Abstract
Background: Musculoskeletal injuries are the most common complaint in active populations. More than 50% of all injuries in sports can be classified as sprains, strains, ruptures, or breaks of musculoskeletal tissues. Nutritional and/or exercise interventions that increase collagen synthesis and strengthen these tissues could have an important effect on injury rates.
Objective: This study was designed to determine whether gelatin supplementation could increase collagen synthesis.
Design: Eight healthy male subjects completed a randomized, double-blinded, crossover-design study in which they consumed either 5 or 15 g of vitamin C–enriched gelatin or a placebo control. After the initial drink, blood was taken every 30 min to determine amino acid content in the blood. A larger blood sample was taken before and 1 h after consumption of gelatin for treatment of engineered ligaments. One hour after the initial supplement, the subjects completed 6 min of rope-skipping to stimulate collagen synthesis. This pattern of supplementation was repeated 3 times/d with ≥6 h between exercise bouts for 3 d. Blood was drawn before and 4, 24, 48, and 72 h after the first exercise bout for determination of amino-terminal propeptide of collagen I content.
Results: Supplementation with increasing amounts of gelatin increased circulating glycine, proline, hydroxyproline, and hydroxylysine, peaking 1 h after the supplement was given. Engineered ligaments treated for 6 d with serum from samples collected before or 1 h after subjects consumed a placebo or 5 or 15 g gelatin showed increased collagen content and improved mechanics. Subjects who took 15 g gelatin 1 h before exercise showed double the amino-terminal propeptide of collagen I in their blood, indicating increased collagen synthesis.
Conclusion: These data suggest that adding gelatin to an intermittent exercise program improves collagen synthesis and could play a beneficial role in injury prevention and tissue repair. This trial was registered at the Australian New Zealand Clinical Trials Registry as ACTRN12616001092482.
Keywords: bone, exercise, ligament, tendon, inury prevention, return to play
See corresponding editorial on page 5.
INTRODUCTION
The structure and function of musculoskeletal tissues, such as tendon, ligaments, cartilage, and bone, are dependent on their collagen-rich extracellular matrix. This matrix, in turn, derives its function from the amount and the crosslinking of this collagen (1, 2) together with the water or mineral within the tissue (3). In disease states or in the presence of a nutritional or genetic insufficiency, a poor extracellular matrix is produced that is unable to withstand the mechanical demands of normal activity (4).
While poor nutrition, genetics, and disease can make connective tissue prone to failure, adequate nutrition together with exercise normally improves the function of the matrix (5). Acute exercise is known to increase collagen synthesis (6–8) as well as the expression of the primary enzyme involved in collagen crosslinking, lysyl oxidase (9). The result is a denser and stiffer tissue after training (10) that is stronger (it fails at a higher load) (11).
With the high stresses placed on these musculoskeletal tissues, injuries are extremely common in sports (12–16), disease (17), and aging (18). Although musculoskeletal injuries are extremely common and have huge personal, competitive, and financial costs, very few advances have been made in preventing and treating these injuries.
To better understand tendon and ligament biology, we have developed a tissue-engineered model that mimics the developing tissue (19–21). Like developing tendons and ligaments, these tissues have more cells and less matrix (22), synthesize collagen at a faster rate (23), have more developmental isoforms of collagen (24), and are much weaker than adult ligaments (21). Despite these differences, engineered ligaments respond to physiologic stimuli in a manner that is quite similar to the native tissue (25–28).
Findings from these engineered tissues that the presence of ascorbic acid (vitamin C) and the amino acid proline can increase collagen production and engineered ligament mechanics (21) together with work by Vieira and colleagues (29), who showed that increasing glycine intake improves the mechanics of Achilles tendons after injury, suggest that a nutritional intervention that increases amino acid components of collagen and the cofactor vitamin C may improve collagen synthesis.
As a result of this background, in the current study we used a randomized, crossover-design protocol to test the hypothesis that consuming gelatin (a food derivative of collagen) and vitamin C combined with exercise could increase collagen synthesis.
METHODS
Subjects, blood sampling, and exercise intervention
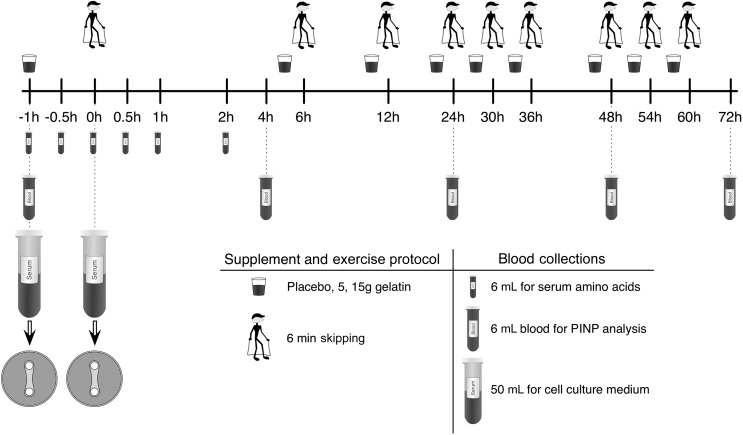
Eight healthy, recreationally active young men (mean ± SEM: 27 ± 6 y, 79.6 ± 12 kg) provided written consent to a protocol that was approved by the Australian Institute of Sport human research ethics committee and that was written in accordance with standards set by the Declaration of Helsinki. The protocol was submitted to the Australian New Zealand Clinical Trials Registry and given the registration number ACTRN12616001092482. Subjects arrived at the laboratory after an overnight fast (Figure 1). The antecubital vein of the arm was cannulated and an initial baseline blood sample collected (time = −1 h). Subjects were then immediately provided with the isocaloric treatment containing 0, 5, or 15 g gelatin dissolved in a flavored low-calorie beverage containing 48 mg vitamin C. Subjects rested for 1 h after the ingestion of the supplement during which period 2 blood samples were taken (−30 min and 0 h). Subjects then completed 6 min of continuous rope skipping to load the musculoskeletal system. After completion of the exercise task, subjects were required to remain in the laboratory in a rested state for the subsequent 4 h, during which time a further 4 blood samples were collected. After the blood sample at 4 h, the cannula was removed, and subjects were allowed to leave the laboratory. On departure, they were provided with a skipping rope and instructed to complete a further 3 sessions/d of 6 min of continuous skipping with a minimum of 6 h between each bout of exercise. The subjects ingested their supplement, which was provided in a blinded manner, 1 h before each rope-skipping session.
FIGURE 1.
Schematic timeline of the study. PINP, N-terminal peptide of pro-collagen I.
Blood samples were drawn from the cannula at 8 time points throughout the acute testing session (at −1, −0.5, 0, 0.5, 1, 2, and 4 h) with 5-mL saline flushes every 30 min to prevent the cannula from clotting. Blood from the collections at −1, −0.5, 0, 0.5, 1, and 2 h was used for amino acid analysis (see below). The blood draws at the −1 and 0 h time points were of a greater volume (50 mL) for use in tissue culture. The blood samples for tissue culture were allowed to clot and were centrifuged (1500 × g; 10 min), and the resulting sterile serum was used in place of fetal bovine serum during the treatment of the engineered ligaments. The subjects returned to the laboratory for blood draws 24, 48, and 72 h after the initial bout of exercise. These blood draws, together with the draw at 4 h, were processed for N-terminal peptide of pro-collagen I (PINP)9 analysis as described below. The PINP blood draw on day 2, 3, and 4 was taken before the subjects completed their first rope-skipping session of the day (12 h after the last exercise bout).
Nutritional supplement
Subjects were provided with 0, 5, 15 g gelatin (Ward McKenzie Pty Ltd.) in an isocaloric beverage. Maltodextrin (Polyjoule) was used to weight- and calorie-match the placebo and gelatin treatments. Subjects were provided with 9 single doses of the dry treatment ingredients sealed in separate envelopes. Subjects were instructed to make the treatment beverage by emptying the contents of each packet into the vitamin C concentrate (low-calorie blackcurrant cordial 80 mL; Ribena light, Lucozade Ribena Suntory Limited; 48 mg vitamin C/80 mL) mixed with 400 mL water in an opaque drink bottle that was provided. Subjects were instructed to consume the beverage as quickly as possible 1 h before exercise. Treatments were randomly assigned to avoid an order effect and were separated by a washout period of 4 d to minimize the effect of the previous treatment. All subjects completed all treatments. Washout was successful because PINP levels were not different in the baseline samples between trials.
Amino acid content of the blood
Whole blood was collected in 6-mL lithium heparin vacutainers. Tubes were immediately centrifuged at 3260 × g at room temperature for 10 min. The resulting plasma was separated into single 2.5-mL cryotubes and stored at −80°C until subsequent analysis. Amino acid concentrations were quantified at the University of California, Davis Proteomics Core facility by HPLC. Briefly, serum samples were hydrolyzed in 6N HCl for 24 h at 110°C, and 50 μL 10% sulfosalicylic acid was added to 200 μL of the hydrolyzed serum samples. This mixture was diluted 1:2.5 in aminoethylation-cysteine buffer, and a 50-μL injection volume was analyzed by using a Hitachi L-8900 High-speed Amino Acid Analyzer (Hitachi High-Technologies). The secondary analysis of the amino acid content was performed under blinded conditions.
Engineered ligament formation
Engineered ligaments were formed by using primary human anterior cruciate ligament cells obtained after receiving written consent for a protocol approved by the Institutional Review Board at the University of California, Davis. The anterior cruciate ligament tissue used in the current study was obtained from 1 healthy, young male donor, and the cells were isolated as described previously (28). The primary fibroblasts were expanded in growth media consisting of DMEM containing 10% fetal bovine serum and 1% penicillin, and aliquots of the cells were frozen until needed for experiments. Frozen cells were thawed and expanded to passage 4–5 before formation of engineered ligaments, as described previously (21). Briefly, 2.5 × 105 cells were embedded in a 1-mL fibrin gel (containing 5.8 U thrombin, 5.72 mg fibrinogen, 20 μg aprotinin, 200 μg 6-aminohexanoic acid) on 35-mm plates containing 2 cylindrical brushite (calcium phosphate) cement anchors placed 12 mm apart. Over the next 4 d, the cells contracted the fibrin into a ligament-like sinew between the 2 brushite anchors (21). Constructs were maintained in 2 mL feed media consisting of growth media supplemented with 5 ng/mL transforming growth factor-β1 (Peprotech), 200 μM ascorbic acid (Sigma), and 50 μM proline (Sigma), which was refreshed every other day.
Engineered ligament treatment and analysis
Once fully formed (day 8 after plating), engineered ligaments were treated for 6 d with experimental media containing blood taken before supplementation (PRE) or 1 h after consuming the supplement containing 0, 5, or 15 g gelatin. The experimental media contained only DMEM, 1% penicillin, and 10% of the serum from the subject. The tissue engineer who treated and tested the engineered ligaments was blinded to the serum treatments. Six constructs per condition per serum donor were treated, and the mean was used for all analysis. Serum from 4 of the 8 subjects was used with the engineered ligaments. All of the trials showed the same response, and the data from subject 5 is shown as a representative sample.
At the end of the 6-d treatment, constructs were tensile-tested as described previously (27). Briefly, the length and cross-sectional area (CSA) of the ligaments were determined by using digital calipers. The anchors were then placed into grips attached to a stepper motor and a force transducer. The ligaments were stretched by using a custom LabView program (National Instrument) at a rate of 0.4 mm/s until failure without preconditioning, and the resulting force-deformation data were used to calculate maximal tensile load (MTL). By using the initial length and CSA, stress and strain were calculated, and from this information the ultimate tensile stress and modulus (stiffness) of the ligaments were established. After tensile testing, constructs were dried and weighed before determining hydroxyproline content (30). Briefly, each sample was hydrolyzed in 6 N HCl and dried at 120°C. The resulting pellet was resuspended in hydroxyproline buffer. An aliquot was diluted 1:4–200 μL, combined with 150 μL chloramine T solution for 20 min, and then combined with 150 μL aldehyde-perchloric acid for 15 min at 60°C. Absorbance was read at 550 nm on an Epoch Microplate Spectrophotometer (BioTek Instruments Limited). Hydroxyproline was converted to collagen mass assuming that collagen contains 13.7% hydroxyproline (28). The collagen fraction was determined by dividing the collagen content by the mass of the tissue.
PINP
Whole blood was collected in a 3.5-mL serum separating tube and allowed to clot for 2 h before centrifugation at 1000 × g for 10 min. Samples were then aliquoted into 0.5-mL cryotubes and immediately frozen at −80°C until processed. These samples were later used to measure PINP concentrations with an ELISA kit (Novatein Biosciences). Samples were measured in duplicate according to manufacturer’s protocol by using a SPECTROstar Nano microplate reader (BMG Labtech). The Intraclass Correlation Coefficients for the ELISA assay is r = 0.984, and the sensitivity of the assay is 1.0 ng/mL. The researcher performing the analysis of PINP was also blinded to the treatment intervention.
Statistics
Amino acid content and PINP levels were analyzed by 2-factor ANOVA with one factor being time and the other being gelatin in the supplement. Post hoc test with the use of Tukey's multiple-comparisons test was performed to determine the effect of the treatments. Engineered ligament data were analyzed by 1-factor ANOVA with Tukey’s post hoc for the different gelatin levels in the supplement (GraphPad Prism, v.5). Data are presented as means ± SEMs, and the significance level was set at α < 0.05 for all comparisons.
RESULTS
Amino acids in the blood
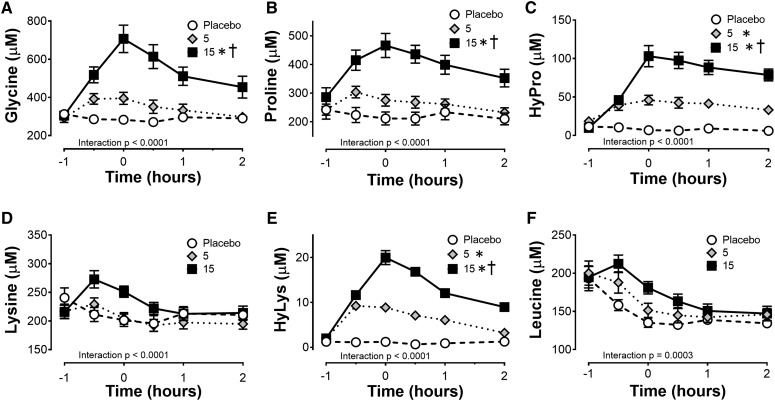
The amino acids that are highly enriched in collagen (glycine, proline, hydroxyproline, and hydroxylysine) increased in a dose-dependent manner peaking 1 h after subjects injested the supplement (Figure 2). Amino acids that had lower enrichment, such as lysine, peaked at 30 min. The greatest change in amino acid concentrations was detected for glycine and proline in the 15-g treatment group, peaking 376 and 162 μmol/L higher than baseline at the 0-h time point, respectively (Figure 2A, B). The minor amino acid components of collagen, hydroxyproline and hydroxylysine, showed a significant treatment effect increasing from almost undetectable at rest to 105 and 19 μmol/L, respectively, in the 15-g gelatin group (Figure 2C, E). In contrast to the amino acids enriched in collagen, the branched-chain amino acid leucine content in the serum significantly decreased over time (Figure 2F) with no differences between treatment groups.
FIGURE 2.
Amino acid concentration in the serum after ingestion of 5 or 15 g vitamin C–enriched gelatin or a placebo control. The amount of (A) glycine, (B) proline, (C) HyPro, (D) lysine, (E) HyLys, and (F) leucine in the blood was determined by HPLC by the University of California, Davis Proteomics Core. The data are presented as means ± SEMs for the 8 subjects who completed the crossover study. The 2-factor ANOVA (treatment and time) indicated a significant time effect for all amino acids tested (P < 0.001). The interaction effect is noted on each graph. Treatment effects are noted beside the group as part of the legend. *Significant treatment effect relative to the placebo, P < 0.05.†Significant treatment effect relative to the 5-g group, P < 0.01. HyPro, hydroxyproline; HyLys, hydroxylysine.
Collagen content in engineered ligaments treated with serum after gelatin supplementation
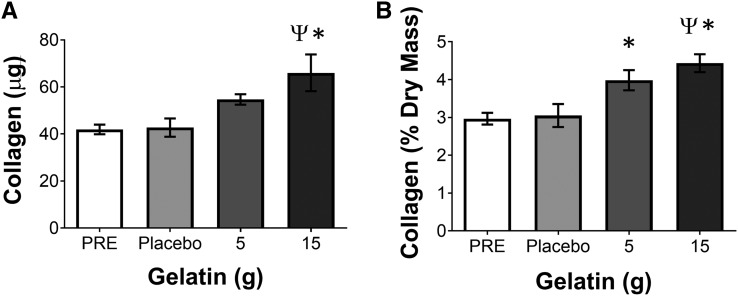
To determine whether consuming gelatin could affect collagen synthesis, we treated engineered ligaments with PRE or the serum isolated from blood collected 1 h after placebo or 5 or 15 g gelatin supplementation. After 6 d of treatment, ligaments engineered with increasing doses of gelatin showed a corresponding increase in collagen content (Figure 3A) and the collagen percent of dry mass (Figure 3B) without a change in engineered tissue CSA, meaning that the packing density of collagen within this developmental tissue model increased. This indicates that the serum from the subjects who had previously consumed increasing amounts of gelatin supported more collagen synthesis and that this effect was dependent on the amount of gelatin within the supplement.
FIGURE 3.
Collagen concentration in engineered ligaments treated with PRE or serum isolated 1 h after ingestion of 5 or 15 g vitamin C–enriched gelatin or a placebo control. The (A) content of collagen and the (B) concentration of collagen (percentage of dry mass of the tissue) were determined after 6 d of treatment with media that were supplemented with 10% of the subject-derived serum. The data are presented as means ± SEMs for 6 constructs treated with the serum from a representative subject. These results were consistent with the use of serum from the 4 subjects who were tested by using this bioassay. *Significant difference from PRE, P < 0.05; ΨSignificant difference from placebo, P < 0.05. PRE, blood taken before supplementation.
Mechanics of engineered ligaments treated with serum after gelatin supplementation
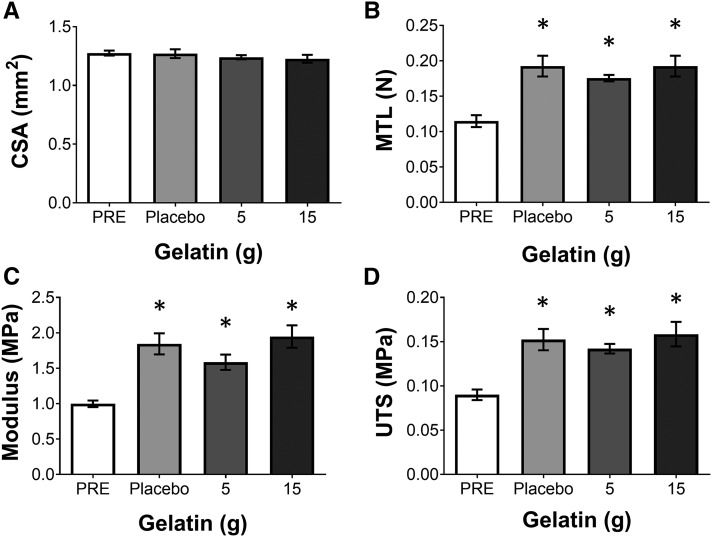
To determine the functional effect of the increase in collagen as a result of treatment with serum from subjects who had consumed gelatin, the engineered ligaments were mechanically tested to failure. None of the sera altered the CSA of the ligaments (Figure 4A). However, the MTL of the ligaments significantly increased for all treatment groups (P < 0.05) (Figure 4B). Because there was an increase in MTL with no change in CSA, the result is that the material properties of modulus (Figure 4C) and ultimate tensile strength (Figure 4D) also significantly increased (P < 0.05) as a result of the supplementation.
FIGURE 4.
Mechanics from engineered ligaments treated with PRE or serum isolated 1 h after ingestion of 5 or 15 g vitamin C–enriched gelatin or a placebo control. The (A) CSA of the engineered ligament as well as the (B) MTL, (C) modulus, and (D) UTS of the engineered ligaments were determined after 6 d of treatment with media that were supplemented with 10% of the subject-derived serum. The data are presented as means ± SEMs for 6 constructs treated with the serum from a representative subject. These results were consistent with the use of serum from the 4 subjects who were tested by using this bioassay. *Significant difference from blood before exercise, P < 0.05. CSA, cross-sectional area; MTL, maximal tensile load; PRE, blood taken before supplementation; UTS, ultimate tensile strength.
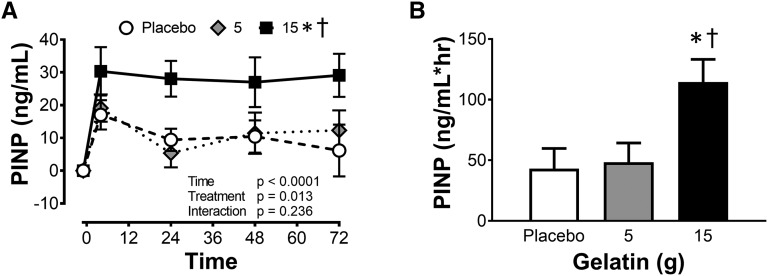
Effects of gelatin supplementation on collagen synthesis in vivo
With the beneficial effects of gelatin supplementation in vitro, we next performed a human study using a serum marker of collagen synthesis during a 72-h supplementation and exercise program. For all 3 treatment groups, PINP concentrations at baseline were 27.9 ± 4 ng/mL. The repeated bouts of 6 min of rope-skipping interspaced with ≥6 h of rest resulted in an increase in the amount of the amino-terminal propeptide of collagen I in the placebo (53.9%) and 5-g gelatin (59.2%) groups (Figure 5A). In the 15g gelatin treatment group, there was a further significant increase (153%) in PINP at 4 h (P < 0.05; Figure 5A). The doubling of PINP concentrations at 4 h was maintained throughout the whole 72 h of treatment. Supplementing with 15 g gelatin resulted in a doubling of the AUC for PINP compared with time (Figure 5B).
FIGURE 5.
Collagen synthesis after exercise and ingestion of placebo or 5 or 15 g gelatin. (A) PINP concentration in the blood of subjects 4, 24, 48, and 72 h after the first exercise bout together with (B) the AUC for PINP concentrations from the placebo or 5- or 15-g gelatin groups. The data are presented as means ± SEMs for the 8 subjects who completed the crossover study. *Significant difference from placebo group, P < 0.05. †Significant treatment effect relative to the 5-g group, P < 0.01. PINP, N-terminal peptide of pro-collagen I.
DISCUSSION
The current study demonstrates for the first time that consuming a gelatin and vitamin C–rich supplement increases the appearance of the amino acid components of collagen within human serum. The enrichment of the serum with these components 1 h after consuming the gelatin supplement was sufficient to increase the collagen content and mechanical properties of engineered ligaments. Further, subjects who consumed high amounts of gelatin 1 h before rope-skipping for 6 min showed twice as much collagen synthesis as either the placebo or the low-gelatin groups.
Supplementation with gelatin has previously been shown to improve connective tissue structure and function. McAlindon and colleagues (31) showed that consuming 10 g collagen hydrolysate/d resulted in an increase in collagen within the knee. In agreement with this finding, a 24-wk randomized clinical trial in athletes showed that collagen hydrolysate significantly decreased knee pain (32). Mouse studies that used 14C-labeled gelatin hydrolysate (33) further demonstrated that, although tracer from proline could be incorporated into skin collagen at the same rate as tracer from gelatin, tracer from the gelatin was incorporated into the collagen of cartilage and muscle twice as much as tracer from proline (33). These data suggest that musculoskeletal collagen synthesis is greater in response to gelatin than to individual amino acids. Here, we show that after ingestion of gelatin in humans, glycine, proline, hydroxyproline, and hydroxylysine peak in the blood after 1 h. In contrast, amino acids in lower enrichment (lysine) or consuming less gelatin resulted in peak levels 30 min after ingesting the supplement. Our data are in agreement with previous work that showed the peak of hydroxyproline in the blood occurred later as the dose of gelatin increased (34). This suggests that the amino acid absorption had peaked at 1 h in the 15-g group and that the exercise should have been performed 30 min after the supplement in the 5-g group.
The current data strongly support the hypothesis that starting an exercise bout 1 h after consuming 15 g gelatin results in greater collagen synthesis in the recovery period after exercise. This is most evident in comparing the PINP concentrations 4 h after exercise in the placebo and 15-g gelatin groups. The data clearly show that gelatin supported collagen synthesis after exercise (Figure 5A, B). Although exercise is known to increase collagen synthesis and the production of PINP in tendons (6–8), it is highly unlikely that the increase in PINP in the blood is due to collagen synthesis in tendon, ligaments, or cartilage. Instead, PINP in the blood is generally used as a marker of bone metabolism because of its higher turnover rate (35, 36). Bone, like tendon, responds to short periods of exercise separated by >6 h of rest (27, 37). Therefore, it is not surprising that short periods of high-impact exercise increased bone collagen synthesis. However, this is the first work that we are aware of that shows that the mechanical increase in bone collagen synthesis could be supported by a nutritional intervention.
Although the in vivo work likely reflects bone collagen synthesis, using an engineered ligament model, we have demonstrated that a similar response is seen in tendons and ligaments treated with serum after supplementation with gelatin. This system has previously been used to model the effect of hormonal changes as a result of the menstrual cycle (26) or heavy resistance exercise (28). In the current study, the experimental media used serum isolated from the same subject at baseline and 1 h after consuming the placebo or 5 or 15 g gelatin supplement. In the presence of serum isolated from the 5- and 15-g of gelatin groups, we observed a stepwise increase in the collagen content of the ligaments. This suggests that the increasing amino acids or another factor that is released into the serum as a result of gelatin ingestion improves collagen synthesis. Because normal DMEM contains supraphysiological levels of many amino acids, the observed effect is likely because of proline, hydroxyproline, or hydroxylysine, which are not components of DMEM. Proline from the serum would have increased the concentration of proline in the feed media from 29 (baseline) to 45 μmol/L (1 h in the 15-g gelatin group), a 60% increase. We have previously shown that the addition of 50 μmol/L proline to feed media can produce a significant increase in collagen content in engineered ligaments (21). Similarly, hydroxyproline and hydroxylysine increased ≥8-fold in the feed media: from 11.5 ± 3.55 and 1.2 ± 0.82 μM, respectively, at baseline (PRE) to 103 ± 13.75 and 20 ± 1.15 μM, respectively, in the 15-g gelatin group at 1 h. Small peptides that contain these trace amino acids can be absorbed into the blood after ingestion of gelatin (38) and may act to directly stimulate fibroblast activity (39). However, because growth factors in the peritendinous fluid are ∼100-fold lower than in the serum (40), how many of these amino acids in the serum would reach a tendon is unclear.
A second interesting finding was that the engineered ligaments showed an increase in mechanics in all of the supplement groups, although the collagen content of the constructs only increased in the gelatin supplemented groups. The Ribena drink used in this study was selected because it is naturally enriched with vitamin C (48.5 mg in the 80 mL given here). Although we did not measure vitamin C concentrations in the serum, it is possible that this component of the supplement had a positive effect on engineered ligament mechanics. Vitamin C is required for collagen synthesis (41). What is less appreciated is that vitamin C can also activate the collagen crosslinking enzymes lysyl oxidase (42) and the prolyl and lysyl hydroxylases (41, 43), and this increases collagen crosslinking (44). An increase in crosslinking within the collagen matrix would result in an increase in mechanical properties without an increase in collagen content consistent with the data presented here.
Finally, although the focus of the manuscript has been on the nutritional intervention, the exercise intervention was effective at increasing collagen synthesis on its own. We have previously shown that an intermittent training stimulus consisting of 10 min of activity followed by 6 h of rest resulted in greater collagen synthesis than continuous activity in engineered ligaments (27). For bone, as few as 40 loads/d delivered in short bouts with 6–8 h of rest maximizes mineralization in rodents (37, 45–50). In humans, as few as 10 maximal vertical jumps/d performed 3 d/wk is enough to increase bone mineral density in female college students (51). This suggests that musculoskeletal tissues, such as tendon, ligament, cartilage, and bone, get a maximal stimulus for collagen synthesis from short periods of activity with long intervening rest periods.
In summary, this report demonstrates, for the first time to our knowledge, that supplementation with gelatin in humans augments collagen synthesis after exercise. The accelerated rate of collagen synthesis was observed as early as 4 h after the first bout (5 h after gelatin supplementation) and was maintained over the 72 h of the study. These data suggest that adding gelatin and vitamin C to an intermittent exercise program could play a beneficial role in injury prevention and tissue repair.
Acknowledgments
The authors’ responsibilities were as follows—GS, MLRR, and KB: designed the research; GS, AL-B, MLRR, and KB: conducted the research; GS, AL-B, BW, and KB: analyzed the data or performed the statistical analysis; KB: had primary responsibility for final content; and all authors: wrote, read, and approved the final version of the manuscript. The authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: CSA, cross-sectional area; MTL, maximal tensile load; PINP, N-terminal peptide of pro-collagen I; PRE, blood taken before supplementation.
REFERENCES
- 1.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 2004;84:649–98. [DOI] [PubMed] [Google Scholar]
- 2.Buehler MJ. Nanomechanics of collagen fibrils under varying cross-link densities: atomistic and continuum studies. J Mech Behav Biomed Mater 2008;1:59–67. [DOI] [PubMed] [Google Scholar]
- 3.Fazzalari NL, Forwood MR, Smith K, Manthey BA, Herreen P. Assessment of cancellous bone quality in severe osteoarthrosis: bone mineral density, mechanics, and microdamage. Bone 1998;22:381–8. [DOI] [PubMed] [Google Scholar]
- 4.Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone 1996;18:281–8. [DOI] [PubMed] [Google Scholar]
- 5.Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 2009;19:500–10. [DOI] [PubMed] [Google Scholar]
- 6.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol 2001;534:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol 1999;521:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol (1985) 2007;102:541–6. [DOI] [PubMed] [Google Scholar]
- 9.Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol (1985) 2009;106:178–86. [DOI] [PubMed] [Google Scholar]
- 10.Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol (1985) 2008;105:805–10. [DOI] [PubMed] [Google Scholar]
- 11.LaCroix AS, Duenwald-Kuehl SE, Lakes RS, Vanderby R Jr. Relationship between tendon stiffness and failure: a metaanalysis. J Appl Physiol (1985) 2013;115:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feeley BT, Kennelly S, Barnes RP, Muller MS, Kelly BT, Rodeo SA, Warren RF. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med 2008;36:1597–603. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins RD, Hulse MA, Wilkinson C, Hodson A, Gibson M. The association football medical research programme: an audit of injuries in professional football. Br J Sports Med 2001;35:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train 2007;42:311–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Woods C, Hawkins R, Hulse M, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football: an analysis of ankle sprains. Br J Sports Med 2003;37:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods C, Hawkins RD, Maltby S, Hulse M, Thomas A, Hodson A. The Football Association Medical Research Programme: an audit of injuries in professional football–analysis of hamstring injuries. Br J Sports Med 2004;38:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes GB Jr, Mann RA. Possible epidemiological factors associated with rupture of the posterior tibial tendon. Foot Ankle 1992;13:70–9. [DOI] [PubMed] [Google Scholar]
- 18.Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med 1999;9:157–60. [DOI] [PubMed] [Google Scholar]
- 19.Paxton JZ, Donnelly K, Keatch RP, Baar K. Engineering the bone-ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng Part A 2009;15:1201–9. [DOI] [PubMed] [Google Scholar]
- 20.Paxton JZ, Donnelly K, Keatch RP, Baar K, Grover LM. Factors affecting the longevity and strength in an in vitro model of the bone-ligament interface. Ann Biomed Eng 2010;38:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paxton JZ, Grover LM, Baar K. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A 2010;16:3515–25. [DOI] [PubMed] [Google Scholar]
- 22.Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci USA 2013;110:6370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calve S, Lytle IF, Grosh K, Brown DL, Arruda EM. Implantation increases tensile strength and collagen content of self-assembled tendon constructs. J Appl Physiol (1985) 2010;108:875–81. [DOI] [PubMed] [Google Scholar]
- 24.Bayer ML, Yeung CY, Kadler KE, Qvortrup K, Baar K, Svensson RB, Magnusson SP, Krogsgaard M, Koch M, Kjaer M. The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials 2010;31:4889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagerty P, Lee A, Calve S, Lee CA, Vidal M, Baar K. The effect of growth factors on both collagen synthesis and tensile strength of engineered human ligaments. Biomaterials 2012;33:6355–61. [DOI] [PubMed] [Google Scholar]
- 26.Lee CA, Lee-Barthel A, Marquino L, Sandoval N, Marcotte GR, Baar K. Estrogen inhibits lysyl oxidase and decreases mechanical function in engineered ligaments. J Appl Physiol 2015;118:1250–7. [DOI] [PubMed] [Google Scholar]
- 27.Paxton JZ, Hagerty P, Andrick JJ, Baar K. Optimizing an intermittent stretch paradigm using ERK1/2 phosphorylation results in increased collagen synthesis in engineered ligaments. Tissue Eng Part A 2012;18:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West DW, Lee-Barthel A, McIntyre T, Shamim B, Lee CA, Baar K. The exercise-induced biochemical milieu enhances collagen content and tensile strength of engineered ligaments. J Physiol 2015;593:4665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira CP, De Oliveira LP, Da Re Guerra F, Dos Santos De Almeida M, Marcondes MC, Pimentel ER. Glycine improves biochemical and biomechanical properties following inflammation of the achilles tendon. Anat Rec (Hoboken) 2015;298:538–45. [DOI] [PubMed] [Google Scholar]
- 30.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–7. [DOI] [PubMed] [Google Scholar]
- 31.McAlindon TE, Nuite M, Krishnan N, Ruthazer R, Price LL, Burstein D, Griffith J, Flechsenhar K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthritis Cartilage 2011;19:399–405. [DOI] [PubMed] [Google Scholar]
- 32.Clark KL, Sebastianelli W, Flechsenhar KR, Aukermann DF, Meza F, Millard RL, Deitch JR, Sherbondy PS, Albert A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr Med Res Opin 2008;24:1485–96. [DOI] [PubMed] [Google Scholar]
- 33.Oesser S, Adam M, Babel W, Seifert J. Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J Nutr 1999;129:1891–5. [DOI] [PubMed] [Google Scholar]
- 34.Imaoka T, Suou T, Hirayama C. A simplified gelatin tolerance test to evaluate gastric and pancreatic proteolytic activities. Res Commun Chem Pathol Pharmacol 1992;78:97–108. [PubMed] [Google Scholar]
- 35.Hale LV, Galvin RJ, Risteli J, Ma YL, Harvey AK, Yang X, Cain RL, Zeng Q, Frolik CA, Sato M, et al. . PINP: a serum biomarker of bone formation in the rat. Bone 2007;40:1103–9. [DOI] [PubMed] [Google Scholar]
- 36.Orum O, Hansen M, Jensen CH, Sorensen HA, Jensen LB, Horslev-Petersen K, Teisner B. Procollagen type I N-terminal propeptide (PINP) as an indicator of type I collagen metabolism: ELISA development, reference interval, and hypovitaminosis D induced hyperparathyroidism. Bone 1996;19:157–63. [DOI] [PubMed] [Google Scholar]
- 37.Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone 2002;30:781–6. [DOI] [PubMed] [Google Scholar]
- 38.Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, Higashi A, Kido Y, Nakabo Y, Ohtsuki K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J Agric Food Chem 2005;53:6531–6. [DOI] [PubMed] [Google Scholar]
- 39.Ohara H, Ichikawa S, Matsumoto H, Akiyama M, Fujimoto N, Kobayashi T, Tajima S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol 2010;37:330–8. [DOI] [PubMed] [Google Scholar]
- 40.Olesen JL, Heinemeier KM, Gemmer C, Kjaer M, Flyvbjerg A, Langberg H. Exercise-dependent IGF-I, IGFBPs, and type I collagen changes in human peritendinous connective tissue determined by microdialysis. J Appl Physiol (1985) 2007;102:214–20. [DOI] [PubMed] [Google Scholar]
- 41.Van Robertson WB, Schwartz B. Ascorbic acid and the formation of collagen. J Biol Chem 1953;201:689–96. [PubMed] [Google Scholar]
- 42.Harris ED, Percival SS. A role for ascorbic acid in copper transport. Am J Clin Nutr 1991;54(6 Suppl):1193S–7S. [DOI] [PubMed] [Google Scholar]
- 43.Murad S, Sivarajah A, Pinnell SR. Prolyl and lysyl hydroxylase activities of human skin fibroblasts: effect of donor age and ascorbate. J Invest Dermatol 1980;75:404–7. [DOI] [PubMed] [Google Scholar]
- 44.Levene CI, Shoshan S, Bates CJ. The effect of ascorbic acid on the cross-linking of collagen during its synthesis by cultured 3 T6 fibroblasts. Biochim Biophys Acta 1972;257:384–8. [DOI] [PubMed] [Google Scholar]
- 45.Honda A, Umemura Y, Nagasawa S. Effect of high-impact and low-repetition training on bones in ovariectomized rats. J Bone Miner Res 2001;16:1688–93. [DOI] [PubMed] [Google Scholar]
- 46.Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodelling. J Biomech 1984;17:897–905. [DOI] [PubMed] [Google Scholar]
- 47.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 1984;66:397–402. [PubMed] [Google Scholar]
- 48.Umemura Y, Ishiko T, Tsujimoto H, Miura H, Mokushi N, Suzuki H. Effects of jump training on bone hypertrophy in young and old rats. Int J Sports Med 1995;16:364–7. [DOI] [PubMed] [Google Scholar]
- 49.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J Bone Miner Res 1997;12:1480–5. [DOI] [PubMed] [Google Scholar]
- 50.Umemura Y, Sogo N, Honda A. Effects of intervals between jumps or bouts on osteogenic response to loading. J Appl Physiol (1985) 2002;93:1345–8. [DOI] [PubMed] [Google Scholar]
- 51.Kato T, Terashima T, Yamashita T, Hatanaka Y, Honda A, Umemura Y. Effect of low-repetition jump training on bone mineral density in young women. J Appl Physiol (1985) 2006;100:839–43. [DOI] [PubMed] [Google Scholar]