Abstract
Background: The effect of a weight-loss intervention on the masses of lean tissues and organs in humans is not well known.
Objective: We studied the effects of a diet and exercise weight-loss intervention on skeletal muscle (SM) mass and selected organs over 2 y using MRI in overweight adults with type 2 diabetes.
Design: Participants were 53 women and 39 men [mean ± SD: age 58 ± 7 y; body mass index (BMI; in kg/m2) 32 ± 3] enrolled in the Look AHEAD (Action for Health in Diabetes) trial and randomly assigned to an intensive lifestyle intervention (ILI) or diabetes support and education (DSE) on whom 2 y of data were collected. MRI-derived measurements of SM, heart, liver, kidney, spleen, and pancreas were acquired.
Results: Adjusted for baseline weight, height, age, sex, and ethnicity, the ILI group weighed (mean ± SE) 6.6 ± 0.7 kg less after 1 y and 5.2 ± 0.7 kg less after 2 y, whereas the DSE group did not change significantly (−0.4 ± 0.6 and −1.0 ± 0.7 kg after 1 and 2 y, respectively; P-interaction < 0.001). Total SM decreased in both groups during year 1 (−1.4 ± 0.2 kg; P < 0.001) with appendicular SM regained during year 2. Liver and spleen masses decreased in the ILI group (−0.12 ± 0.02 and −0.006 ± 0.003 kg, respectively) but were unchanged in the DSE group (0.00 ± 0.02 and 0.004 ± 0.003 kg, respectively). Kidney mass decreased by 0.013 ± 0.003 kg (P < 0.001) over 2 y in both groups.
Conclusions: Decreases in liver (in Caucasians but not African Americans) and spleen were detected after a 6.2-kg weight reduction compared with a control group. SM and kidney mass decreased in both groups. Appendicular SM was regained during the second year whereas trunk SM was not. No evidence of a disproportionate loss of high–metabolic rate organs (heart, liver, kidney, spleen) compared with SM was found.
Keywords: type 2 diabetes, magnetic resonance imaging, body composition, skeletal muscle, organ mass, weight loss, Look AHEAD trial
INTRODUCTION
Although the usual prescription for obese and overweight persons is to lose weight, the unstated objective is actually for them to lose fat. Nevertheless, except in rare circumstances, intentional fat loss is accompanied by unintentional loss of lean tissue. Studies that examined the composition of weight loss found typically 60–80% fat and 20–40% fat-free mass; however, the proportions can vary widely depending on degree of caloric restriction, length of intervention, activity level, adiposity, age, sex, and ethnicity [for a recent review see Heymsfield et al. (1)]. Less skeletal muscle (SM)10 is needed to support the physical load of a reduced fat mass but excessive muscle loss may degrade physical function. This concern is magnified in aging populations in whom sarcopenia is a known hazard, and preservation of physical mobility can mean the difference between independent living and institutionalization.
Furthermore, the effects of weight loss interventions on other constituents of lean mass (heart, liver, kidney, lungs, and brain) are not well known. An issue of ongoing interest is whether reductions in resting energy expenditure (REE) seen after weight loss could be accounted for by a disproportionate loss of high metabolic rate (HMR) tissue and organs (brain, liver, kidney, heart, spleen) compared with low metabolic rate tissue (muscle, skin) thereby changing the composition of total lean body mass (LBM). Limited studies in animals (2, 3) have shown that caloric restriction (40–50% of ad libitum intake) results in a reduction in the size of the heart, liver, kidney and spleen but not the brain.
Detailed descriptions of organ-level changes in humans after a weight-loss intervention have been reported. In one study (4) after a 9% weight loss over 3 mo, investigators observed decreases of 4–6% in the masses of the heart, kidney, and liver, whereas the brain remained unchanged and SM decreased by 3.1%. In a subsequent study (5) with a 24-mo follow-up of participants who maintained an 11% weight loss, the brain and liver did not change, heart mass decreased 26%, kidneys 19%, and SM 5.7%. In the most recent study (6), 3 wk of caloric restriction after 1 wk of overfeeding resulted in 4.2 kg of weight loss, SM decrease of 5%, liver 13%, kidneys 8%, and brain 3%, but the heart was unchanged. The differences in findings among the studies suggest that effects on organs differ depending on subject characteristics, intensity and duration of intervention, and follow-up interval.
We investigated the loss of SM and selected organ masses during a weight-loss intervention in persons with type 2 diabetes in a randomized, parallel-groups, 2-y longitudinal study ancillary to the Look AHEAD (Action for Health in Diabetes) trial (7). The 2-y duration of our study allowed us to address whether changes observed soon after weight loss persist during a subsequent 1-y period of relative weight stability or partial regain.
METHODS
Participants who enrolled in the Look AHEAD trial at the New York, NY, and Pittsburgh, PA, sites in 2002 and 2003 were invited to enroll in this ancillary study after random assignment and before initiation of any intervention. Look AHEAD is a clinical trial of a diet and exercise weight-loss intervention for the prevention of cardiovascular disease in men and women with type 2 diabetes, aged 45–74 y, and with a BMI (in kg/m2) ≥25 as previously described (7, 8). Preintervention (baseline, T0), 1-y (T12), and 2-y (T24) follow-up data were used in this analysis. Information on duration of diabetes (length of time since diagnosis) and whether the patient was taking medication or insulin was obtained from the parent trial. Selected results from the Look AHEAD trial (9–13) and some findings of this MRI ancillary study have been published (8, 14, 15). All studies were approved by the Institutional Review Board of St. Luke's-Roosevelt Hospital or the University of Pittsburgh, and each subject gave written consent before data were collected.
Men and women with a BMI 25 to <41; of African American, Asian, Caucasian, and Hispanic ethnicity; and for whom valid MRI organ and tissue measurements were obtained at the baseline visit and ≥1 follow-up MRI visit after baseline were included in this analysis.
Organ and tissue measures
Body weight was measured to the nearest 0.1 kg (Weight Tronix) and height to the nearest 0.5 cm with the use of a stadiometer (Holtain).
MRI
LBM, the sum of SM, liver, kidney, spleen, pancreas, and residual tissue (skin, lungs, gastrointestinal tract, brain, bone), was measured by using whole-body multislice MRI as previously described (16, 17). Subjects at both sites (New York and Pittsburgh) were placed on a 1.5 T scanner (6× Horizon; General Electric) platform with arms extended above head. A whole-body MRI protocol with the use of an axial T1-weighted spin echo sequence with 10-mm thickness, 40-mm interslice gap, and a 40 × 40–cm2 field of view was used to quantify SM mass. The scans at both sites were acquired by using a single protocol on similar scanners, which were also in use for clinical purposes.
Organ mass measurement
Liver, kidneys, spleen, and pancreas volumes were measured by MRI by using an axial T1-weighted spin echo sequence with 5-mm slice thickness and no interslice gap throughout the abdominal region by using acquisition and segmentation analysis techniques that have been described previously (16, 17). The volume of the left ventricle was measured by cardiac-gated MRI. The protocol consisted of 8–12 contiguous, short-axis image locations along the short axis of the left ventricle, with the use of the electrocardiographic R wave to determine the image that represented end-diastole for volume analysis. Detailed explanation of the method has been given previously (16–18). A single radiologist, blinded to group assignment, analyzed all cases. In a series of 10 normal subjects, the mean intraobserver variability for estimating left ventricular mass was 5.13% ± 2.9% (18). Left ventricular mass was multiplied by 1.5 to reflect total cardiac mass (19).
Image analyses
SliceOmatic image analysis software (version 4.2; Tomovision) was used to analyze images on a PC workstation (Gateway). MRI volume estimates were converted to mass by using the assumed density of 1.04 kg/L for SM, the liver, spleen, kidney, and pancreas and 1.03 kg/L for the heart (20). These densities assume typical/average amounts of intramyocellular lipid as found in Snyder et al. (20). All scans in this study were read by the same technician blinded to group assignment in the Image Analysis Laboratory of the New York Obesity Nutrition Research Center. The CVs for 2 readings of the same scan by the same analyst for SM, the kidney, liver, and spleen in a previous pediatric sample were 2.4%, 12.3%, 1.7%, and 19.7%, respectively.
Statistical analysis
Descriptive statistics (number, mean, SD, SE) were calculated for subject characteristics at T0, and least squares means for changes from T0 to T12 and to T24 for body weight and lean tissue components. Differences between treatment and control groups at T0 and characteristics of subjects who were not included in the analysis compared with those who were included were tested by using Student’s t tests for continuous variables and Fisher’s exact test for categorical variables. The primary analysis for investigation of longitudinal changes in individual compartments was maximum-likelihood regression modeling with 2 between-subject groups [diabetes support and education (DSE) compared with intensive lifestyle intervention (ILI)], repeated measures at 3 visit times (T0, T12, and T24) coded as a categorical variable, and group-by-visit (time) interactions. The study was powered (β = 0.8) to detect differences of ∼12% (moderate effect sizes) between ILI and DSE changes during the first year, α = 0.05, 2-tailed. Analyses were also run with sex and ethnicity (dummy coded) and weight, height, and age (continuous variables) as covariates and with models testing whether covariates interacted with group and time.
Model assumptions were tested and in case of violations analyses were carried out with transformed data (e.g., log spleen mass) or excluding outliers identified by visual inspection of residual scatterplots. Sensitivity analyses compared results with and without influential cases identified by Cook’s D and restricted likelihood distance statistics and with data from only subjects who completed the study.
Statistical calculations were performed by using SAS (v9.4; SAS Institute Inc.) and STATA (v11.0). The level of significance for all statistical tests was 0.05, with Tukey-Kramer adjusted P values used for comparisons of group means or within-group changes after a significant overall F test. Values are expressed as means ± SEs.
RESULTS
Demographic and body composition characteristics
Of 138 subjects initially enrolled in this ancillary study, 92 participants provided useable MRI measurements at T0 and at a subsequent visit. T0 characteristics of excluded men (n = 22) were not significantly different from those included, but excluded women (n = 24) were older (61 compared with 57 y old; P = 0.02) and had a larger pancreas (mean ± SD, 46 ± 15 compared with 39 ± 12 g, P = 0.04). Subject demographic characteristics and unadjusted mean values for organ and tissue components at T0 are shown in Table 1. Of the subjects included in this analysis, men and women in the DSE group were not significantly different by Student’s t test from men and women in the ILI group on any measure at T0.
TABLE 1.
Baseline subject characteristics1
| Women |
Men |
|||
| DSE | ILI | DSE | ILI | |
| Subjects | 26 | 27 | 25 | 14 |
| Ethnicity | ||||
| AA | 14 | 10 | 3 | 2 |
| Caucasian | 9 | 12 | 20 | 12 |
| Hispanic | 2 | 3 | 1 | |
| Asian | 1 | 2 | 1 | |
| Age, y | 57 ± 6 (45–68) | 57 ± 6 (45–73) | 59 ± 7 (45–75) | 59 ± 8 (47–71) |
| Weight, kg | 85 ± 13 (64–110) | 82 ± 14 (63–114) | 99 ± 13 (74–119) | 93 ± 8 (80–107) |
| Height, cm | 161 ± 7 (149–180) | 162 ± 7 (149–179) | 177 ± 8 (165–191) | 175 ± 6 (162–183) |
| BMI, kg/m2 | 33 ± 3 (26–40) | 31 ± 4 (25–40) | 32 ± 3 (27–36) | 30 ± 3 (26–35) |
| SM, kg | 20.3 ± 3.5 (12.7–28.8) | 20.6 ± 3.0 (16.3–28.3) | 31.7 ± 4.4 (25.0–41.0) | 29.7 ± 2.8 (24.1–35.7) |
| Liver, kg | 1.73 ± 0.37 (1.03–2.73) | 1.76 ± 0.50 (0.87–2.91) | 2.09 ± 0.50 (1.27–3.78) | 1.90 ± 0.40 (1.32–2.76) |
| Spleen, kg | 0.18 ± 0.13 (0.04–0.60) | 0.21 ± 0.20 (0.07–0.88) | 0.31 ± 0.14 (0.10–0.54) | 0.26 ± 0.12 (0.06–0.44) |
| Kidney, kg | 0.39 ± 0.07 (0.22–0.51) | 0.38 ± 0.11 (0.21–0.64) | 0.50 ± 0.07 (0.40–0.66) | 0.46 ± 0.08 (0.38–0.61) |
| Heart, kg | 0.22 ± 0.04 (0.14–0.33) | 0.22 ± 0.03 (0.15–0.29) | 0.29 ± 0.06 (0.15–0.39) | 0.29 ± 0.06 (0.21–0.38) |
| Pancreas, g | 38 ± 13 (13-61) | 39 ± 12 (13–59) | 49 ± 16 (19–86) | 49 ± 24 (8–103) |
All values are n or means ± SDs (ranges). Ethnicity was self identified. Within sex, DSE was not significantly different from ILI by using Student’s t test on any measurement. AA, African American; DSE, diabetes support and education; ILI, intensive lifestyle intervention; SM, skeletal muscle.
Least squares means of T0 values and changes from T0 to T12 and T0 to T24 adjusted for age, height, initial weight, sex, and ethnicity are shown in Table 2 for the variables discussed below.
TABLE 2.
T0 values and changes in body composition at T12 and T241
| DSE |
ILI |
|||||
| T0 | T0–T12 | T0–T24 | T0 | T0–T12 | T0–T24 | |
| Subjects,2 n | 51–49 | 51–47 | 34–27 | 41–40 | 41–37 | 31–27 |
| Weight, kg | 90.1 ± 0.66 | −0.44 ± 0.60 | −0.97 ± 0.69 | 89.8 ± 0.73 | −6.63 ± 0.673,4 | −5.21 ± 0.743,5 |
| Skeletal muscle, kg | 25.4 ± 0.4 | −1.22 ± 0.206 | −1.15 ± 0.257,8 | 25.2 ± 0.5 | −1.49 ± 0.236 | −0.63 ± 0.267,8 |
| Arm | 3.16 ± 0.09 | −0.09 ± 0.056 | −0.03 ± 0.078 | 3.11 ± 0.10 | −0.14 ± 0.066 | 0.06 ± 0.078 |
| Trunk | 12.4 ± 0.24 | −0.46 ± 0.186 | −0.90 ± 0.237 | 12.3 ± 0.27 | −0.88 ± 0.236 | −1.20 ± 0.237 |
| Leg | 9.80 ± 0.19 | −0.65 ± 0.116 | 0.03 ± 0.158 | 9.79 ± 0.22 | −0.48 ± 0.136 | 0.42 ± 0.158 |
| Liver, kg | 1.77 ± 0.05 | −0.00 ± 0.02 | −0.03 ± 0.02 | 1.70 ± 0.06 | −0.12 ± 0.023,5 | −0.11 ± 0.039 |
| Caucasian | 2.01 ± 0.07 | 0.02 ± 0.03 | 0.03 ± 0.03 | 2.00 ± 0.07 | −0.14 ± 0.033,10 | −0.12 ± 0.033,5 |
| AA | 1.60 ± 0.06 | −0.01 ± 0.03 | −0.08 ± 0.037 | 1.44 ± 0.07 | −0.05 ± 0.04 | −0.05 ± 0.047 |
| Spleen,11 kg | 0.150 ± 0.024 | 0.004 ± 0.003 | 0.003 ± 0.004 | 0.139 ± 0.027 | −0.006 ± 0.00310 | −0.005 ± 0.003 |
| Kidney, kg | 0.44 ± 0.01 | 0.00 ± 0.00 | −0.01 ± 0.007 | 0.42 ± 0.01 | −0.01 ± 0.00 | −0.02 ± 0.007 |
| Heart, kg | 0.252 ± 0.007 | −0.001 ± 0.004 | −0.004 ± 0.005 | 0.258 ± 0.008 | −0.009 ± 0.005 | −0.010 ± 0.006 |
| Pancreas,2 g | 44 ± 3 | 6 ± 26 | 2 ± 28 | 43 ± 4 | 3 ± 26 | 0 ± 28 |
Values are n (range) or least-squares means ± SEs from maximum-likelihood linear regression models with between-subject groups (DSE compared with ILI), time as a repeated measure, and time-by-group interactions adjusted for T0 weight, height, age, sex, and ethnicity. AA, African American; DSE, diabetes support and education; ILI, intensive lifestyle intervention; T0, baseline; T12, 1 y; T24, 2 y.
Number of cases providing data at each study visit, except for pancreas where Ns are DSE 41, 44, 23; ILI 40, 36, 26.
Significance level of within-group changes from zero, 3P < 0.001, 9P < 0.01.
ILI change versus corresponding DSE change, 4P < 0.001, 5P < 0.05, 10P < 0.01.
P < 0.05 for T0–T12 pooled DSE and ILI change.
P < 0.05 for T0–T24 pooled DSE and ILI change.
P < 0.05 for T12–T24 pooled DSE and ILI change.
Spleen SEs are back-transformed from log values.
Body weight
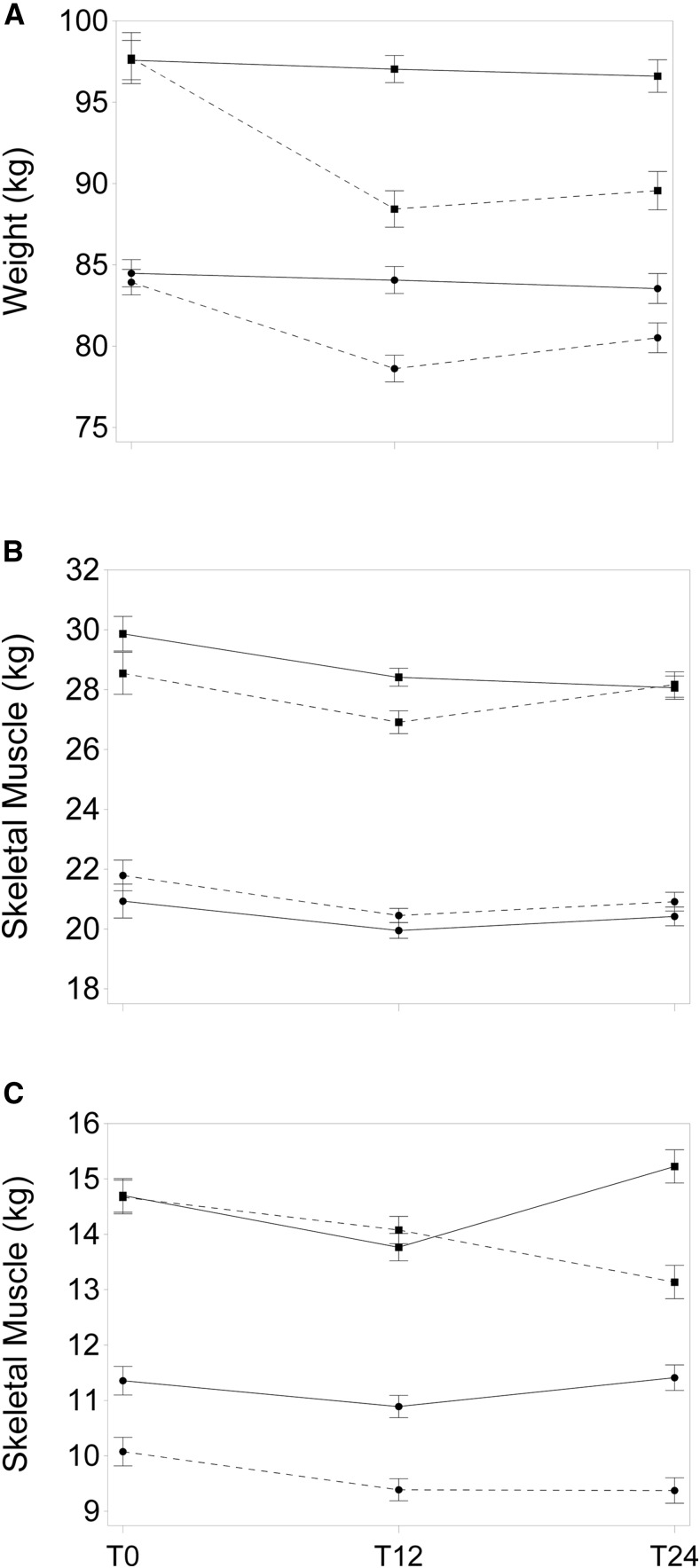
The DSE group showed no statistically significant changes in weight over the duration of the study, whereas the ILI group lost weight during the first year and remained below starting weight at T24 (Table 2). A significant interaction of sex, time, and group (P = 0.028) indicated that the weight loss of women in the ILI group at T12 (−5.2 kg; 95% CI: −6.8, −3.6 kg) was smaller than the corresponding change in men (−9.3 kg; 95% CI: −11.5, −7.1 kg) (Figure 1A). The interaction remained significant (P = 0.033) when T0 weight was added to the model, suggesting that the effect is not a consequence of the different initial mean weights of men and women and the tendency for larger weight loss to be related to larger initial weight.
FIGURE 1.
Least-squares mean T0, T12, and T24 body weight ± SE of changes from T0 for men (■) and women (●) in the DSE (solid line) and ILI (dashed line) treatment groups adjusted within sex group for weight, height, age, and ethnicity at T0 (sex-by-group-by-time, P-interaction = 0.033) (A). Least-squares mean ± SE of changes from baseline of skeletal muscle in men (■) and women (●) in the DSE (solid line) and ILI (dashed line) groups adjusted for baseline weight, height, age, and ethnicity (sex-by-group-by-time, P-interaction = 0.04) (B). Least-squares mean ± SE of changes from baseline of skeletal muscle in men (■) and women (●) in appendicular (solid line) and truncal (dashed line) regions of the body, adjusted for baseline weight, height, age, sex, and ethnicity (sex-by-region-by-time, P-interaction = 0.001) (C). Least-squares means are from maximum-likelihood linear regression models with between-subject groups (DSE and ILI), time as a repeated measure, and time-by-group interactions. DSE, diabetes support and education; ILI, intensive lifestyle intervention; T0, baseline; T12, 1 y; T24, 2 y.
SM
Adjusted for age, height, weight, and ethnicity, men had more SM than women at T0 (29.6 ± 0.5 compared with 22.1 ± 0.4 kg; P < 0.001). A significant group-by-sex-by-time interaction (P < 0.05) indicated that the amounts of SM loss and regain differed by sex, mainly because men in the ILI group lost and regained more SM than women did, but the general pattern of loss and regain was similar (Figure 1B). Despite the greater loss of weight in the ILI group than in the DSE group, the changes in SM did not differ significantly by treatment group. SM decreased significantly in both groups during the first year by ∼1.4 kg (95% CI: −1.1, −1.7 kg) and remained significantly below T0 at the end of the second year by ∼0.9 kg (95% CI: −0.5, −1.2 kg). When SM changes were examined by region, trunk SM was reduced at T12 and remained low or continued to decrease in both the ILI and DSE groups for the remainder of the study whereas appendicular SM (arm and leg), after a decline at T12, fully recovered its T0 mass by T24. As with total SM, this pattern was similar in both sexes, although the loss and regain were more pronounced in men (sex-by-region-by-time P-interaction < 0.001; Figure 1C).
Liver
Treatment effects on the liver differed by ethnicity (P-interaction = 0.03). In Caucasians, a significant group-by-time interaction was the result of a large liver decrease in the ILI group during the first year and no further change during the second year, whereas the DSE group remained unchanged throughout. In African Americans, the decrease in liver mass in the ILI group did not differ significantly from that in the DSE group during the first year. Rather, the mean liver mass of African Americans in both groups declined over the duration of the study from 1.52 to 1.45 kg (−0.07 ± 0.03 kg). The Asian and Hispanic sample sizes were too small to permit estimates. Table 2 shows liver results for the full sample as well as separate values for Caucasians and African Americans.
Across the entire sample, liver mass was smaller by 0.011 ± 0.005 (P = 0.03) per year of T0 age, larger by 0.014 ± 0.003 (P = 0.001) for each additional kilogram of body weight, and differed by ethnicity: adjusted for baseline weight, height, age, and sex, African Americans had a smaller liver mass (1.56 kg; 95% CI: 1.69, 1.43 kg) than Caucasians did (1.97 kg; 95% CI: 2.04, 1.86 kg).
Spleen
Spleen mass was log transformed because of nonconstant error variance in regression models. Spleen mass changes differed between the DSE and ILI groups during the first year with DSE values increasing nonsignificantly whereas ILI values decreased (Table 2). Differences were no longer significant after the second year. At T0, Caucasian spleen mass was >2 times that of African American spleen mass (0.31 ± 0.02 compared with 0.13 ± 0.03 kg; P < 0.001) adjusted for weight, height, age, and sex. Liver and spleen mass correlated (r = 0.64; P < 0.001) at T0 in Caucasians. In the full sample, changes in spleen mass were associated with changes in liver mass during the first year (P < 0.05).
Kidney
There was a mean decrease of 0.013 ± 0.003 kg (P < 0.001) in kidney mass during the study unrelated to treatment group that differed in timing by sex (interaction P = 0.005). Men’s kidney mass decreased by 0.015 ± 0.004 kg (P < 0.002) during the first year whereas women’s was unchanged (−0.003 ± 0.003 kg); during the second year, women’s kidney mass decreased by 0.015 ± 0.004 kg (P < 0.0.002) whereas the men’s did not change (−0.003 ± 0.005 kg). The reason for this difference in the timing of kidney changes is unknown.
Kidney mass at T0 was larger in men (0.46 ± 0.02 kg) than in women (0.37 ± 0.02 kg; P < 0.01) after adjustment for age, height, weight, and ethnicity.
Heart
Changes in cardiac mass over time were not statistically significant and did not differ by treatment group. There was an association of heart size with T0 weight showing an additional 0.002 ± 0.000 kg (P < 0.001) of cardiac mass for each additional kilogram of body weight, and a difference in cardiac mass by sex after adjusting for weight (men: 0.27 ± 0.01 kg, women: 0.23 ± 0.01 kg; P < 0.01).
Pancreas
The ILI and DSE groups showed similar changes: an increase of ∼5 ± 1.4 g from an initial mean value of 45 ± 3.0 g during the first year, then a decrease of ∼4 ± 1.7 g in the second year (both changes P < 0.05). Pancreas mass was negatively associated with duration of diabetes at T0 (regression coefficient −0.5, SE 0.02; P < 0.05) but was not related to sex, weight, age, or ethnicity.
Proportionality of organ sizes after weight reduction
We investigated whether the HMR organs that decreased significantly in size during the first year of the study (liver, spleen, and kidney in men) changed in the same proportion as SM, which has a relatively low metabolic rate at rest. This was done by testing whether, for a given amount of SM, the amount of the HMR organ was similar at T0 and after 1 y of intervention. That is, we looked for an interaction of SM and visit time (T0, T12) in the regression equation predicting organ mass from treatment group, visit, subject, and covariates. An interaction effect would indicate that a given amount of SM is associated with a smaller amount of organ mass at the second visit compared with the first, hence a disproportionate decrease. We also calculated for the ILI groups the simple proportional change in SM in the first year compared with the proportional change in each HMR organ during the same interval.
We found no significant effect or trend in the interactions for the SM by time in the regressions for liver, spleen, or kidney, suggesting that the relation of amount of SM to amount of organ was not different at T0 and T12. Comparison of simple proportion change in SM compared with liver, spleen, or kidney in the ILI group during the first year also showed no trends toward disproportionate change in HMR organs (change in SM −6.4%, liver −5.9%, spleen −4.3%, and kidney −1.5%).
DISCUSSION
The results reported here show how the masses of several important lean organs and tissues were affected by the weight loss intervention of the Look AHEAD trial in comparison with changes in a control group. The novel findings of the current study include 1) the differential impact of weight loss secondary to ILI on different organs including a decrease in spleen mass but not SM, heart, pancreas, and kidney; 2) ethnic differences in loss of liver mass associated with weight loss; 3) sexual dimorphism in loss of kidney mass; and 4) loss of pancreas mass proportional to disease duration.
SM decreased significantly in both groups during the first year, but the amount of SM loss was not significantly different by treatment. This was an unexpected finding given the 6.2-kg mean group difference in weight loss but may be simply an issue of statistical power. Alternatively, this finding suggests that ILI, which included a component of increased physical activity, spared the loss of SM. The regional analysis of SM change suggested that appendicular SM, an important component for functionality and mobility, may be regained shortly after the weight loss phase during a period of relative stability or slow weight regain, whereas trunk SM remains depressed. A similar result for weight regainers was reported by Bosy-Westphal et al. (21). Nevertheless, the mean loss of ∼1.4 kg of SM by participants during the first year of this study may have implications for physical function and mobility of elderly persons with type 2 diabetes. The rebound in appendicular SM may be temporary, and rebound notwithstanding, total SM remained ∼0.9 kg below initial values at the end of the study.
Liver mass decreased in the ILI group compared with the DSE group, but this loss was largely confined to Caucasian patients. The African Americans in the sample showed a gradual mean decrease in both groups over 2 y. Ethnic differences in lean tissue organs including liver in both patients with type 2 diabetes mellitus and healthy control subjects have been previously reported by our group (14). Although in African Americans liver size was 13% larger in patients with type 2 diabetes mellitus than in healthy control subjects, in Caucasians livers were 40% larger. It is plausible that Caucasians in this sample had more hepatic steatosis than African Americans did (22).
Organ fat infiltration in diabetes and obesity has been well documented by magnetic resonance spectroscopy and computed tomography studies, and moderate weight loss has been shown to significantly reduce intrahepatic lipid (12, 23, 24). Thus, a reduction of intrahepatic lipid rather than a loss of hepatocytes may explain the decrease in mass. We cannot confirm this explanation because intrahepatic lipid was not measured in this study. One study that did measure liver mass and intrahepatic lipid longitudinally with weight loss reported a decrease in intrahepatic lipid that was correlated (r = 0.44) with liver mass and in a subgroup accounted for 85% of the decrease in liver mass (4).
Spleen mass changes also differed as a function of treatment, with no change in the DSE group compared with a decrease in the ILI group. Tsushima and Endo (25) reported an association between degree of fatty infiltration in the liver and spleen mass in a cross-sectional investigation and proposed that increased portal venous pressure may be the cause of the enlarged spleen. Inasmuch as hepatic fatty infiltration may have been reversed by the lifestyle intervention, our findings of a correlation in the amount of decrease in liver and spleen masses in the sample over the first year are consistent with cross-sectional reports.
A reduction in cardiac mass by the intervention was not detected within the power of our study. This contrasts with results from other research groups that reported 5% and 26% changes in cardiac mass (4, 5), but those interventions produced greater weight loss; therefore, this apparent lack of agreement may relate to differences in intensity and duration of intervention.
Decreased pancreas volume by 8–33% in type 2 diabetic patients compared with control subjects has been reported (26, 27), but research on the causes of the differences and the nature of longitudinal changes is scant. Our coincidental finding of a small significant negative association of size with duration of illness is novel. A similar but nonsignificant association was reported by Macauley et al. (28). Cross-sectionally, a positive association of pancreas volume with a better HOMA-IR index has been observed, suggesting possible superior β-cell function in a larger pancreas (28).
As in the parent trial, we found a sex difference in the degree of success at weight loss (29). The reason for the greater loss in men than in women is not fully known. Similar differences have been observed in other studies (30, 31). Explanations tend to invoke sex differences in the proportions of fat mass and fat-free mass in body composition (32).
Significance for post–weight loss REE
An issue of ongoing interest is whether reductions in REE adjusted for LBM observed after weight loss could be accounted for by a disproportionate loss of HMR tissue, thereby changing the composition of total LBM (5, 6, 33, 34).
With one exception (6), studies agree that in weight-loss interventions with moderate caloric restriction, brain size does not decrease (2–5). Furthermore, if the decrease in liver size is largely a reduction in hepatic fat as suggested elsewhere (4), then the change in liver mass may not signal an equivalent diminution of metabolically active cell mass. Similarly, if the change in spleen size is a consequence of reduced portal vein pressure, then that change also may not reflect a reduction in active cell mass. That leaves the heart and kidneys as the HMR organs that may show disproportionate shrinkage. In this study, reduction in cardiac mass as a function of treatment was not detected, and kidney mass maintained the same relation to SM mass after weight loss as before. Thus, aside from a possible contribution by the liver, owing to the loss of some active cell mass beyond the decrease in mass attributable to loss of hepatic fat, the hypothesis of a disproportionate decrease in HMR organs as an explanation for post–weight loss REE decline is not supported in this study. Nevertheless, the detailed analyses of the role of disproportionate organ mass changes by Müller et al. (6) provide a valuable framework for further investigation into adaptive thermogenesis.
Study limitations
Our sample consists of older, obese, and overweight persons with type 2 diabetes; extrapolation to other populations may not be warranted. The reduced-weight groups in other studies (4, 5) lost more weight than did our ILI group, and HMR organ change may require a certain threshold of weight loss before it becomes disproportionate to SM. The conversion of organ volumes to mass assumed a typical density and did not take into account possible ethnic or individual differences in lipid content.
Conclusions
SM and kidney mass (and liver mass in African Americans) decreased in both intervention and control groups, spleen mass (and liver mass in Caucasians) decreased in the intervention but not the control group, and no treatment-related changes were detected in heart and pancreas size during a lifestyle intervention that produced a 6.2-kg weight reduction after 1 y. Appendicular SM lost during the first year was regained during the second year whereas trunk SM was not. No evidence was found for disproportionate loss of HMR organs compared with SM.
Acknowledgments
The members of the MRI Ancillary Study Working Group of the Look AHEAD Trial are: Dympna Gallagher, David E Kelley, Stanley Heshka, John Thornton, Lawrence Boxt, Isaiah Janumala, Lance Davidson, F Xavier Pi-Sunyer, Jennifer Patricio, and Juliet Mancino.
The authors’ responsibilities were as follows—DEK, XP-S, EL, and SH: contributed to the design of the parent Look AHEAD trial; DEK and XP-S: were principal investigators of the parent trial at the New York and Pittsburgh sites and were responsible for data collection and administrative supervision at their respective sites; DG and SH: designed this ancillary study and wrote the manuscript; DG: supervised and managed data collection for this study and had primary responsibility for the final content; DG, JT, and SH: analyzed the data; LB: assisted in quality control of MRI data collection; EN: assisted in the acquisition of patient data at the Memphis Look AHEAD site; IJ: analyzed all of the MRI scans; and all authors: read and approved the final manuscript. The authors declared no conflicts of interest related to the study.
Footnotes
Abbreviations used: DSE, diabetes support and education; HMR, high metabolic rate; ILI, intensive lifestyle intervention; LBM, lean body mass; REE, resting energy expenditure; SM, skeletal muscle; T0, baseline; T12, 1 y; T24, 2 y.
REFERENCES
- 1.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev 2014;15:310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rompala RE, Johnson DE, Rumpler WV, Phetteplace HW, Specht SM, Parker CF. Energy utilization and organ mass of Targhee sheep selected for rate and efficiency of gain and receiving high and low planes of nutrition. J Anim Sci 1991;69:1760–5. [DOI] [PubMed] [Google Scholar]
- 3.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center: caloric intake and aging. N Engl J Med 1997;337:986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosy-Westphal A, Kossel E, Goele K, Later W, Hitze B, Settler U, Heller M, Gluer CC, Heymsfield SB, Müller MJ. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. Am J Clin Nutr 2009;90:993–1001. [DOI] [PubMed] [Google Scholar]
- 5.Pourhassan M, Bosy-Westphal A, Schautz B, Braun W, Gluer CC, Müller MJ. Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. Am J Clin Nutr 2014;99:779–91. [DOI] [PubMed] [Google Scholar]
- 6.Müller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Gluer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr 2015;102:807–19. [DOI] [PubMed] [Google Scholar]
- 7.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–28. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, Pi-Sunyer FX, Heshka S. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 2009;89:807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, Foreyt JP, Hill JO, Trence DL, Vitolins MZ. One-year Weight Losses in the Look AHEAD Study: factors associated with success. Obesity (Silver Spring) 2009;17:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakicic JM, Jaramillo SA, Balasubramanyam A, Bancroft B, Curtis JM, Mathews A, Pereira M, Regensteiner JG, Ribisl PM, Look ASG. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond) 2009;33:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasarica M, Tchoukalova YD, Heilbronn LK, Fang X, Albu JB, Kelley DE, Smith SR, Ravussin E, Look AARG. Differential effect of weight loss on adipocyte size subfractions in patients with type 2 diabetes. Obesity (Silver Spring) 2009;17:1976–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, Pi-Sunyer FX, Ravussin E. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes 2010;59:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pownall HJ, Bray GA, Wagenknecht LE, Walkup MP, Heshka S, Hubbard VS, Hill J, Kahn SE, Nathan DM, Schwartz AV, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring) 2015;23:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson LE, Kelley DE, Heshka S, Thornton J, Pi-Sunyer FX, Boxt L, Balasubramanyam A, Gallagher D; MRI Ancillary Study Subgroup of the Look AHEAD Research Group. Skeletal muscle and organ masses differ in overweight adults with type 2 diabetes. J Appl Physiol (1985) 2014;117:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher D, Heshka S, Kelley DE, Thornton J, Boxt L, Pi-Sunyer FX, Patricio J, Mancino J, Clark JM. Changes in adipose tissue depots and metabolic markers following a 1-year diet and exercise intervention in overweight and obese patients with type 2 diabetes. Diabetes Care 2014;37:3325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol 1998;275:E249–58. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N, Elia M. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr 2006;83:1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz J, Whang J, Boxt LM, Barst RJ. Estimation of right ventricular mass in normal subjects and in patients with primary pulmonary hypertension by nuclear magnetic resonance imaging. J Am Coll Cardiol 1993;21:1475–81. [DOI] [PubMed] [Google Scholar]
- 19.Jones RS. The weight of the heart and its chambers in hypertensive cardiovascular disease with and without failure. Circulation 1953;7:357–69. [DOI] [PubMed] [Google Scholar]
- 20.Snyder WS, Cooke MJ, Mnassett ES, Larhansen LT, Howells GP, Tipton IH. Report of the Task Group on Reference Man. Oxford (United Kingdom): Pergamon; 1975. [Google Scholar]
- 21.Bosy-Westphal A, Schautz B, Lagerpusch M, Pourhassan M, Braun W, Goele K, Heller M, Gluer CC, Müller MJ. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes (Lond) 2013;37:1371–7. [DOI] [PubMed] [Google Scholar]
- 22.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–95. [DOI] [PubMed] [Google Scholar]
- 23.Kelley DE, Kuller LH, McKolanis TM, Harper P, Mancino J, Kalhan S. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care 2004;27:33–40. [DOI] [PubMed] [Google Scholar]
- 24.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsushima Y, Endo K. Spleen enlargement in patients with nonalcoholic fatty liver: correlation between degree of fatty infiltration in liver and size of spleen. Dig Dis Sci 2000;45:196–200. [DOI] [PubMed] [Google Scholar]
- 26.Alzaid A, Aideyan O, Nawaz S. The size of the pancreas in diabetes mellitus. Diabet Med 1993;10:759–63. [DOI] [PubMed] [Google Scholar]
- 27.Migdalis IN, Voudouris G, Kalogeropoulou K, Iliopoulou V, Koutoulidis K, Samartzis M. Size of the pancreas in non-insulin-dependent diabetic patients. J Med 1991;22:179–86. [PubMed] [Google Scholar]
- 28.Macauley M, Percival K, Thelwall PE, Hollingsworth KG, Taylor R. Altered volume, morphology and composition of the pancreas in type 2 diabetes. PLoS One 2015;10:e0126825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, Krakoff J, Otto A, Ryan DH, Vitolins MZ. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Vollmer WM, Gullion CM, Funk K, Smith P, et al. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA 2008;299:1139–48. [DOI] [PubMed] [Google Scholar]
- 31.Williams RL, Wood LG, Collins CE, Callister R. Effectiveness of weight loss interventions–is there a difference between men and women: a systematic review. Obes Rev 2015;16:171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millward DJ, Truby H, Fox KR, Livingstone MB, Macdonald IA, Tothill P. Sex differences in the composition of weight gain and loss in overweight and obese adults. Br J Nutr 2014;111:933–43. [DOI] [PubMed] [Google Scholar]
- 33.Hsu A, Heshka S, Janumala I, Song MY, Horlick M, Krasnow N, Gallagher D. Larger mass of high-metabolic-rate organs does not explain higher resting energy expenditure in children. Am J Clin Nutr 2003;77:1506–11. [DOI] [PubMed] [Google Scholar]
- 34.Javed F, He Q, Davidson LE, Thornton JC, Albu J, Boxt L, Krasnow N, Elia M, Kang P, Heshka S, et al. Brain and high metabolic rate organ mass: contributions to resting energy expenditure beyond fat-free mass. Am J Clin Nutr 2010;91:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]