Handler JA, Brian WR. Effect of aging on mixed-function oxidation and conjugation by isolated perfused rat livers. Biochem Pharmacol 1997;54:159–64. [DOI] [PubMed] [Google Scholar]
- 58.Argikar UA, Remmel RP. Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab Dispos 2009;37:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plewka A, Kaminski M, Plewka D. The influence of age and some inducers on UDP-glucuronyltransferase activity. Exp Gerontol 1997;32:305–13. [DOI] [PubMed] [Google Scholar]
- 60.Fu ZD, Csanaky IL, Klaassen CD. Effects of aging on mRNA profiles for drug-metabolizing enzymes and transporters in livers of male and female mice. Drug Metab Dispos 2012;40:1216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kawase A, Ito A, Yamada A, Iwaki M. Age-related changes in mRNA levels of hepatic transporters, cytochrome P450 and UDP-glucuronosyltransferase in female rats. Eur J Drug Metab Pharmacokinet 2015;40:239–44. [DOI] [PubMed] [Google Scholar]
- 62.Dai P, Luo F, Wang Y, Jiang H, Wang L, Zhang G, Zhu L, Hu M, Wang X, Lu L, et al. . Species- and gender-dependent differences in the glucuronidation of a flavonoid glucoside and its aglycone determined using expressed UGT enzymes and microsomes. Biopharm Drug Dispos 2015;36:622–35. [DOI] [PubMed] [Google Scholar]
- 63.Bolling BW, Court MH, Blumberg JB, Chen CY. The kinetic basis for age-associated changes in quercetin and genistein glucuronidation by rat liver microsomes. J Nutr Biochem 2010;21:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, et al. . Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 2004;75:807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 2006;60:141–51. [DOI] [PubMed] [Google Scholar]
- 67.Wu AH, Tseng CC, Van Den Berg D, Yu MC. Tea intake, COMT genotype, and breast cancer in Asian-American women. Cancer Res 2003;63:7526–9. [PubMed] [Google Scholar]
- 68.Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Lovegrove JA, Minihane AM. The impact of the catechol-O-methyltransferase genotype on vascular function and blood pressure after acute green tea ingestion. Mol Nutr Food Res 2012;56:966–75. [DOI] [PubMed] [Google Scholar]
- 69.Dostal AM, Samavat H, Espejo L, Arikawa AY, Stendell-Hollis NR, Kurzer MS. Green tea extract and catechol-O-methyltransferase genotype modify fasting serum insulin and plasma adiponectin concentrations in a randomized controlled trial of overweight and obese postmenopausal women. J Nutr 2016;146:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.An SH, Chang BC, Lee KE, Gwak HS. Influence of UDP-glucuronosyltransferase polymorphisms on stable warfarin doses in patients with mechanical cardiac valves. Cardiovasc Ther 2015;33:324–8. [DOI] [PubMed] [Google Scholar]
- 71.Burchell B. Genetic variation of human UDP-glucuronosyltransferase: implications in disease and drug glucuronidation. Am J Pharmacogenomics 2003;3:37–52. [DOI] [PubMed] [Google Scholar]
- 72.Chang JL, Bigler J, Schwarz Y, Li SS, Li L, King IB, Potter JD, Lampe JW. UGT1A1 polymorphism is associated with serum bilirubin concentrations in a randomized, controlled, fruit and vegetable feeding trial. J Nutr 2007;137:890–7. [DOI] [PubMed] [Google Scholar]
- 73.Gammal RS, Court MH, Haidar CE, Iwuchukwu OF, Gaur AH, Alvarellos M, Guillemette C, Lennox JL, Whirl-Carrillo M, Brummel S, et al. . Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for UGT1A1 and atazanavir prescribing. Clin Pharmacol Ther 2016;99:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JY, Cheong HS, Park BL, Kim LH, Namgoong S, Kim JO, Kim HD, Kim YH, Chung MW, Han SY, et al. . Comprehensive variant screening of the UGT gene family. Yonsei Med J 2014;55:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lampe JW, Bigler J, Horner NK, Potter JD. UDP-glucuronosyltransferase (UGT1A1*28 and UGT1A6*2) polymorphisms in Caucasians and Asians: relationships to serum bilirubin concentrations. Pharmacogenetics 1999;9:341–9. [DOI] [PubMed] [Google Scholar]
- 76.Navarro SL, Peterson S, Chen C, Makar KW, Schwarz Y, King IB, Li SS, Li L, Kestin M, Lampe JW. Cruciferous vegetable feeding alters UGT1A1 activity: diet- and genotype-dependent changes in serum bilirubin in a controlled feeding trial. Cancer Prev Res (Phila) 2009;2:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero-Lorca A, Novillo A, Gaibar M, Bandres F, Fernandez-Santander A. Impacts of the glucuronidase genotypes UGT1A4, UGT2B7, UGT2B15 and UGT2B17 on tamoxifen metabolism in breast cancer patients. PLoS One 2015;10:e0132269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saracino MR, Bigler J, Schwarz Y, Chang JL, Li S, Li L, White E, Potter JD, Lampe JW. Citrus fruit intake is associated with lower serum bilirubin concentration among women with the UGT1A1*28 polymorphism. J Nutr 2009;139:555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scherer D, Koepl LM, Poole EM, Balavarca Y, Xiao L, Baron JA, Hsu L, Coghill AE, Campbell PT, Kleinstein SE, et al. . Genetic variation in UGT genes modify the associations of NSAIDs with risk of colorectal cancer: colon cancer family registry. Genes Chromosomes Cancer 2014;53:568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stingl JC, Bartels H, Viviani R, Lehmann ML, Brockmoller J. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: a quantitative systematic review. Pharmacol Ther 2014;141:92–116. [DOI] [PubMed] [Google Scholar]
- 81.Yong M, Schwartz SM, Atkinson C, Makar KW, Thomas SS, Newton KM, Aiello Bowles EJ, Holt VL, Leisenring WM, Lampe JW. Associations between polymorphisms in glucuronidation and sulfation enzymes and mammographic breast density in premenopausal women in the United States. Cancer Epidemiol Biomarkers Prev 2010;19:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, et al. . The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 1995;333:1171–5. [DOI] [PubMed] [Google Scholar]
- 83.Basten GP, Bao Y, Williamson G. Sulforaphane and its glutathione conjugate but not sulforaphane nitrile induce UDP-glucuronosyl transferase (UGT1A1) and glutathione transferase (GSTA1) in cultured cells. Carcinogenesis 2002;23:1399–404. [DOI] [PubMed] [Google Scholar]
- 84.Siess MH, Le Bon AM, Suschetet M. Dietary modification of drug-metabolizing enzyme activities: dose-response effect of flavonoids. J Toxicol Environ Health 1992;35:141–52. [DOI] [PubMed] [Google Scholar]
- 85.Dai D, Tang J, Rose R, Hodgson E, Bienstock RJ, Mohrenweiser HW, Goldstein JA. Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther 2001;299:825–31. [PubMed] [Google Scholar]
- 86.Cermak R. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin Drug Metab Toxicol 2008;4:17–35. [DOI] [PubMed] [Google Scholar]
- 87.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci 2006;78:2116–30. [DOI] [PubMed] [Google Scholar]
- 88.Rodríguez-Fragoso L, Martinez-Arismendi JL, Orozco-Bustos D, Reyes-Esparza J, Torres E, Burchiel SW. Potential risks resulting from fruit/vegetable-drug interactions: effects on drug-metabolizing enzymes and drug transporters. J Food Sci 2011;76:R112–24. [DOI] [PubMed] [Google Scholar]
- 89.Srinivas NR. Recent trends in preclinical drug-drug interaction studies of flavonoids–review of case studies, issues and perspectives. Phytother Res 2015;29:1679–91. [DOI] [PubMed] [Google Scholar]
- 90.Bailey DG, Arnold JM, Munoz C, Spence JD. Grapefruit juice–felodipine interaction: mechanism, predictability, and effect of naringin. Clin Pharmacol Ther 1993;53:637–42. [DOI] [PubMed] [Google Scholar]
- 91.Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol 2006;46:1390–416. [DOI] [PubMed] [Google Scholar]
- 92.Jiménez-Girón A, Queipo-Ortuno MI, Boto-Ordonez M, Munoz-Gonzalez I, Sanchez-Patan F, Monagas M, Martin-Alvarez PJ, Murri M, Tinahones FJ, Andres-Lacueva C, et al. . Comparative study of microbial-derived phenolic metabolites in human feces after intake of gin, red wine, and dealcoholized red wine. J Agric Food Chem 2013;61:3909–15. [DOI] [PubMed] [Google Scholar]
- 93.Bell JR, Donovan JL, Wong R, Waterhouse AL, German JB, Walzem RL, Kasim-Karakas SE. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am J Clin Nutr 2000;71:103–8. [DOI] [PubMed] [Google Scholar]
- 94.Donovan JL, Kasim-Karakas S, German JB, Waterhouse AL. Urinary excretion of catechin metabolites by human subjects after red wine consumption. Br J Nutr 2002;87:31–7. [DOI] [PubMed] [Google Scholar]
- 95.Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev 2014;72:429–52. [DOI] [PubMed] [Google Scholar]
- 96.Tamura M, Nakagawa H, Tsushida T, Hirayama K, Itoh K. Effect of pectin enhancement on plasma quercetin and fecal flora in rutin-supplemented mice. J Food Sci 2007;72:S648–51. [DOI] [PubMed] [Google Scholar]
- 97.Kimura T, Higaki K. Gastrointestinal transit and drug absorption. Biol Pharm Bull 2002;25:149–64. [DOI] [PubMed] [Google Scholar]
- 98.Scheidt HA, Pampel A, Nissler L, Gebhardt R, Huster D. Investigation of the membrane localization and distribution of flavonoids by high-resolution magic angle spinning NMR spectroscopy. Biochim Biophys Acta 2004;1663:97–107. [DOI] [PubMed] [Google Scholar]
- 99.Mullen W, Edwards CA, Serafini M, Crozier A. Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J Agric Food Chem 2008;56:713–9. [DOI] [PubMed] [Google Scholar]
- 100.Ortega N, Reguant J, Romero MP, Macia A, Motilva MJ. Effect of fat content on the digestibility and bioaccessibility of cocoa polyphenol by an in vitro digestion model. J Agric Food Chem 2009;57:5743–9. [DOI] [PubMed] [Google Scholar]
- 101.Lesser S, Cermak R, Wolffram S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J Nutr 2004;134:1508–11. [DOI] [PubMed] [Google Scholar]
- 102.Guo Y, Mah E, Davis CG, Jalili T, Ferruzzi MG, Chun OK, Bruno RS. Dietary fat increases quercetin bioavailability in overweight adults. Mol Nutr Food Res 2013;57:896–905. [DOI] [PubMed] [Google Scholar]
- 103.Giunta B, Hou H, Zhu Y, Salemi J, Ruscin A, Shytle RD, Tan J. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci Lett 2010;471:134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brett GM, Hollands W, Needs PW, Teucher B, Dainty JR, Davis BD, Brodbelt JS, Kroon PA. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br J Nutr 2009;101:664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vallejo F, Larrosa M, Escudero E, Zafrilla MP, Cerda B, Boza J, Garcia-Conesa MT, Espin JC, Tomas-Barberan FA. Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans. J Agric Food Chem 2010;58:6516–24. [DOI] [PubMed] [Google Scholar]
- 106.Tomás-Navarro M, Vallejo F, Sentandreu E, Navarro JL, Tomas-Barberan FA. Volunteer stratification is more relevant than technological treatment in orange juice flavanone bioavailability. J Agric Food Chem 2014;62:24–7. [DOI] [PubMed] [Google Scholar]
- 107.Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva Pinto M. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 2013;75:588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rubió L, Macia A, Motilva MJ. Impact of various factors on pharmacokinetics of bioactive polyphenols: an overview. Curr Drug Metab 2014;15:62–76. [DOI] [PubMed] [Google Scholar]
- 109.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol 1999;37:937–42. [DOI] [PubMed] [Google Scholar]
- 110.Nielsen IL, Chee WS, Poulsen L, Offord-Cavin E, Rasmussen SE, Frederiksen H, Enslen M, Barron D, Horcajada MN, Williamson G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J Nutr 2006;136:404–8. [DOI] [PubMed] [Google Scholar]
- 111.Lévèques A, Actis-Goretta L, Rein MJ, Williamson G, Dionisi F, Giuffrida F. UPLC-MS/MS quantification of total hesperetin and hesperetin enantiomers in biological matrices. J Pharm Biomed Anal 2012;57:1–6. [DOI] [PubMed] [Google Scholar]
- 112.Ottaviani JI, Momma TY, Heiss C, Kwik-Uribe C, Schroeter H, Keen CL. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radic Biol Med 2011;50:237–44. [DOI] [PubMed] [Google Scholar]
- 113.Del Rio D, Borges G, Crozier A. Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr 2010;104(Suppl 3):S67–90. [DOI] [PubMed] [Google Scholar]
- 114.van Duynhoven J, Vaughan EE, Jacobs DM, Kemperman RA, van Velzen EJ, Gross G, Roger LC, Possemiers S, Smilde AK, Dore J, et al. . Metabolic fate of polyphenols in the human superorganism. Proc Natl Acad Sci USA 2011;108(Suppl 1):4531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cermak R, Breves GM. In vitro degradation of the flavonol quercetin and of quercetin glycosides in the porcine hindgut. Arch Anim Nutr 2006;60:180–9. [DOI] [PubMed] [Google Scholar]
- 116.de Ferrars RM, Czank C, Zhang Q, Botting NP, Kroon PA, Cassidy A, Kay CD. The pharmacokinetics of anthocyanins and their metabolites in humans. Br J Pharmacol 2014;171:3268–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. . A core gut microbiome in obese and lean twins. Nature 2009;457:480–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr 2007;137(3 Suppl 2):751S–5S. [DOI] [PubMed] [Google Scholar]
- 119.Simons AL, Renouf M, Hendrich S, Murphy PA. Human gut microbial degradation of flavonoids: structure-function relationships. J Agric Food Chem 2005;53:4258–63. [DOI] [PubMed] [Google Scholar]
- 120.Roowi S, Mullen W, Edwards CA, Crozier A. Yoghurt impacts on the excretion of phenolic acids derived from colonic breakdown of orange juice flavanones in humans. Mol Nutr Food Res 2009;53(Suppl 1):S68–75. [DOI] [PubMed] [Google Scholar]
- 121.Axelson M, Setchell KD. The excretion of lignans in rats–evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett 1981;123:337–42. [DOI] [PubMed] [Google Scholar]
- 122.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 2002;132:3577–84. [DOI] [PubMed] [Google Scholar]
- 123.Setchell KD, Brown NM, Summer S, King EC, Heubi JE, Cole S, Guy T, Hokin B. Dietary factors influence production of the soy isoflavone metabolite s-(-)equol in healthy adults. J Nutr 2013;143:1950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr 2009;89:1664S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Atkinson C, Newton KM, Aiello Bowles EJ, Lehman CD, Stanczyk FZ, Westerlind KC, Li L, Lampe JW. Daidzein-metabolizing phenotypes in relation to mammographic breast density among premenopausal women in the United States. Breast Cancer Res Treat 2009;116:587–94. [DOI] [PubMed] [Google Scholar]
- 126.Atkinson C, Newton KM, Yong M, Stanczyk FZ, Westerlind KC, Li L, Lampe JW. Daidzein-metabolizing phenotypes in relation to bone density and body composition among premenopausal women in the United States. Metabolism 2012;61:1678–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pawlowski JW, Martin BR, McCabe GP, McCabe L, Jackson GS, Peacock M, Barnes S, Weaver CM. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: a randomized crossover trial. Am J Clin Nutr 2015;102:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mosele JI, Macia A, Motilva MJ. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: a review. Molecules 2015;20:17429–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5. [DOI] [PubMed] [Google Scholar]
- 130.Jacobs DM, Deltimple N, van Velzen E, van Dorsten FA, Bingham M, Vaughan EE, van Duynhoven J. (1)H NMR metabolite profiling of feces as a tool to assess the impact of nutrition on the human microbiome. NMR Biomed 2008;21:615–26. [DOI] [PubMed] [Google Scholar]
- 131.Mosele JI, Gosalbes MJ, Macia A, Rubio L, Vazquez-Castellanos JF, Jimenez Hernandez N, Moya A, Latorre A, Motilva MJ. Effect of daily intake of pomegranate juice on fecal microbiota and feces metabolites from healthy volunteers. Mol Nutr Food Res 2015;59:1942–53. [DOI] [PubMed] [Google Scholar]
- 132.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, et al. . Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- 133.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011;140:976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boto-Ordóñez M, Urpi-Sarda M, Queipo-Ortuno MI, Tulipani S, Tinahones FJ, Andres-Lacueva C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: a randomized clinical trial. Food Funct 2014;5:1932–8. [DOI] [PubMed] [Google Scholar]
- 135.Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015;64:2847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anhê FF, Roy D, Pilon G, Dudonne S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, et al. . A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015;64:872–83. [DOI] [PubMed] [Google Scholar]
- 137.Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Strom K, Ahrne S, Holm C, Molin G, Berger K. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 2012;9:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kemperman RA, Gross G, Mondot S, Possemiers S, Marzorati M, Van de Wiele T, Doré J, Vaughan EE. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res Int 2013;53:659–69. [Google Scholar]
- 139.Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal 2013;18:1818–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.di Gesso JL, Kerr JS, Zhang Q, Raheem S, Yalamanchili SK, O’Hagan D, Kay CD, O’Connell MA. Flavonoid metabolites reduce tumor necrosis factor-alpha secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol Nutr Food Res 2015;59:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amin HP, Czank C, Raheem S, Zhang Q, Botting NP, Cassidy A, Kay CD. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol Nutr Food Res 2015;59:1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr 2008;138:1067–73. [DOI] [PubMed] [Google Scholar]
- 143.Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann N Y Acad Sci 2009;1165:267–73. [DOI] [PubMed] [Google Scholar]
- 144.Amasheh M, Luettig J, Amasheh S, Zeitz M, Fromm M, Schulzke JD. Effects of quercetin studied in colonic HT-29/B6 cells and rat intestine in vitro. Ann N Y Acad Sci 2012;1258:100–7. [DOI] [PubMed] [Google Scholar]
- 145.Balentine DA, Dwyer JT, Erdman JW Jr, Ferruzzi MG, Gaine PC, Harnly JM, Kwik-Uribe CL. Recommendations on reporting requirements for flavonoids in research. Am J Clin Nutr 2015;101:1113–25. [DOI] [PubMed] [Google Scholar]