Abstract
Background: Animal studies have shown that a high intake of galactose, a breakdown product of lactose, increases ovarian toxicity. Few epidemiologic studies, to our knowledge, have examined the association between dairy intake and fertility, and they have had conflicting findings.
Objective: We prospectively evaluated dairy intake in relation to fecundability among women who were planning for pregnancy.
Design: Data were derived from preconception cohort studies in Denmark (Snart Foraeldre) and North America [PRESTO (Pregnancy Study Online)] in which women completed a validated food-frequency questionnaire 10 d after enrollment. The dietary intake of dairy foods and their constituents was calculated based on reported frequencies, mean serving sizes, and standard recipes for mixed foods. Outcome data were updated every 8 wk for 12 mo or until reported conception. Analyses were restricted to 2426 women attempting pregnancy for ≤6 cycles at study entry. Fecundability ratios (FRs) and 95% CIs were estimated with the use of proportional probabilities regression models adjusted for potential confounders.
Results: FRs for total dairy intake (≥18 compared with <7 servings/wk) were 1.37 (95% CI: 1.05, 1.78) among 1126 Snart Foraeldre participants and 1.04 (95% CI: 0.78, 1.38) among 1300 PRESTO participants (pooled FR: 1.11; 95% CI: 0.94, 1.31). The elevated FR for total dairy intake among Snart Foraeldre participants was limited to milk consumption and found only among women aged <30 y. There was no clear association between low- or high-fat dairy intake and fecundability in either cohort. Although there was little evidence of an association between dietary intake of calcium, potassium, magnesium, or vitamin D and fecundability, a greater consumption of phosphorus and lactose was associated with slightly higher fecundability in both cohorts.
Conclusions: Associations between dairy intake and fecundability were generally small and inconsistent across cohorts. Our findings do not support the hypotheses that a greater consumption of high-fat dairy improves fertility or that a greater consumption of lactose or low-fat dairy harms fertility.
Keywords: fertility, Internet, prospective studies, dairy, lactose, calcium
INTRODUCTION
Approximately 10–15% of couples experience infertility, clinically defined as the inability to conceive after 12 mo of unprotected intercourse (1). Few modifiable risk factors for infertility have been identified. Because fertility treatments are expensive, have modest success rates, and create a psychological burden for those affected, it is important to identify modifiable causes of reduced fertility, such as diet.
Animal studies have shown that a high intake of galactose, a breakdown product of lactose, increases the risk of ovarian toxicity (2–4). Moreover, women with galactosemia, a hereditary disease that affects galactose metabolism, are at increased risk of premature ovarian failure (5–7). To date, only a few studies to our knowledge have examined the association between dairy intake and fertility in women, and findings have been inconclusive (8–11). An ecologic study reported an inverse correlation between per-capita milk consumption and fertility rates (8). In contrast, a small case-control study reported that milk intake was associated with a reduced risk of infertility (9). The largest study, a prospective cohort study of female nurses, found that ovulatory infertility was positively associated with low-fat dairy intake and inversely associated with high-fat dairy intake but found little association with lactose consumption (10). A study of 232 women attending a fertility clinic reported an inverse association between total dairy intake and live birth rates, but only among women aged ≥35 y (11).
In light of the inconsistent results from previous studies, we assessed the influence of dairy consumption on time to pregnancy (TTP)6 in 2 preconception cohort studies: Snart Foraeldre (Denmark) and PRESTO (Pregnancy Study Online) (North America). We also evaluated the micronutrients commonly found in dairy products, including calcium, phosphorus, potassium, magnesium, vitamin D, and lactose. Dietary recommendations in the United States are to consume ≥3 daily servings of low-fat milk or equivalent dairy products (12). Canada has similar recommendations (2–3 servings of milk or milk alternatives each day) (13). Denmark has one of the highest levels of per-capita milk intake in the world (296 kg/y), followed closely by the United States (254 kg/y) and Canada (207 kg/y) (14). Danish recommendations include the intake of low-fat dairy products equivalent to 0.25–0.50 L/d (1–2 cups/d) (15). Thus, clarification of the extent to which dairy intake influences fertility has important public health implications.
METHODS
Study population
The Snart Foraeldre (Soon Parents) study is an Internet-based prospective cohort study of pregnancy planners in Denmark. Snart Foraeldre is an expansion of the earlier Snart Gravid (Soon Pregnant) study (16, 17). Recruitment for Snart Foraeldre began in 2011 with advertisements placed on Danish health-related websites and blogs. Eligible women were aged 18–45 y, residents of Denmark, planning a pregnancy, in a stable relation with a male partner, and not receiving fertility treatment. Enrollment and primary data collection were conducted via online self-administered questionnaires. Beginning in January 2013, 10 d after enrollment, participants were invited to complete a comprehensive food-frequency questionnaire (FFQ) designed specifically for this population (18).
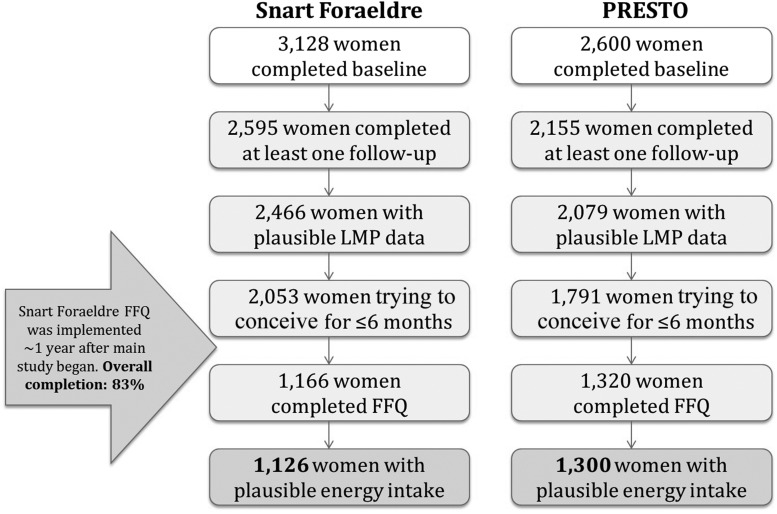
Of 3128 Snart Foraeldre participants, we excluded 533 who did not complete ≥1 follow-up questionnaire, 52 whose last menstrual period (LMP) was >6 mo before study entry, and 77 who had missing or implausible LMP information or who were pregnant at study entry. Furthermore, in an effort to avoid misclassifying diet because of subfertility, we limited our analyses to the 2053 women who had been trying to conceive for ≤6 cycles at study entry. Among these, 1166 women completed the FFQ once it was implemented (83% completion). We then excluded 24 women with implausible total energy intake (<600 or >3800 kcal/d) and 16 who had >12 missing food items on the FFQ. The final analytic sample included 1126 women. Snart Foraeldre was approved by the Danish Data Protection Agency and the Boston University Medical Center Institutional Review Board.
PRESTO (19) is also an Internet-based prospective cohort study of pregnancy planners in the United States and Canada modeled after Snart Foraeldre. Recruitment began in June 2013. Eligible women were aged 21–45 y, planning a pregnancy, not receiving fertility treatments, and in a stable relation with a male partner. PRESTO participants were invited to complete a baseline questionnaire and the National Cancer Institute’s Dietary Health Questionnaire (DHQ) II (20), a web-based FFQ, 10 d after enrollment (63% completion). In total, 2600 eligible participants completed the baseline questionnaire. We excluded 445 women with no follow-up data, 35 whose baseline LMP was >6 mo before study entry, and 41 with missing or implausible LMP data or who were already pregnant at study entry. Of the 1791 remaining women who had been trying to achieve pregnancy for ≤6 cycles at study entry, 471 did not complete the FFQ. We excluded 20 women with implausible total energy intake (<600 or >3800 kcal/d) for a final analytic sample of 1300 women. PRESTO was approved by the Boston University Medical Center Institutional Review Board.
In both cohorts, the online baseline questionnaire ascertained information on demographics, lifestyle and behavioral factors, and reproductive and medical history. To update exposure and covariate data over time and to ascertain changes in pregnancy status, self-administered online follow-up questionnaires were completed every 8 wk for 12 mo or until a reported conception. Participants in both studies provided online informed consent. Figure 1 presents a flowchart of analytic exclusions for each cohort.
FIGURE 1.
Flowchart of participant exclusions in the Snart Foraeldre and PRESTO dairy analyses, 2013–2016. Women excluded for having implausible LMP data included those whose baseline LMP was >6 mo before the date of study entry, those whose LMP date was later than the date the baseline questionnaire was completed, those who were pregnant at study entry and had been pregnant for >1 menstrual cycle length, and nonpregnant women who provided no new LMP during follow-up. FFQ, food-frequency questionnaire; LMP, last menstrual period; PRESTO, Pregnancy Study Online.
Assessment of dairy intake
Total dairy intake was estimated with the use of the nutrient composition of all food items on the FFQ and validated in each population (18, 20). Total dairy intake was calculated by summing all servings of dairy from individual foods and mixed recipes. Dairy items composed primarily of fat (i.e., butter, cream, sour cream, or cream cheese) were not included. High-fat dairy intake was calculated by summing all servings of whole milk, evaporated and condensed milk, whole-milk yogurt, regular cheese, regular ice cream, chocolate milk, and high-fat dairy products in mixed recipes. Low-fat dairy intake was calculated by summing all servings of skim, 0.5%, 1%, and 2% milk, reduced-fat chocolate milk, low-fat yogurt, cottage and ricotta cheese, low-fat cheese, low-fat ice cream, frozen yogurt and sherbet, and low-fat dairy products in mixed recipes. In the Snart Foraeldre dietary validation study, deattenuated correlation coefficients comparing the FFQ to 4-d food records were 0.49 for high-fat dairy intake and 0.66 for low-fat dairy intake (18). In the DHQ validation study, deattenuated correlation coefficients comparing the FFQ to repeated 24-h dietary recalls were 0.78 for total dairy intake, 0.84 for milk, and 0.62 for cheese (20).
We calculated dietary intake of micronutrients found in dairy foods, including calcium, phosphorus, potassium, vitamin D, magnesium, and lactose, with the use of the Danish nutrient database in Snart Foraeldre and the National Cancer Institute’s Diet*Calc software version 1.5.0 in PRESTO (21). In multivariable models, we controlled separately for the use of multivitamins and nutritional supplements, which were ascertained on the baseline questionnaire (see Data analysis).
Assessment of TTP
We calculated TTP with the use of data from the baseline and follow-up questionnaires. Women with regular menstrual cycles, defined as the ability to predict when one’s next menstrual period would start, were asked to report their usual menstrual cycle length. Among women with irregular cycles, we estimated menstrual cycle length based on LMP date at baseline and prospectively reported LMP dates during follow-up. We estimated TTP with the use of the following formula: (days of trying to conceive at study entry/cycle length) + [(LMP date from most recent follow-up questionnaire − date of baseline questionnaire)/cycle length] + 1 (22).
Assessment of covariates
Information on potential confounders (including age, race/ethnicity, education, height, weight, physical activity, smoking, alcohol consumption, last method of contraception, parity, and use of supplements or multivitamins) was reported on the baseline questionnaire. We calculated BMI as kg/m2. In Snart Foraeldre, total metabolic equivalents (METs) per week were calculated with the use of the short-form International Physical Activity Questionnaire by summing the MET-hours from walking, moderate physical activity, and vigorous physical activity (h/wk × 3.3 METs, 4 METs, and 8 METs, respectively) (23). In PRESTO, total MET-hours per week were calculated by multiplying the mean number of hours per week spent engaging in various activities by METs estimated from the Compendium of Physical Activities (24). The values for the various activities have been published elsewhere (25). The potential confounders examined for the 2 cohorts were identical except for race/ethnicity (ascertained in PRESTO only) and education, which was ascertained differently across the 2 studies.
Data analysis
We first performed parallel analyses across the 2 cohorts and then harmonized the data to carry out a pooled analysis. For ease of comparison, the same categories for servings of dairy intake were used in each cohort and were based on the underlying distribution of nutrient intake in both cohorts combined. Women contributed at-risk menstrual cycles to the analysis from study entry until a reported pregnancy (68.8% in Snart Foraeldre and 63.2% in PRESTO) or a censoring event (initiation of fertility treatment: 6.2% in Snart Foraeldre and 11.1% in PRESTO; loss to follow-up: 16.5% in Snart Foraeldre and 11.1% in PRESTO; or 12 cycles of attempts: 8.5% in Snart Foraeldre and 14.6% in PRESTO), whichever came first. Couples that did not conceive within 12 cycles of attempted conception were censored at 12 cycles because that is the typical amount of time after which couples seek infertility treatment (26). To account for the variation in attempts at study entry (range: 0–6 cycles) and to reduce bias caused by left truncation (27), we analyzed observed cycles only with the use the Anderson-Gill data structure (28). For example, if a woman enrolled with 3 cycles of attempts and then conceived during her sixth cycle, she would contribute cycles 4–6 to the analysis. We used proportional probabilities regression models to estimate fecundability ratios (FRs), defined as the ratio of the cycle-specific probability of conception comparing exposed with unexposed women (29, 30). This model controls for the decline in fecundability over time by adjusting for binary indicators of the cycle number at risk. We evaluated trends by modeling each exposure with the use of a single continuous variable measured in units of cup equivalents. We reported the trend by calculating the FR and 95% CI for a unit increase in each dairy variable. We also analyzed restricted cubic splines for nonlinear associations.
Potential confounders were selected based on the literature and an assessment of a causal diagram. We included potential risk factors for subfertility that were associated with dairy intake. Final models were adjusted for age, BMI, smoking history, parity, alcohol use, physical activity, last method of contraception, and current regular use of multivitamins or folic acid. PRESTO models were additionally adjusted for race/ethnicity and education. Snart Foraeldre models were additionally adjusted for vocational training. Models for dairy foods were adjusted for energy intake by including a continuous total energy intake variable (kilocalories per day) in the regression models. Models for individual dietary micronutrients (e.g., calcium) were adjusted for total energy intake with the use of the nutrient residual method (31), as well as for individual supplements of relevance (e.g., calcium supplements in analyses of dietary calcium). We also examined the calcium:phosphorus ratio because it is an indicator of calcium bioavailability (32). Models of high- and low-fat dairy intake and individual dairy foods (milk, cheese, yogurt) were further adjusted for total dairy intake to assess the effect of substituting 1 type of dairy food for another. In pooled analyses, regression models included a term for the cohort (PRESTO compared with Snart Foraeldre), and the race of all Snart Foraeldre participants was categorized as white. Education variables were harmonized into a single variable as follows: ≤12 y education = no vocational training, semiskilled worker, basic training, or fundamental education; 13–15 y education = <3 y higher education; 16 y education = 3–4 y higher education; and ≥17 y education = >4 y higher education.
In secondary analyses, we evaluated the extent to which the relation between dairy intake and fecundability varied by pregnancy attempts at study entry (<3 compared with 3–6 cycles) and age (<30 compared with ≥30 y). We assessed effect measure modification by conducting stratified analyses and calculating the FRs across strata along with their 95% CIs. Our rationale for stratifying the data by attempts at enrollment was to assess the extent to which reverse causation could have explained our results (e.g., if subfertility caused a change in dairy intake). Because the prevalence of ovulatory infertility increases with age, we stratified the data by age to better approximate the outcome examined in NHS (Nurses’ Health Study) II (10). Out of concern that parity could be a causal intermediate (33, 34), models were stratified by parity and fit with and without control for parity.
We used multiple imputation to impute missing covariate data (35). Covariate missingness in Snart Foraeldre ranged from 0% (age, dairy intake, and energy intake) to 6% for calcium supplements. In PRESTO, covariate missingness ranged from 0% (age, education, dairy intake, supplement use, and energy) to 0.8% for physical activity. Within each cohort, we used PROC MI to create 5 imputed data sets based on imputation models with ≥100 covariates. We combined coefficients and SEs across the imputed data sets with the use of PROC MIANALYZE. Analyses were conducted with the use of SAS version 9.4 (SAS Institute) (36).
RESULTS
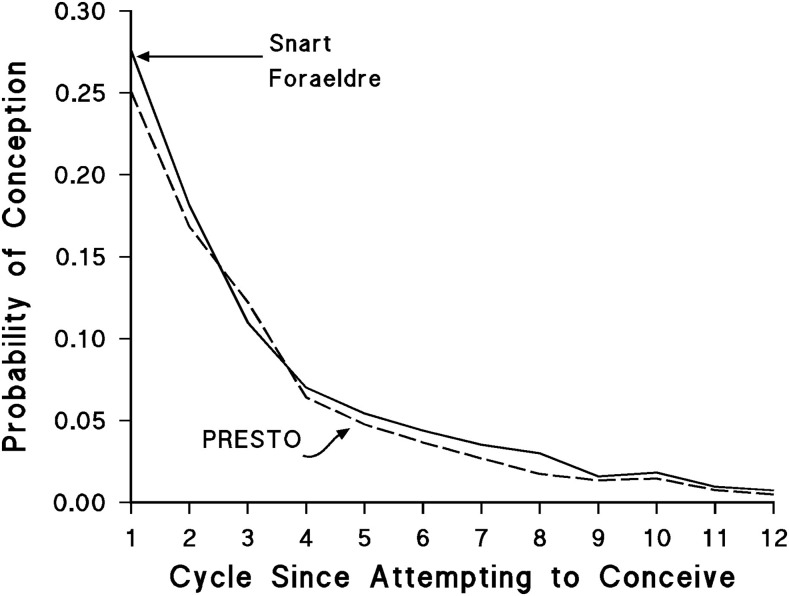
From 2013 to 2016, 1126 Snart Foraeldre participants contributed 774 pregnancies and 4307 menstrual cycles of pregnancy attempts, and 1300 PRESTO participants contributed 822 pregnancies and 5649 menstrual cycles of attempts. Fecundability was higher in Snart Foraeldre than in PRESTO for most cycles, but the differences were small (Figure 2). The FR comparing overall fecundability in PRESTO with Snart Foraeldre was 0.85 (95% CI: 0.76, 0.95) after adjusting for all covariates plus total dairy intake.
FIGURE 2.
Conditional probability of conception per menstrual cycle of attempt time stratified by cohort. PRESTO, Pregnancy Study Online.
Daily servings of dairy foods (means ± SDs) were consistently higher in Snart Foraeldre than in PRESTO: total dairy (2.32 ± 1.17 compared with 1.39 ± 0.82), high-fat dairy (0.76 ± 0.48 compared with 0.75 ± 0.60), low-fat dairy (1.56 ± 1.29 compared with 0.62 ± 0.72), milk (1.14 ± 0.92 compared with 0.64 ± 0.79), cheese (0.72 ± 0.78 compared with 0.52 ± 0.30), and yogurt (0.41 ± 0.35 compared with 0.23 ± 0.25). The top 3 foods contributing to total dairy consumption in Snart Foraeldre were “mini milk” (0.5% fat content), skim milk (<0.5% fat content), and 27% milk-fat cheese; in PRESTO, the respective foods were full-fat cheese, low-fat yogurt, and low-fat cheese. In Snart Foraeldre, total dairy intake was positively associated with caffeine intake and education and inversely associated with intercourse frequency (Table 1). In PRESTO, dairy intake was positively associated with parity, early menarche, and cycle irregularity and inversely associated with education, nonwhite race/ethnicity, and smoking.
TABLE 1.
Baseline characteristics of 2426 women in Snart Foraeldre and PRESTO by weekly dairy intake, 2013–20161
| Snart Foraeldre (n = 1126) |
PRESTO (n = 1300) |
|||||||
| Dairy consumption, cup equivalents/wk |
Dairy consumption, cup equivalents/wk |
|||||||
| Characteristics | <7 | 7–9 | 10–17 | ≥18 | <7 | 7–9 | 10–17 | ≥18 |
| n | 119 | 176 | 439 | 392 | 458 | 364 | 382 | 96 |
| Age, y | 27.2 ± 4.1 | 28.0 ± 4.2 | 28.6 ± 4.1 | 28.7 ± 4.4 | 29.9 ± 3.9 | 30.4 ± 4.0 | 30.3 ± 4.0 | 29.9 ± 4.2 |
| Partner’s age, y | 30.2 ± 4.7 | 30.7 ± 5.2 | 30.8 ± 5.2 | 30.4 ± 5.0 | 32.5 ± 5.5 | 32.0 ± 4.6 | 32.1 ± 4.9 | 31.8 ± 5.5 |
| Nonwhite or Hispanic, % | — | — | — | — | 14.5 | 13.9 | 9.8 | 7.2 |
| Less than college degree,2 % | 35.7 | 24.9 | 18.8 | 21.6 | 18.9 | 15.3 | 14.5 | 26.7 |
| Regular multivitamin intake, % | 61.3 | 53.5 | 57.8 | 63.3 | 85.5 | 89.7 | 87.0 | 82.9 |
| Age at menarche <12 y, % | 16.9 | 9.3 | 12.6 | 12.9 | 19.6 | 22.1 | 24.0 | 26.1 |
| Irregular cycles, % | 22.0 | 15.6 | 17.0 | 16.9 | 13.4 | 13.6 | 14.0 | 21.1 |
| Parous, % | 31.7 | 34.8 | 31.4 | 37.9 | 26.2 | 25.2 | 28.4 | 42.0 |
| BMI, kg/m2 | 23.7 ± 5.2 | 24.2 ± 5.1 | 24.3 ± 5.0 | 23.8 ± 4.7 | 25.5 ± 5.8 | 26.1 ± 6.3 | 26.4 ± 6.6 | 27.0 ± 8.1 |
| Exercise, MET-h/wk | 61.5 ± 66.1 | 66.8 ± 70.3 | 61.0 ± 64.1 | 71.3 ± 68.1 | 42.0 ± 28.1 | 41.6 ± 27.8 | 41.3 ± 30.8 | 34.9 ± 21.7 |
| Current smoker, % | 6.1 | 7.7 | 5.5 | 4.9 | 5.2 | 2.2 | 4.1 | 0.9 |
| Alcohol intake, drinks/wk | 2.4 | 2.8 | 2.7 | 2.4 | 3.9 ±4.4 | 3.4 ± 4.4 | 3.0 ± 3.7 | 2.8 ± 5.7 |
| Caffeine intake, mg/d | 196.8 ± 237.1 | 165.5 ± 190.1 | 208.3 ± 239.9 | 222.5 ± 232.3 | 110.6 ± 103.4 | 113.6 ± 103.3 | 118.1 ± 106.5 | 99.0 ± 110.9 |
| Intercourse frequency, % | ||||||||
| <1 time/wk | 18.4 | 18.8 | 17.2 | 14.0 | 16.6 | 21.7 | 23.1 | 18.5 |
| ≥4 times/wk | 25.5 | 15.1 | 17.3 | 16.5 | 14.5 | 11.9 | 16.1 | 13.7 |
| Trying to improve chances | 61.5 | 76.2 | 73.7 | 71.4 | 74.2 | 75.3 | 73.9 | 75.7 |
| Last method of contraception, % | ||||||||
| Hormonal | 57.1 | 61.8 | 62.0 | 61.5 | 39.2 | 42.1 | 42.3 | 46.1 |
| Barrier | 36.7 | 35.3 | 34.4 | 35.2 | 41.7 | 38.8 | 40.3 | 34.6 |
All values are means ± SDs unless otherwise indicated. Baseline enrollment took place from 2013 to 2015 in both cohorts. Follow-up continued through 2016. With the exception of age, all characteristics were age-standardized to the cohort at baseline. One cup = 250 mL. MET-h, metabolic equivalent hours; PRESTO, Pregnancy Study Online.
Equivalent to no vocational training, semiskilled worker, basic training, or fundamental education in Denmark.
In Snart Foraeldre, total dairy (≥18 compared with <7 servings/wk—FR: 1.37; 95% CI: 1.05, 1.78) and milk consumption (≥14 compared with <4 servings/wk—FR: 1.39; 95% CI: 1.07, 1.79) was positively associated with fecundability (Table 2). In PRESTO, there was little association between fecundability and total dairy, high-fat dairy, low-fat dairy, or milk consumption, but cheese intake was associated with higher fecundability. FRs corresponding to cheese consumption of 2–3, 4–6, and ≥7 servings/wk relative to <2 servings/wk were 1.10 (95% CI: 0.92, 1.31), 1.29 (95% CI: 1.05, 1.57), and 1.39 (95% CI: 1.02, 1.89), respectively. Only the positive association between milk and fecundability persisted in pooled analyses. Omitting parity from the models made little difference in the FRs (data not shown).
TABLE 2.
Dairy intake and fecundability in Snart Foraeldre and PRESTO, 2013–20161
| Snart Foraeldre (n = 1126) |
PRESTO (n = 1300) |
||||||||
| Exposure, cup equivalents/wk | Pregnancies, n | Cycles, n | Unadjusted FR3 | Adjusted FR4 | Pregnancies, n | Cycles, n | Unadjusted FR3 | Adjusted FR4 | Pooled cohorts’ adjusted FR2 |
| Total dairy | |||||||||
| <7 | 74 | 464 | Reference | Reference | 293 | 1935 | Reference | Reference | Reference |
| 7–9 | 119 | 675 | 1.15 (0.89, 1.50) | 1.18 (0.90, 1.54) | 232 | 1553 | 0.99 (0.84, 1.16) | 0.96 (0.82, 1.13) | 1.02 (0.89, 1.16) |
| 10–17 | 298 | 1717 | 1.17 (0.92, 1.48) | 1.19 (0.93, 1.52) | 235 | 1722 | 0.92 (0.77, 1.09) | 0.92 (0.77, 1.09) | 0.99 (0.87, 1.13) |
| ≥18 | 283 | 1451 | 1.35 (1.04, 1.75) | 1.37 (1.05, 1.78) | 62 | 439 | 0.98 (0.75, 1.29) | 1.04 (0.78, 1.38) | 1.11 (0.94, 1.31) |
| High-fat dairy | |||||||||
| <4 | 314 | 1799 | Reference | Reference | 356 | 2513 | Reference | Reference | Reference |
| 4–6 | 303 | 1639 | 1.06 (0.91, 1.22) | 1.04 (0.89, 1.21) | 278 | 1849 | 1.04 (0.90, 1.21) | 1.07 (0.92, 1.25) | 1.06 (0.95, 1.18) |
| 7–9 | 93 | 487 | 1.08 (0.86, 1.35) | 1.10 (0.88, 1.38) | 110 | 705 | 1.16 (0.95, 1.43) | 1.23 (0.99, 1.53) | 1.14 (0.98, 1.34) |
| ≥10 | 64 | 382 | 0.97 (0.74, 1.27) | 0.90 (0.68, 1.18) | 78 | 582 | 1.01 (0.79, 1.30) | 1.07 (0.81, 1.41) | 0.95 (0.78, 1.15) |
| Low-fat dairy | |||||||||
| <4 | 130 | 739 | Reference | Reference | 489 | 3275 | Reference | Reference | Reference |
| 4–6 | 160 | 935 | 1.01 (0.82, 1.25) | 0.98 (0.77, 1.25) | 193 | 1341 | 1.00 (0.86, 1.17) | 0.97 (0.82, 1.15) | 0.97 (0.85, 1.11) |
| 7–13 | 255 | 1446 | 1.03 (0.85, 1.25) | 0.99 (0.75, 1.29) | 106 | 799 | 0.89 (0.73, 1.08) | 0.88 (0.70, 1.11) | 0.95 (0.81, 1.12) |
| ≥14 | 229 | 1187 | 1.14 (0.93, 1.41) | 0.90 (0.65, 1.25) | 34 | 234 | 1.03 (0.75, 1.41) | 1.10 (0.73, 1.66) | 0.96 (0.77, 1.21) |
| Milk | |||||||||
| <4 | 243 | 1582 | Reference | Reference | 503 | 3476 | Reference | Reference | Reference |
| 4–6 | 184 | 958 | 1.22 (1.03, 1.45) | 1.22 (1.02, 1.47) | 177 | 1070 | 1.12 (0.96, 1.31) | 1.19 (0.99, 1.43) | 1.21 (1.07, 1.37) |
| 7–13 | 148 | 846 | 1.16 (0.96, 1.40) | 1.19 (0.96, 1.48) | 106 | 828 | 0.90 (0.74, 1.10) | 0.98 (0.76, 1.26) | 1.08 (0.92, 1.27) |
| ≥14 | 199 | 921 | 1.43 (1.20, 1.71) | 1.39 (1.07, 1.79) | 36 | 275 | 0.96 (0.70, 1.32) | 0.88 (0.53, 1.46) | 1.27 (1.02, 1.58) |
| Cheese | |||||||||
| <2 | 118 | 754 | Reference | Reference | 175 | 1321 | Reference | Reference | Reference |
| 2–3 | 289 | 1511 | 1.19 (0.98, 1.45) | 1.11 (0.91, 1.36) | 354 | 2487 | 1.07 (0.90, 1.27) | 1.10 (0.92, 1.31) | 1.10 (0.97, 1.26) |
| 4–6 | 248 | 1293 | 1.19 (0.97, 1.46) | 1.15 (0.92, 1.43) | 236 | 1451 | 1.23 (1.02, 1.48) | 1.29 (1.05, 1.57) | 1.21 (1.04, 1.41) |
| ≥7 | 119 | 749 | 1.01 (0.78, 1.30) | 0.94 (0.72, 1.23) | 57 | 390 | 1.22 (0.91, 1.63) | 1.39 (1.02, 1.89) | 1.08 (0.88, 1.31) |
| Yogurt | |||||||||
| <1 | 161 | 983 | Reference | Reference | 414 | 2830 | Reference | Reference | Reference |
| 1–3 | 420 | 2185 | 1.12 (0.95, 1.33) | 1.03 (0.87, 1.23) | 350 | 2344 | 1.03 (0.90, 1.17) | 1.01 (0.88, 1.16) | 1.03 (0.92, 1.15) |
| ≥4 | 193 | 1139 | 1.02 (0.84, 1.24) | 0.99 (0.80, 1.22) | 58 | 475 | 0.87 (0.67, 1.12) | 0.79 (0.60, 1.04) | 0.94 (0.80, 1.09) |
Values in parentheses are 95% CIs. One cup = 250 mL. FR, fecundability ratio; PRESTO, Pregnancy Study Online.
Adjusted for total energy intake, age, BMI, smoking history, parity, alcohol intake, last method of contraception, physical activity, regular multivitamin use, and study cohort.
Adjusted for total energy intake.
Adjusted for total energy intake, age, BMI, smoking history, parity, alcohol intake, last method of contraception, physical activity, and regular multivitamin use. PRESTO models were additionally adjusted for race/ethnicity and education. Snart Foraeldre models were additionally adjusted for vocational training. High- and low-fat dairy, cheese, milk, and yogurt models were additionally adjusted for total dairy intake.
We stratified by pregnancy attempts at study entry to account for potential changes in diet that may have occurred because of difficulty conceiving (Table 3). In Snart Foraeldre, total dairy intake remained positively associated with fecundability only among women with <3 cycles of attempts at entry. In PRESTO, there was little association with dairy intake in either stratum. When the data were pooled, total dairy intake was not appreciably associated with fecundability in either stratum of attempt time; the FRs across age strata were close to 1.0.
TABLE 3.
Dairy intake and fecundability stratified by attempt time at study entry, 2013–20161
| Snart Foraeldre (n = 1126) |
PRESTO (n = 1300) |
||||||
| Exposure, cup equivalents/wk | Pregnancies, n | Cycles, n | Adjusted FR3 | Pregnancies, n | Cycles, n | Adjusted FR3 | Pooled cohorts’ adjusted FR2 |
| <3 Cycles of attempt at study entry | |||||||
| Total dairy | |||||||
| <7 | 52 | 356 | Reference | 215 | 1293 | Reference | Reference |
| 7–9 | 89 | 470 | 1.26 (0.92, 1.73) | 175 | 1087 | 0.97 (0.81, 1.16) | 1.03 (0.88, 1.20) |
| 10–17 | 216 | 1221 | 1.20 (0.90, 1.61) | 172 | 1217 | 0.88 (0.72, 1.08) | 0.98 (0.84, 1.14) |
| ≥18 | 214 | 1039 | 1.45 (1.06, 1.99) | 43 | 308 | 1.00 (0.73, 1.38) | 1.13 (0.94, 1.37) |
| High-fat dairy | |||||||
| <4 | 226 | 1276 | Reference | 261 | 1670 | Reference | Reference |
| 4–6 | 220 | 1192 | 1.00 (0.83, 1.19) | 209 | 1382 | 1.03 (0.86, 1.23) | 1.02 (0.90, 1.16) |
| 7–9 | 73 | 347 | 1.13 (0.87, 1.46) | 80 | 449 | 1.28 (0.99, 1.66) | 1.20 (1.00, 1.44) |
| ≥10 | 52 | 271 | 1.00 (0.74, 1.36) | 55 | 404 | 1.08 (0.77, 1.50) | 0.97 (0.78, 1.21) |
| Low-fat dairy | |||||||
| <4 | 90 | 516 | Reference | 367 | 2288 | Reference | Reference |
| 4–6 | 116 | 696 | 0.97 (0.72, 1.31) | 137 | 867 | 1.00 (0.82, 1.22) | 0.99 (0.84, 1.16) |
| 7–13 | 199 | 1040 | 0.98 (0.70, 1.37) | 77 | 598 | 0.87 (0.66, 1.13) | 0.98 (0.81, 1.18) |
| ≥14 | 166 | 834 | 0.82 (0.55, 1.23) | 24 | 152 | 1.03 (0.64, 1.66) | 0.94 (0.72, 1.23) |
| Between 3 and 6 cycles of attempt at study entry | |||||||
| Total dairy | |||||||
| <7 | 22 | 108 | Reference | 78 | 642 | Reference | Reference |
| 7–9 | 30 | 205 | 0.81 (0.48, 1.39) | 57 | 466 | 1.07 (0.77, 1.48) | 1.00 (0.76, 1.31) |
| 10–17 | 82 | 496 | 0.94 (0.59, 1.49) | 63 | 505 | 1.08 (0.77, 1.51) | 1.01 (0.77, 1.32) |
| ≥18 | 69 | 412 | 1.05 (0.64, 1.71) | 19 | 131 | 1.02 (0.61, 1.73) | 1.08 (0.78, 1.50) |
| High-fat dairy | |||||||
| <4 | 88 | 523 | Reference | 95 | 843 | Reference | Reference |
| 4–6 | 83 | 447 | 1.16 (0.86, 1.56) | 69 | 467 | 1.23 (0.91, 1.66) | 1.18 (0.96, 1.45) |
| 7–9 | 20 | 140 | 0.94 (0.58, 1.50) | 30 | 256 | 1.06 (0.69, 1.63) | 0.99 (0.72, 1.36) |
| ≥10 | 12 | 111 | 0.70 (0.38, 1.28) | 23 | 178 | 1.12 (0.67, 1.88) | 0.87 (0.60, 1.27) |
| Low-fat dairy | |||||||
| <4 | 40 | 223 | Reference | 122 | 987 | Reference | Reference |
| 4–6 | 44 | 239 | 1.09 (0.71, 1.66) | 56 | 474 | 0.92 (0.66, 1.28) | 0.96 (0.74, 1.25) |
| 7–13 | 56 | 406 | 0.89 (0.55, 1.42) | 29 | 201 | 1.20 (0.76, 1.90) | 0.95 (0.69, 1.31) |
| ≥14 | 63 | 353 | 1.09 (0.60, 1.97) | 10 | 82 | 0.75 (0.34, 1.66) | 1.06 (0.67, 1.68) |
Values in parentheses are 95% CIs. One cup = 250 mL. For the pooled results, the ratio of FRs for a 1-unit increase in dairy variables within strata of attempts and 95% CIs for this ratio were 0.98 (0.89, 1.08) for total dairy, 1.06 (0.87, 1.30) for high-fat dairy, and 0.92 (0.78, 1.07) for low-fat dairy. FR, fecundability ratio; PRESTO, Pregnancy Study Online.
Adjusted for total energy intake, age, BMI, smoking history, parity, alcohol intake, last method of contraception, physical activity, regular multivitamin use, and study cohort.
Adjusted for total energy intake, age, and BMI. PRESTO models were additionally adjusted for race/ethnicity and education. High- and low-fat dairy were additionally adjusted for total dairy intake. Snart Foraeldre models were additionally adjusted for vocational training.
Results for total dairy and high-fat dairy intake were inconsistent across age (Table 4). In Snart Foraeldre, total dairy intake was positively associated with fecundability among women aged <30 y only, and this finding persisted in pooled analyses, albeit driven entirely by the Snart Foraeldre results. In PRESTO, a modest positive association between high-fat dairy intake and fecundability was observed among women aged <30 y (≥10 compared with <4 servings/wk—FR: 1.30; 95% CI: 0.86, 1.96) but not ≥30 y. The association was markedly attenuated in pooled analyses, with FRs across strata close to 1.0.
TABLE 4.
Dairy intake and fecundability stratified by age at baseline, 2013–20161
| Snart Foraeldre (n = 1126) |
PRESTO (n = 1300) |
||||||
| Exposure, cup equivalents/wk | Pregnancies, n | Cycles, n | Adjusted FR3 | Pregnancies, n | Cycles, n | Adjusted FR3 | Pooled cohorts’ adjusted FR2 |
| Age <30 y | |||||||
| Total dairy | |||||||
| <7 | 51 | 364 | Reference | 141 | 872 | Reference | Reference |
| 7–9 | 86 | 414 | 1.48 (1.08, 2.03) | 107 | 725 | 0.96 (0.76, 1.21) | 1.17 (0.98, 1.41) |
| 10–17 | 192 | 1114 | 1.28 (0.95, 1.72) | 117 | 783 | 0.96 (0.75, 1.23) | 1.10 (0.92, 1.31) |
| ≥18 | 174 | 909 | 1.52 (1.10, 2.10) | 35 | 242 | 1.05 (0.73, 1.50) | 1.23 (0.99, 1.53) |
| High-fat dairy | |||||||
| <4 | 209 | 1160 | Reference | 165 | 1136 | Reference | Reference |
| 4–6 | 201 | 1112 | 0.96 (0.80, 1.16) | 137 | 890 | 1.15 (0.92, 1.43) | 1.04 (0.90, 1.20) |
| 7–9 | 57 | 316 | 0.98 (0.74, 1.30) | 57 | 337 | 1.39 (1.02, 1.90) | 1.14 (0.93, 1.40) |
| ≥10 | 36 | 213 | 0.85 (0.60, 1.21) | 41 | 259 | 1.30 (0.86, 1.96) | 1.00 (0.77, 1.29) |
| Low-fat dairy | |||||||
| <4 | 93 | 571 | Reference | 242 | 1551 | Reference | Reference |
| 4–6 | 107 | 589 | 1.11 (0.83, 1.48) | 81 | 550 | 1.00 (0.77, 1.30) | 1.04 (0.87, 1.25) |
| 7–13 | 162 | 860 | 1.10 (0.79, 1.53) | 60 | 396 | 1.02 (0.74, 1.42) | 1.08 (0.86, 1.34) |
| ≥14 | 141 | 781 | 0.87 (0.58, 1.32) | 17 | 125 | 0.92 (0.52, 1.63) | 0.89 (0.66, 1.21) |
| Age ≥30 y | |||||||
| Total dairy | |||||||
| <7 | 23 | 100 | Reference | 152 | 1063 | Reference | Reference |
| 7–9 | 33 | 261 | 0.64 (0.39, 1.06) | 125 | 828 | 1.00 (0.80, 1.24) | 0.88 (0.72, 1.07) |
| 10–17 | 106 | 603 | 0.82 (0.53, 1.28) | 118 | 939 | 0.89 (0.70, 1.13) | 0.88 (0.72, 1.09) |
| ≥18 | 109 | 542 | 0.97 (0.61, 1.54) | 27 | 197 | 0.99 (0.65, 1.50) | 0.98 (0.75, 1.28) |
| High-fat dairy | |||||||
| <4 | 105 | 639 | Reference | 191 | 1377 | Reference | Reference |
| 4–6 | 102 | 527 | 1.15 (0.87, 1.50) | 141 | 959 | 0.99 (0.80, 1.23) | 1.07 (0.91, 1.27) |
| 7–9 | 36 | 171 | 1.17 (0.80, 1.72) | 53 | 368 | 1.10 (0.80, 1.51) | 1.17 (0.91, 1.49) |
| ≥10 | 28 | 169 | 0.95 (0.61, 1.47) | 37 | 323 | 0.92 (0.62, 1.36) | 0.91 (0.68, 1.21) |
| Low-fat dairy | |||||||
| <4 | 37 | 168 | Reference | 247 | 1724 | Reference | Reference |
| 4–6 | 53 | 346 | 0.79 (0.51, 1.22) | 112 | 791 | 0.98 (0.78, 1.22) | 0.92 (0.75, 1.12) |
| 7–13 | 93 | 586 | 0.68 (0.43, 1.09) | 46 | 403 | 0.83 (0.59, 1.16) | 0.83 (0.64, 1.07) |
| ≥14 | 88 | 406 | 0.83 (0.48, 1.45) | 17 | 109 | 1.14 (0.65, 2.02) | 1.06 (0.74, 1.51) |
Values in parentheses are 95% CIs. One cup = 250 mL. For the pooled results, the ratio of FRs for a 1-unit increase in dairy variables within strata of age and 95% CIs for this ratio were 1.00 (0.91, 1.10) for total dairy, 1.07 (0.87, 1.32) for high-fat dairy, and 0.92 (0.82, 1.04) for low-fat dairy. FR, fecundability ratio; PRESTO, Pregnancy Study Online.
Adjusted for total energy intake, age, BMI, smoking history, parity, alcohol intake, last method of contraception, physical activity, regular multivitamin use, and study cohort.
Adjusted for total energy intake, age, and BMI. PRESTO models were additionally adjusted for race/ethnicity and education. Snart Foraeldre models were additionally adjusted for vocational training.
Although there were no notable associations in either cohort between fecundability and dietary calcium, calcium:phosphorus ratio, potassium, magnesium, or vitamin D intake, FRs for phosphorus (≥1400 compared with <1200 mg/d) ranged from 1.28 to 1.30 in each cohort and was 1.18 in pooled analyses (Table 5). Adjusting for individual supplements in each respective model did not change the FRs appreciably (data not shown). Likewise, further adjusting for protein (grams per day), an important contributor to phosphorus intake, made little difference in the phosphorus association (data not shown). Finally, FRs for lactose intake (≥30 compared with <10 g/d) ranged from 1.24 to 1.25 in each cohort and was 1.15 in pooled analyses.
TABLE 5.
Dietary intake of individual nutrients and fecundability in Snart Foraeldre and PRESTO, 2013–20161
| Snart Foraeldre (n = 1126) |
PRESTO (n = 1300) |
||||||||
| Exposure | Pregnancies, n | Cycles, n | Unadjusted FR3 | Adjusted FR4 | Pregnancies, n | Cycles, n | Unadjusted FR3 | Adjusted FR4 | Pooled cohorts’ adjusted FR2 |
| Calcium, mg/d | |||||||||
| <800 | 116 | 728 | Reference | Reference | 371 | 2572 | Reference | Reference | Reference |
| 800–999 | 200 | 1156 | 1.07 (0.87, 1.32) | 1.09 (0.89, 1.34) | 263 | 1779 | 1.05 (0.91, 1.21) | 1.01 (0.87, 1.16) | 1.03 (0.91, 1.15) |
| ≥1000 | 458 | 2423 | 1.17 (0.97, 1.40) | 1.20 (1.00, 1.45) | 188 | 1298 | 1.04 (0.89, 1.22) | 1.07 (0.90, 1.26) | 1.10 (0.98, 1.23) |
| Phosphorus, mg/d | |||||||||
| <1200 | 87 | 618 | Reference | Reference | 644 | 4430 | Reference | Reference | Reference |
| 1200–1399 | 222 | 1238 | 1.23 (0.98, 1.54) | 1.19 (0.95, 1.50) | 134 | 967 | 0.98 (0.83, 1.17) | 1.00 (0.84, 1.18) | 1.05 (0.93, 1.20) |
| ≥1400 | 465 | 2451 | 1.31 (1.06, 1.61) | 1.30 (1.05, 1.60) | 44 | 252 | 1.22 (0.93, 1.61) | 1.28 (0.97, 1.69) | 1.18 (1.02, 1.36) |
| Calcium:potassium ratio | |||||||||
| <0.8 | 553 | 3130 | Reference | Reference | 474 | 3292 | Reference | Reference | Reference |
| 0.8–0.99 | 210 | 1105 | 1.09 (0.95, 1.26) | 1.11 (0.96, 1.28) | 289 | 1859 | 1.09 (0.95, 1.24) | 1.07 (0.93, 1.22) | 1.08 (0.98, 1.19) |
| ≥1.00 | 11 | 72 | 0.84 (0.49, 1.44) | 0.85 (0.49, 1.48) | 59 | 498 | 0.91 (0.71, 1.17) | 0.98 (0.75, 1.26) | 0.95 (0.76, 1.20) |
| Potassium, mg/d | |||||||||
| <3000 | 244 | 1439 | Reference | Reference | 706 | 4805 | Reference | Reference | Reference |
| 3000–3499 | 252 | 1377 | 1.07 (0.91, 1.25) | 1.06 (0.91, 1.25) | 86 | 618 | 0.98 (0.80, 1.20) | 1.01 (0.82, 1.24) | 1.04 (0.92, 1.17) |
| ≥3500 | 278 | 1491 | 1.07 (0.92, 1.25) | 1.08 (0.92, 1.27) | 30 | 226 | 0.93 (0.66, 1.30) | 0.96 (0.68, 1.34) | 1.03 (0.90, 1.18) |
| Magnesium, mg/d | |||||||||
| <300 | 203 | 1209 | Reference | Reference | 440 | 3248 | Reference | Reference | Reference |
| 300–399 | 455 | 2510 | 1.06 (0.91, 1.23) | 1.01 (0.86, 1.19) | 342 | 2071 | 1.18 (1.04, 1.34) | 1.12 (0.98, 1.28) | 1.08 (0.98, 1.20) |
| ≥400 | 116 | 588 | 1.13 (0.92, 1.39) | 1.11 (0.90, 1.38) | 40 | 330 | 0.93 (0.69, 1.26) | 0.93 (0.69, 1.27) | 1.09 (0.92, 1.28) |
| Lactose, g/d | |||||||||
| <10 | 133 | 828 | Reference | Reference | 417 | 2893 | Reference | Reference | Reference |
| 10–19 | 328 | 1828 | 1.12 (0.93, 1.34) | 1.14 (0.95, 1.38) | 303 | 1995 | 1.05 (0.92, 1.20) | 1.04 (0.91, 1.19) | 1.06 (0.95, 1.18) |
| 20–29 | 202 | 1078 | 1.16 (0.95, 1.42) | 1.15 (0.94, 1.40) | 64 | 531 | 0.89 (0.70, 1.14) | 0.93 (0.72, 1.19) | 1.03 (0.89, 1.18) |
| ≥30 | 111 | 573 | 1.21 (0.97, 1.52) | 1.24 (0.99, 1.56) | 38 | 230 | 1.13 (0.84, 1.53) | 1.25 (0.92, 1.70) | 1.15 (0.98, 1.37) |
| Vitamin D, IU/d | |||||||||
| <100 | 200 | 1187 | Reference | Reference | 235 | 1607 | Reference | Reference | Reference |
| 100–199 | 447 | 2389 | 1.05 (0.92, 1.20) | 0.99 (0.86, 1.13) | 427 | 2891 | 1.02 (0.88, 1.18) | 0.99 (0.86, 1.15) | 0.98 (0.89, 1.08) |
| ≥200 | 127 | 731 | 1.00 (0.70, 1.41) | 1.04 (0.73, 1.47) | 160 | 1151 | 1.00 (0.84, 1.21) | 1.02 (0.85, 1.23) | 0.99 (0.85, 1.16) |
Values in parentheses are 95% CIs. FR, fecundability ratio; PRESTO, Pregnancy Study Online.
Adjusted for total energy intake, age, BMI, smoking history, parity, alcohol intake, physical activity, last method of contraception, regular multivitamin use, and study cohort.
Adjusted for total energy intake.
Adjusted for total energy intake, age, BMI, smoking history, parity, alcohol intake, physical activity, last method of contraception, and regular multivitamin use. PRESTO models were additionally adjusted for race/ethnicity and education. Snart Foraeldre models were additionally adjusted for vocational training.
DISCUSSION
In this study of Danish and North American pregnancy planners, associations between dairy consumption and fecundability were inconsistent. In the Snart Foraeldre cohort, intakes of total dairy and milk were associated with increased fecundability. However, the association between total dairy intake and fecundability was found only among young women aged <30 y. This finding persisted in pooled analyses across the cohorts, but there was no dose-response relation. In the PRESTO cohort, there was little association between fecundability and dairy intake, although cheese and high-dairy intake was associated with increased fecundability, but only among women aged <30 y. However, neither of these findings persisted in the pooled analysis. Finally, there was little evidence of an association between fecundability and dietary consumption of calcium, potassium, or vitamin D in either cohort. In contrast, intakes of phosphorus and lactose were weakly positively associated with fecundability in both cohorts.
Four prior studies have evaluated the association between dairy intake and female fertility. An ecologic study of 31 countries found a positive correlation between per-capita milk consumption and age-related declines in fertility (8). Like all ecologic studies, the analysis lacked individual-level data on potential confounders. Results from a subsequent case-control study among couples attending a Wisconsin health clinic conflicted with the ecologic data (9). Women who consumed ≥3 glasses milk/d had a 70% lower risk of infertility than nonconsumers (9). As in all case-control studies in which participants are queried about exposures after their infertility diagnosis, recall bias is a possible threat to validity. In NHS II, a prospective cohort study of US nurses, ovulatory infertility was inversely associated with high-fat dairy and positively associated with low-fat dairy; lactose intake was not markedly associated with risk (10). Finally, in a 2016 prospective cohort study of women aged 24–44 y undergoing infertility treatment, dairy food intake was not associated with implantation or clinical pregnancy but was positively associated with live births among women aged ≥35 y (11). The association was not influenced by dairy fat content or specific kinds of dairy foods.
Comparing our results with previous studies is challenging because of methodologic differences. For example, NHS II (10) examined diet in relation to the risk of ovulatory infertility, but we examined all types of subfertility. If dairy intake influences ovulatory infertility but not other types of infertility (e.g., tubal blockage or endometriosis), results from our study might be attenuated. It is unclear why NHS II found a stronger inverse association between high-fat dairy and ovulatory infertility among older women (>32 y), whereas PRESTO found a stronger association between high-fat dairy and fecundability among younger women (<30 y). If polycystic ovarian syndrome is the primary cause of ovulatory dysfunction among younger women and diminished ovarian reserve is the primary cause of ovulatory dysfunction among older women, findings among older women should have been similar across the studies. Although the ecologic (8), case-control (9), and fertility clinic (11) studies are comparable to our study in terms of outcome definition (i.e., all-cause infertility), these studies differ in their timing of dietary intake measurement relative to outcome assessment and modeling of the outcome (dichotomization of TTP infertility compared with an evaluation of the full range of TTP). For instance, in fertility clinic populations (11), the timing of dietary assessment may have been several months or years after the diagnosis of infertility, whereas for most women in this cohort, diet was assessed soon after conception attempts began and before the diagnosis of infertility. Our study agrees with most (10, 11) but not all (8) previous studies in providing support against the hypothesis of ovarian toxicity via galactose-lactose pathways (2–4).
Diet is difficult to measure. Although there were reasonable correlations between the FFQ and dietary recalls in each of the validation studies (18, 20), some misclassification is inevitable. Because diet was assessed prospectively before the occurrence of infertility, any misclassification in our study is likely to be nondifferential, which would attenuate associations in the extreme categories of dairy intake toward the null. In contrast, the misclassification of diet in previous studies (9–11) could have been differential, biasing results in an unpredictable direction. For instance, infertile women may change dietary patterns to be more healthful in an effort to improve fertility. Given that low-fat dairy consumption is considered a healthful dietary practice in the United States, this phenomenon may have produced a spurious positive association between low-fat dairy intake and infertility in studies in which the timing of dietary ascertainment could have been months after women began trying to conceive (but before an infertility diagnosis) (10).
Residual confounding cannot be ruled out as a possible explanation of our findings. Although we controlled for a wide range of potential confounders, there may have been other important confounders strongly associated with dairy intake and fecundability that were unmeasured in our study. For example, the positive association observed between total dairy intake and fecundability in Snart Foraeldre could have resulted from residual confounding by healthy lifestyles not captured by measured variables such as exercise, adiposity, and energy intake. Confounding by male characteristics, including diet, is also possible given that couples’ diets tend to be correlated (37), and male factors may account for ≤50% subfertility. Whether these phenomena would influence results more in one cohort but not the other is unclear. Studies of dairy intake and male fertility have been equivocal (38–40). Although chance variation is the most likely explanation for variation in results across cohorts, another contributing factor could be greater exposure to antibiotics (41–43) and estrogens (44–47) from commercial dairy products in North America.
Strengths of this study include enrollment during the preconception period, with more than two-thirds of women enrolled during their first 3 cycles of attempted pregnancy. Information was collected on a wide range of potential confounders, including exercise, body size, and indicators of socioeconomic status. FFQs were validated in each population, and dietary data were collected before conception. The relative validity of selected nutrients was high. Despite all the shortcomings of FFQs, they still remain the most feasible method for assessing long-term dietary patterns in large epidemiologic studies (31, 48, 49).
Internet-based recruitment has been criticized because the characteristics of those with and without Internet access differ, and among those with Internet access, those who participate in studies may differ from those who do not (50). However, this recruitment method would not affect the validity of the study results unless the relation between diet and fertility differed substantially between Internet users and nonusers, which is unlikely (51). Furthermore, other studies (52–54) have shown that even when participation at cohort entry is related to characteristics such as age, parity, or smoking, measures of association are not biased because of self-selection. Concerns about selection bias stemming from length-biased recruitment of women with longer TTPs—or misclassification caused by changes in diet over time as a result of subfertility—can be assessed by stratifying by attempts at study entry (e.g., <3 compared with 3–6 cycles) and, if needed, controlled by restricting couples with fewer attempts at study entry. In this analysis, we observed little evidence of such bias.
In conclusion, our study showed a complex and inconsistent relation between dairy intake and fecundability among Danish and North American pregnancy planners. Total dairy and milk consumption was associated with increased fecundability in Denmark, where most milk consumed is of the low-fat variety. Nevertheless, the data showed no clear association between low- or high-fat dairy consumption and fecundability in either cohort. Although there was little evidence of an association between fecundability and dietary intake of calcium, potassium, magnesium, or vitamin D in either cohort, greater intakes of phosphorus and lactose were associated with slightly higher fecundability in both cohorts, contrary to the hypothesis that greater lactose intake harms fertility. Our findings for high- and low-fat dairy intake do not agree with previous studies, thus implying that it is premature to recommend that women increase their intake of high-fat dairy products to improve fecundity (55).
Acknowledgments
We thank Thomas Jensen and Michael Bairos for technical support with developing the web-based infrastructure of the studies; Anders Riis for data management; Vibeke Knudsen, Ellen Trolle, and Tue Christensen for assistance with the development, testing, and processing of the Danish FFQ; Amy Subar and Ken Bishop for sharing the DHQ II with our cohort; and Craig McKinnon for assistance importing the DHQ II dietary data.
The authors’ responsibilities were as follows—LAW, EMM, KJR, HTS, and EEH: designed the research; LAW and AKW: analyzed the dietary data; LAW, AKW, and KAH: coded the outcome and covariate data; LAW: took the lead in writing the manuscript and had primary responsibility for the final content; and all authors: conducted the research and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: DHQ, Dietary Health Questionnaire; FFQ, food-frequency questionnaire; FR, fecundability ratio; LMP, last menstrual period; MET, metabolic equivalent; NHS, Nurses’ Health Study; PRESTO, Pregnancy Study Online; TTP, time to pregnancy.
REFERENCES
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324-31 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swartz WJ, Mattison DR. Galactose inhibition of ovulation in mice. Fertil Steril 1988;49:522–6. [PubMed] [Google Scholar]
- 3.Bandyopadhyay S, Chakrabarti J, Banerjee S, Pal AK, Bhattacharyya D, Goswami SK, Chakravarty BN, Kabir SN. Prenatal exposure to high galactose adversely affects initial gonadal pool of germ cells in rats. Hum Reprod 2003;18:276–82. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay S, Chakrabarti J, Banerjee S, Pal AK, Goswami SK, Chakravarty BN, Kabir SN. Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Hum Reprod 2003;18:2031–8. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman FR, Kogut MD, Donnell GN, Goebelsmann U, March C, Koch R. Hypergonadotropic hypogonadism in female patients with galactosemia. N Engl J Med 1981;304:994–8. [DOI] [PubMed] [Google Scholar]
- 6.Waggoner DD, Buist NR, Donnell GN. Long-term prognosis in galactosaemia: results of a survey of 350 cases. J Inherit Metab Dis 1990;13:802–18. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero NV, Singh RH, Manatunga A, Berry GT, Steiner RD, Elsas LJ II. Risk factors for premature ovarian failure in females with galactosemia. J Pediatr 2000;137:833–41. [DOI] [PubMed] [Google Scholar]
- 8.Cramer DW, Xu H, Sahi T. Adult hypolactasia, milk consumption, and age-specific fertility. Am J Epidemiol 1994;139:282–9. [DOI] [PubMed] [Google Scholar]
- 9.Greenlee AR, Arbuckle TE, Chyou PH. Risk factors for female infertility in an agricultural region. Epidemiology 2003;14:429–36. [DOI] [PubMed] [Google Scholar]
- 10.Chavarro JE, Rich-Edwards JW, Rosner B, Willett WC. A prospective study of dairy foods intake and anovulatory infertility. Hum Reprod 2007;22:1340–7. [DOI] [PubMed] [Google Scholar]
- 11.Afeiche MC, Chiu YH, Gaskins AJ, Williams PL, Souter I, Wright DL, Hauser R, Chavarro JE. Dairy intake in relation to in vitro fertilization outcomes among women from a fertility clinic. Hum Reprod 2016;31:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans [Internet]. 8th ed. December 2015. [cited 2016 Nov 10]. Available from: http://health.gov/dietaryguidelines/2015/.
- 13.Health Canada. Eating well with Canada’s food guide: a resource for educators and communicators [Internet]. Available from: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/basics-base/quantit-eng.php.
- 14.FAO. Milk consumption—excluding butter [Internet] [cited 2015 Aug 2]. Available from: http://faostat.fao.org/site/610/DesktopDefault.aspx?PageID=610#ancor.
- 15.Ministry of Environment and Food of Denmark. Danish food guidelines [Internet]. [cited 2016 Nov 10]. Available from: https://www.foedevarestyrelsen.dk/english/Food/Nutrition/The_dietary_recommendations/Pages/default.aspx.
- 16.Huybrechts KF, Mikkelsen EM, Christensen T, Riis AH, Hatch EE, Wise LA, Sorensen HT, Rothman KJ. A successful implementation of e-epidemiology: the Danish pregnancy planning study ‘Snart-Gravid’. Eur J Epidemiol 2010;25:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish web-based pregnancy planning study—‘Snart-Gravid’. Int J Epidemiol 2009;38:938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knudsen VK, Hatch EE, Cueto H, Tucker KL, Wise L, Christensen T, Mikkelsen EM. Relative validity of a semi-quantitative, web-based FFQ used in the ‘Snart Foraeldre’ cohort—a Danish study of diet and fertility. Public Health Nutr 2016;19:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA, et al. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol 2015;29:360–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol 2001;154:1089–99. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Diet history questionnaire: Diet*Calc software [Internet]. [cited 2012 Oct 19]. Available from: http://riskfactor.cancer.gov/DHQ/dietcalc.
- 22.Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sorensen HT, Huybrechts KF, Hatch EE. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol 2011;174:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 25.McKinnon CJ, Hatch EE, Rothman KJ, Mikkelsen EM, Wesselink AK, Hahn KA, Wise LA. Body mass index, physical activity and fecundability in a North American preconception cohort study. Fertil Steril 2016;106:451–9. [DOI] [PubMed] [Google Scholar]
- 26.Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol 1986;124:470–80. [DOI] [PubMed] [Google Scholar]
- 27.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol 2013;27:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer-Verlag; 2000. [Google Scholar]
- 29.Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol 1989;129:1072–8. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 31.Willett W. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 32.Calvo MS. The effects of high phosphorus intake on calcium homeostasis. Adv Nutr Res 1994;9:183–207. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol 1993;137:1–8. [DOI] [PubMed] [Google Scholar]
- 34.Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. “Toward a clearer definition of confounding” revisited with directed acyclic graphs. Am J Epidemiol 2012;176:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou XH, Eckert GJ, Tierney WM. Multiple imputation in public health research. Stat Med 2001;20:1541–9. [DOI] [PubMed] [Google Scholar]
- 36.SAS Institute. SAS/STAT 9.4 user’s guide. Cary (NC): SAS Institute; 2014. [Google Scholar]
- 37.Kolonel LN, Lee J. Husband-wife correspondence in smoking, drinking, and dietary habits. Am J Clin Nutr 1981;34:99–104. [DOI] [PubMed] [Google Scholar]
- 38.Afeiche M, Williams PL, Mendiola J, Gaskins AJ, Jorgensen N, Swan SH, Chavarro JE. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod 2013;28:2265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, Hauser R, Chavarro JE. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril 2014;101:1280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia W, Chiu YH, Afeiche MC, Williams PL, Ford JB, Tanrikut C, Souter I, Hauser R, Chavarro JE. Impact of men’s dairy intake on assisted reproductive technology outcomes among couples attending a fertility clinic. Andrology 2016;4:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver SP, Murinda SE, Jayarao BM. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodborne Pathog Dis 2011;8:337–55. [DOI] [PubMed] [Google Scholar]
- 42.Levy S. Reduced antibiotic use in livestock: how Denmark tackled resistance. Environ Health Perspect 2014;122:A160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landers TF, Cohen B, Wittum TE, Larson EL. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep 2012;127:4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chem 1998;62:7–20. [Google Scholar]
- 45.Malekinejad H, Scherpenisse P, Bergwerff AA. Naturally occurring estrogens in processed milk and in raw milk (from gestated cows). J Agric Food Chem 2006;54:9785–91. [DOI] [PubMed] [Google Scholar]
- 46.Safe SH. Environmental and dietary estrogens and human health: is there a problem? Environ Health Perspect 1995;103:346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganmaa D, Qin LQ, Wang PY, Tezuka H, Teramoto S, Sato A. A two-generation reproduction study to assess the effects of cows’ milk on reproductive development in male and female rats. Fertil Steril 2004;82(Suppl 3):1106–14. [DOI] [PubMed] [Google Scholar]
- 48.Willett W. Invited commentary: OPEN questions. Am J Epidemiol 2003;158:22–4. [Google Scholar]
- 49.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 50.Keiding N, Slama R. Commentary: time-to-pregnancy in the Real World. Epidemiology 2015;26:119–21. [DOI] [PubMed] [Google Scholar]
- 51.Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol 2013;42:1012–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatch EE, Hahn KA, Wise LA, Mikkelsen EM, Kumar R, Fox MP, Brooks DR, Riis AH, Sorensen HT, Rothman KJ. Evaluation of selection bias in an internet-based study of pregnancy planners. Epidemiology 2016;27:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nohr EA, Frydenberg M, Henriksen TB, Olsen J. Does low participation in cohort studies induce bias? Epidemiology 2006;17:413–8. [DOI] [PubMed] [Google Scholar]
- 54.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol 2009;23:597–608. [DOI] [PubMed] [Google Scholar]
- 55.Chavarro JE, Willett WC, Skerrett PJ. The fertility diet: groundbreaking research reveals natural ways to boost ovulation & improve your chances of getting pregnant. New York: McGraw-Hill; 2007. [Google Scholar]