Abstract
Background: Limited knowledge regarding the reproducibility of biomarkers in 24-h urine samples has hindered the collection and use of the samples in epidemiologic studies.
Objective: We aimed to evaluate the reproducibility of various markers in repeat 24-h urine samples.
Design: We calculated intraclass correlation coefficients (ICCs) of biomarkers measured in 24-h urine samples that were collected in 3168 participants in the NHS (Nurses’ Health Study), NHSII (Nurses’ Health Study II), and Health Professionals Follow-Up Study.
Results: In 742 women with 4 samples each collected over the course of 1 y, ICCs for sodium were 0.32 in the NHS and 0.34 in the NHSII. In 2439 men and women with 2 samples each collected over 1 wk to ≥1 mo, the ICCs ranged from 0.33 to 0.68 for sodium at various intervals between collections. The urinary excretion of potassium, calcium, magnesium, phosphate, sulfate, and other urinary markers showed generally higher reproducibility (ICCs >0.4). In 47 women with two 24-h urine samples, ICCs ranged from 0.15 (catechin) to 0.75 (enterolactone) for polyphenol metabolites. For phthalates, ICCs were generally ≤0.26 except for monobenzyl phthalate (ICC: 0.55), whereas the ICC was 0.39 for bisphenol A (BPA). We further estimated that, for the large majority of the biomarkers, the mean of three 24-h urine samples could provide a correlation of ≥0.8 with true long-term urinary excretion.
Conclusions: These data suggest that the urinary excretion of various biomarkers, such as minerals, electrolytes, most polyphenols, and BPA, is reasonably reproducible in 24-h urine samples that are collected within a few days or ≤1 y. Our findings show that three 24-h samples are sufficient for the measurement of long-term exposure status in epidemiologic studies.
Keywords: biomarker, bisphenol A, reproducibility, sodium, 24-h urine sample
See corresponding editorial on page 8.
INTRODUCTION
Urine specimens are uniquely valuable for epidemiologic research to assess various exposures, including dietary factors, environmental pollutants, endogenous metabolites, and other molecules that are excreted by the kidneys. Besides this versatility of urine samples, compared with blood samples or tissues, the collection of spot urine samples is noninvasive, easy to implement, and subject to smaller collection efforts. However, the excretion of many urinary markers, such as sodium, bisphenol A (BPA),10 and flavonoid metabolites, is subject to substantial day-to-day variability (1–4). These markers, when measured in spot urine samples, have poor time integration and, therefore, may only reflect very short-term exposure. Furthermore, it is difficult to accurately infer the absolute excretion of biomarkers with the use of spot urine samples even after adjustment for dilution with the use of the urinary creatinine concentration, which is also subject to within-person variability (5, 6) and is influenced by individuals’ meat intakes (4). Therefore, in epidemiologic studies, 24-h urine samples are preferred over spot urine samples to reduce the extraneous variability of biomarkers that is due to the timing of sample collection and very short-term changes in exposures (4, 7). This may largely explain the contradicting observations regarding sodium excretion in relation to cardiovascular disease shown in 2 recent studies that used spot urine samples (8) compared with 24-h urine samples (9) to assess long-term sodium intake. However, data regarding the reproducibility of biomarkers with various half-lives in 24-h urine samples are still sparse. In addition, few studies have evaluated the reproducibility in samples that have been collected within various time intervals or have accounted for participant characteristics, such as total energy intake, body weight, physical activity, and other factors that are usually important in epidemiologic studies.
In the current analysis, we evaluated the reproducibility of a variety of biomarkers in multiple 24-h urine samples that were collected from men and women who were participating in the NHS (Nurses’ Health Study), NHSII (Nurses’ Health Study II), and HPFS (Health Professionals Follow-Up Study). The data in these studies allowed us to examine the reproducibility in various time-frames of sample collection and to adjust for factors that might have resulted in the extraneous variability of marker concentrations. We also used these findings to estimate the number of 24-h urine samples that would provide a reasonably strong correlation with true long-term average urinary excretion.
METHODS
Study population
The NHS was established in 1976 when 121,700 female registered nurses, aged 30–55 y, completed a questionnaire on their medical history and lifestyle characteristics. The NHSII was initiated in 1989 and consists of 116,430 female registered nurses, aged 25–42 y, who completed a questionnaire that was similar to that used in the NHS. The HPFS is a cohort study of 51,529 male US health professionals, aged 40–75 y, who were enrolled in 1986. In all 3 cohorts, similar questionnaires have been administered biennially to update information on lifestyle, medication, and disease status. Every 2–4 y, validated semiquantitative food-frequency questionnaires (SFFQs) (10) have been used to update diet data since 1980 in the NHS, 1991 in the NHSII, and 1986 in the HPFS.
The WLVS (Women’s Lifestyle Validation Study), which is a substudy within the NHS and NHSII cohorts, is one of the 3 studies that comprised the Multi-Cohort Eating and Activity Study for Understanding Reporting Error, which aimed to evaluate measurement errors that were associated with self-administered dietary and physical activity assessments. NHS and NHSII participants were eligible to participate in the WLVS if they responded to SFFQs in the cohort follow-up in 2006–2007; were free of cardiovascular, cancer, or major neurologic diseases in 2006–2007; had previously provided blood samples; had access to broadband Internet; and were not planning to make substantial changes in diet or physical activity levels. A total of 5509 nurses, aged 45–80 y, were randomly selected and invited via e-mail, regular US mail, or both. Of these nurses, 2423 (44%) individuals responded to the invitation, and 796 subjects consented to participate and received information on the timeline and activities.
The KSS (Kidney Stone Study) was conducted in participants in the NHS, NHSII, and HPFS to examine risk factors in relation to kidney stones (11). Of 3350 participants in the KSS, 2530 men and women who were <75 y of age and did not report having a history of cancer or cardiovascular disease in 2003 provided two 24-h urine samples during 2003–2007. In the current analysis, we excluded samples if the estimated volume was missing (sample n = 71 in the WLVS) or values of covariates were missing, including body weight, height, physical activity, total energy intake, and total energy expenditure (TEE) (in the WLVS only) (n = 149 in the WLVS; n = 91 in the KSS). We further excluded 1 WLVS sample for which the estimated volume was >10 L. We applied the following exclusion criteria for participants: missing urinary excretion of sodium and potassium (n = 18 in the WLVS) or a lack of multiple assessments of these markers (n = 10 in the WLVS). After these exclusions, 742 WLVS participants and 2439 KSS participants remained in the analysis. Note that there were 13 women who participated in both studies although statistical analyses were conducted in each study separately.
This study was approved by the Human Subjects Committees of the Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital. Informed consent was obtained from all study participants.
Twenty-four-hour urine-sample collection
In WLVS, participants were asked to complete several assessments and sample collections allocated into 4 phases that were evenly spanned over 4 seasons (Supplemental Table 1). At each phase, WLVS participants were asked to provide a 24-h urine sample. Participants were instructed to collect and pour all voids within 24 h into a 4-L container, measure the total urine volume, and pour a 50-mL aliquot into a sample tube after inverting the container 10 times. The tubes were shipped in a styrofoam box that was cooled with a cold pack via an overnight courier service (FedEx; FedEx Corp) to Fisher BioServices Inc. for processing. A short questionnaire was sent along with the sample-collection kit to obtain the start and end times of collection, the total volume collected, the estimated volume lost, and medication use.
In the KSS, participants were asked to provide two 24-h urine samples, preferably within 1 wk. The 24-h urine collections in the KSS were performed with the use of the system provided by Mission Pharmacal (11). In brief, a kit that contained all necessary supplies, including a specially designed toilet hat for women and a urinal for men, a 4-L container with a lithium-impregnated sponge, and small vials, was sent to participants. On completion of the collection, participants were instructed to pour samples into the small vials, which were returned to Mission Pharmacal in a prepaid self-addressed FedEx mailer. The total volume was calculated on the basis of the concentration of lithium that was placed in the collection containers before collections.
Measurements of urinary markers
In the WLVS, the urinary excretion of sodium and potassium was measured with the use of ion-selective–electrode methods with the Olympus AU400 analyzer (Olympus). Creatinine concentrations were measured with the use of a modified Jaffe kinetic assay (Olympus). In the KSS, sodium and potassium were determined directly with the use of flame-emission photometry; calcium and magnesium were measured with the use of an atomic absorption spectrophotometer; creatinine, uric acid, citrate, and phosphorus were measured with the use of a Cobas centrifugal analyzer (Roche Diagnostics); and oxalate was analyzed with the use of ion chromatography. Blind, split, quality-control samples were used to show that the overall CVs for all markers in these samples were <10%.
In 50 WLVS participants, we measured the urinary excretion of enterodiol, enterolactone, caffeic acid, ferulic acid, resveratrol, quercetin, naringenin, hesperetin, kaempferol, isorhamnetin, catechin, epicatechin, dihydrodaidzein, daidzein, dihydrogenistein, genistein, O-desmethylangolensin, phthalic acid, monoethyl phthalate, mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), monobutyl phthalate (MBP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), monobenzyl phthalate (MBzP), mono-(2-ethylhexyl) phthalate, and BPA in two 24-h urine samples that were collected at phases 1 and 2. Methods for the measurement of these markers have been described in detail elsewhere (2, 12). Briefly, polyphenol metabolites and phthalates were analyzed with the use of Orbitrap liquid chromatography–mass spectrometry (model Exactive; Thermo Electron), and BPA was assayed with the use of tandem liquid chromatography–mass spectrometry (model TSQ Ultra; Thermo Electron). Overall laboratory CVs were <10% for creatinine, enterolactone, naringenin, daidzein, genistein, phthalic acid, MEHHP, MECPP, and MBzP; 10–19% for catechin, caffeic acid, ferulic acid, isorhamnetin, enterodiol, hesperetin, dihydrodaidzein, dihydrogenistein, kaempferol, monoethyl phthalate, MBP, MEOHP, and BPA; and 20–36% for epicatechin, quercetin, O-desmethylangolensin, and resveratrol. For each marker, we derived the total excretion per day by multiplying the concentrations of markers by the total volume. For this analysis, we applied the same exclusion criteria as those for other WLVS participants, and 47 women were included for data analysis after exclusions.
Assessments of covariates
In the WLVS, current body weight and height were obtained with the use of a self-administered questionnaire at each phase. Diet was assessed at phases 1 and 4 with the use of the same validated SFFQ as was used in the cohort follow-up, which included >130 food items. Participants were asked how often, on average, they consumed a specified portion of a given food or beverage in the past year. Total energy intake was calculated by multiplying the frequency of consumption by the energy content of each item and summing the energy across all contributing foods. At phases 1 and 4, we inquired about the average time per week in the past year that was spent on leisure-time physical activities including walking, jogging, running, bicycling, lap swimming, tennis, aerobics, squash or racquet ball, weight training, outdoor work, lower-intensity exercise, standing, and sitting (13). For each question, there were 13 possible response categories that ranged from 0 to ≥40 h/wk. Furthermore, we inquired about the number of flights of stairs that were climbed each day and the usual walking pace. On the basis of these data, we calculated energy expenditure in metabolic equivalent tasks that were measured in hours per week. At phase 1, TEE was evaluated with the use of the doubly labeled water (DLW) method. Urine specimens were assessed with the use of mass spectroscopic analysis for deuterium and heavy oxygen. The relative decrease in deuterium and oxygen concentrations was used to calculate daily TEE as described by Schoeller et al. (14). A subset of 100 women repeated the DLW assessments at 6, 9, or 12 mo after the first DLW measurement. In the current analysis, values of total energy, physical activity, and TEE that were assessed at phase 1 were carried forward to phase 2 (or phases 3 and 4 for TEE in participants who did not have the second DLW assessment), and the second assessments of these covariates were assigned to phases 3 and 4.
In the KSS, covariates were assessed with the use of questionnaires at baseline or during follow-up in each cohort. Standing height was inquired for in the baseline questionnaire. Current body weight was self-reported in the biennial follow-up questionnaires with high accuracy (15). Total energy intake was derived on the basis of the SFFQ assessments quadrennially. Physical activity was assessed with the use of a questionnaire that was similar to that used in the WLVS every 2–4 y, and the total weekly metabolic equivalent task hours were calculated. In the current analysis, we used covariate assessments that were closest to the urine sample collection in the KSS.
Statistical analyses
We calculated cohort-specific intraclass correlation coefficients (ICCs) of the excretion of urinary markers in multiple 24-h urine samples to evaluate the reproducibility of the markers. To improve normality, we log transformed all values of urinary markers. Because our primary interest in nutritional epidemiology is energy-adjusted dietary variables (16, 17), we adjusted for the effects of variation in total energy intake on the variability of urinary excretion of the markers by calculating a series of residuals with the use of linear regression. Thus, the residuals of urinary markers were independent of the extraneous variability caused by these covariates (10). We also considered body weight, height, physical activity, and TEE (in the WLVS only) because these variables are direct measurements of, or closely related to, energy intake. We used the residuals to calculate the ICCs. We calculated reliability indexes (RIs) with the use of the following formula (18, 19):
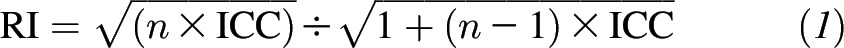 |
where n represents the number of repeated samples. The RI measures the correlation between a mean of n measurements and the true underlying value of an exposure of interest (i.e., the mean of a very large number of repeat measurements). To estimate the number of samples that were needed to achieve an RI at a given ICC, we used the following formula (18, 19):
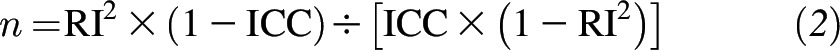 |
All statistical analyses were performed with SAS software (version 9.4; SAS Institute Inc.). A 2-sided P < 0.05 was considered significant.
RESULTS
Baseline characteristics of WLVS and KSS participants are shown in Table 1. Participants from the parent cohorts (NHS or NHSII) had, in general, similar distributions of lifestyle and anthropometric variables between the WLVS and KSS. The apparent differences in physical activity levels and baseline medical histories of existing chronic diseases between the WLVS and KSS were due to the use of different questionnaires for physical activity assessments and exclusion criteria, respectively. The urinary excretion of sodium was higher in KSS women than in WLVS women, whereas the opposite outcome was observed for potassium excretion. The urinary excretion of polyphenols, phthalates, and BPA that was measured in 47 WLVS participants is shown in Supplemental Table 2.
TABLE 1.
Baseline characteristics of study participants in the WLVS and KSS1
| WLVS |
KSS |
||||
| Characteristic | NHS (n = 336) | NHSII (n = 406) | HPFS (n = 698) | NHS (n = 818) | NHSII (n = 923) |
| Age, y | 71.8 ± 4.3 | 55.6 ± 5.2 | 66.9 ± 6.7 | 68.6 ± 6.2 | 50.7 ± 5.0 |
| Smoking status,2 % | |||||
| Never smoked | 46.3 | 70.9 | 54.8 | 48.4 | 69.5 |
| Past smoker | 52.2 | 25.9 | 41.0 | 45.0 | 25.0 |
| Current smoker | 1.5 | 3.2 | 4.2 | 6.6 | 5.5 |
| Alcohol intake,2 g/d | 10.0 ± 13.0 | 6.9 ± 10.1 | 12.5 ± 15.3 | 5.1 ± 9.8 | 4.2 ± 7.4 |
| Height, cm | 162.0 ± 6.5 | 165.5 ± 7.0 | 178.6 ± 6.1 | 164.0 ± 5.9 | 164.5 ± 6.4 |
| Weight, kg | 68.4 ± 12.9 | 73.2 ± 16.7 | 84.0 ± 13.3 | 71.9 ± 15.1 | 73.6 ± 18.0 |
| BMI,2 kg/m2 | 26.1 ± 4.7 | 26.7 ± 5.8 | 26.3 ± 3.7 | 26.8 ± 5.4 | 27.2 ± 6.5 |
| Total energy,2 kcal/d | 1927 ± 524 | 1964 ± 607 | 2089 ± 640 | 1723 ± 514 | 1844 ± 557 |
| Physical activity,2 MET-h/wk | 107 ± 47 | 124 ± 56 | 44 ± 43 | 22 ± 25 | 21 ± 31 |
| Menopausal status,2 % | |||||
| Menopause | 100 | 81.0 | — | 100.0 | 49.8 |
| Current hormone use3 | 24.5 | 22.8 | — | 20.8 | 22.9 |
| Medical history, % | |||||
| Ischemic heart disease | 0 | 0.6 | 6.9 | 6.2 | 1.1 |
| Hypertension | 47.3 | 29.8 | 41.0 | 57.6 | 23.7 |
| High cholesterol | 69.9 | 49.5 | 59.7 | 68.0 | 41.0 |
| Cancer | 0.9 | 1.0 | 8.5 | 13.6 | 4.7 |
| Diabetes | 3.3 | 5.9 | 6.5 | 10.8 | 4.6 |
| Urinary measurements4 | |||||
| Total volume, L/d | 1.9 ± 0.6 | 2.1 ± 0.8 | 1.7 ± 0.6 | 1.8 ± 0.6 | 1.8 ± 0.7 |
| Creatinine, mg/d | 1033 ± 187 | 1286 ± 241 | 1678 ± 370 | 1059 ± 231 | 1229 ± 255 |
| Sodium, mg/d | 2643 ± 758 | 3103 ± 892 | 4131 ± 1360 | 3151 ± 1136 | 3478 ± 1184 |
| Potassium, mg/d | 2463 ± 661 | 2416 ± 726 | 2952 ± 889 | 2284 ± 730 | 2174 ± 745 |
| Magnesium, mg/d | — | — | 122 ± 44 | 100 ± 39 | 99 ± 36 |
| Phosphate, mg/d | — | — | 1032 ± 300 | 738 ± 221 | 850 ± 256 |
| Sulfate, mg/d | — | — | 23.2 ± 7.5 | 15.8 ± 5.7 | 16.9 ± 5.5 |
| Uric acid, mg/d | — | — | 571 ± 196 | 421 ± 134 | 500 ± 140 |
| Calcium, mg/d | — | — | 184 ± 95 | 194 ± 100 | 206 ± 89 |
| Citrate, mg/d | — | — | 718 ± 306 | 615 ± 289 | 755 ± 292 |
| Oxalate, mg/d | — | — | 37.5 ± 10.9 | 27.9 ± 8.6 | 26.5 ± 8.6 |
Values are means ± SD unless otherwise indicated. Baseline was 2011 for the WLVS and 2003–2007 for the KSS. HPFS, Health Professionals Follow-Up Study; KSS, Kidney Stone Study; MET-h, metabolic equivalent task hours; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; WLVS, Women’s Lifestyle Validation Study.
Estimates were based on nonmissing data.
In postmenopausal women only.
Estimates were based on the average urinary excretion of multiple assessments.
Table 2 presents ICCs of sodium and potassium excretion as well as the volume of 24-h urine samples and creatinine excretion in the WLVS. Of these 4 urinary variables, the total urine volume showed the highest ICCs; on the basis of all 4 samples, ICCs were 0.63 in the NHS and 0.68 in the NHSII. ICCs of sodium excretion were the lowest; they were 0.32 in the NHS and 0.34 in the NHSII for 4 samples. The reproducibility was modest for the urinary excretion of creatinine and potassium with ICCs in the range from 0.48 to 0.53 for all 4 samples. The ICCs of these urinary markers, when calculated on the basis of 2 or 3 samples, did not differ substantially from the ICCs for all 4 samples. In addition, when ICCs were calculated on the basis of creatinine- and energy-adjusted residuals of sodium and potassium excretion, the values for sodium were modestly lower and, for potassium, were not appreciably different. Note that adjustment for total energy intake that was estimated with the use of DBW had virtually no influence on ICCs. RIs for sodium excretion were in the range from 0.61 to 0.82. In contrast, RIs for potassium excretion were in the range from 0.79 to 0.90 for various numbers of samples.
TABLE 2.
ICCs and corresponding RIs for sodium and potassium in WLVS participants1
| NHS (n = 336), draws |
NHSII (n = 406), draws |
|||||||||||||||||
| All | 1 and 2 | 2 and 3 | 3 and 4 | 1 and 4 | 1–3 | 1, 2, and 4 | 1, 3, and 4 | 2–4 | All | 1 and 2 | 2 and 3 | 3 and 4 | 1 and 4 | 1–3 | 1, 2, and 4 | 1, 3, and 4 | 2–4 | |
| ICC | ||||||||||||||||||
| Volume, L/d | 0.63 | 0.61 | 0.63 | 0.67 | 0.63 | 0.62 | 0.63 | 0.64 | 0.65 | 0.68 | 0.67 | 0.72 | 0.70 | 0.69 | 0.69 | 0.68 | 0.67 | 0.70 |
| Creatinine, mg/d | 0.48 | 0.48 | 0.51 | 0.42 | 0.43 | 0.49 | 0.48 | 0.45 | 0.49 | 0.53 | 0.43 | 0.59 | 0.58 | 0.61 | 0.50 | 0.52 | 0.54 | 0.59 |
| Sodium, mg/d | 0.32 | 0.23 | 0.36 | 0.36 | 0.26 | 0.31 | 0.28 | 0.33 | 0.36 | 0.34 | 0.27 | 0.40 | 0.37 | 0.38 | 0.30 | 0.34 | 0.32 | 0.39 |
| Residual 1 | 0.29 | 0.20 | 0.32 | 0.31 | 0.27 | 0.28 | 0.25 | 0.31 | 0.31 | 0.27 | 0.26 | 0.31 | 0.27 | 0.28 | 0.24 | 0.29 | 0.22 | 0.31 |
| Residual 2 | 0.28 | 0.20 | 0.31 | 0.31 | 0.25 | 0.27 | 0.24 | 0.30 | 0.31 | 0.26 | 0.25 | 0.31 | 0.26 | 0.27 | 0.23 | 0.28 | 0.20 | 0.30 |
| Residual 3 | 0.28 | 0.19 | 0.31 | 0.31 | 0.25 | 0.27 | 0.24 | 0.30 | 0.30 | 0.26 | 0.25 | 0.31 | 0.26 | 0.27 | 0.23 | 0.28 | 0.20 | 0.30 |
| Potassium, mg/d | 0.48 | 0.49 | 0.48 | 0.46 | 0.42 | 0.49 | 0.48 | 0.46 | 0.49 | 0.49 | 0.45 | 0.54 | 0.51 | 0.51 | 0.48 | 0.49 | 0.48 | 0.52 |
| Residual 1 | 0.52 | 0.50 | 0.51 | 0.54 | 0.48 | 0.51 | 0.51 | 0.51 | 0.53 | 0.53 | 0.52 | 0.57 | 0.53 | 0.55 | 0.54 | 0.54 | 0.52 | 0.55 |
| Residual 2 | 0.51 | 0.50 | 0.50 | 0.54 | 0.48 | 0.50 | 0.51 | 0.50 | 0.53 | 0.51 | 0.51 | 0.54 | 0.51 | 0.53 | 0.52 | 0.52 | 0.50 | 0.52 |
| Residual 3 | 0.51 | 0.50 | 0.50 | 0.54 | 0.47 | 0.50 | 0.51 | 0.50 | 0.53 | 0.51 | 0.51 | 0.54 | 0.51 | 0.53 | 0.52 | 0.52 | 0.50 | 0.52 |
| RI | ||||||||||||||||||
| Sodium, mg/d | 0.81 | 0.61 | 0.73 | 0.73 | 0.64 | 0.76 | 0.73 | 0.77 | 0.79 | 0.82 | 0.65 | 0.76 | 0.73 | 0.74 | 0.75 | 0.78 | 0.77 | 0.81 |
| Residual 1 | 0.79 | 0.58 | 0.69 | 0.69 | 0.65 | 0.73 | 0.71 | 0.76 | 0.76 | 0.77 | 0.64 | 0.69 | 0.65 | 0.66 | 0.69 | 0.74 | 0.67 | 0.75 |
| Residual 2 | 0.78 | 0.57 | 0.69 | 0.69 | 0.64 | 0.73 | 0.70 | 0.75 | 0.75 | 0.76 | 0.63 | 0.69 | 0.64 | 0.65 | 0.68 | 0.74 | 0.66 | 0.75 |
| Residual 3 | 0.78 | 0.57 | 0.69 | 0.68 | 0.63 | 0.72 | 0.70 | 0.75 | 0.75 | 0.76 | 0.63 | 0.69 | 0.64 | 0.65 | 0.68 | 0.74 | 0.66 | 0.75 |
| Potassium, mg/d | 0.89 | 0.81 | 0.81 | 0.79 | 0.77 | 0.86 | 0.86 | 0.85 | 0.86 | 0.89 | 0.79 | 0.84 | 0.82 | 0.82 | 0.86 | 0.86 | 0.86 | 0.88 |
| Residual 1 | 0.90 | 0.82 | 0.82 | 0.84 | 0.80 | 0.87 | 0.87 | 0.87 | 0.88 | 0.91 | 0.83 | 0.85 | 0.83 | 0.84 | 0.88 | 0.88 | 0.88 | 0.89 |
| Residual 2 | 0.90 | 0.81 | 0.82 | 0.84 | 0.80 | 0.87 | 0.87 | 0.87 | 0.88 | 0.90 | 0.82 | 0.84 | 0.82 | 0.83 | 0.87 | 0.87 | 0.87 | 0.88 |
| Residual 3 | 0.90 | 0.81 | 0.82 | 0.84 | 0.80 | 0.87 | 0.87 | 0.87 | 0.88 | 0.90 | 0.82 | 0.84 | 0.82 | 0.83 | 0.87 | 0.87 | 0.87 | 0.88 |
 . Original values of urinary markers and total volumes were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). Residual 3 was adjusted as for residual 2 and for total energy expenditure measured with the use of doubly labeled water (kilocalories per day). ICC, intraclass correlation coefficient; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RI, reliability index; WLVS, Women’s Lifestyle Validation Study.
. Original values of urinary markers and total volumes were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). Residual 3 was adjusted as for residual 2 and for total energy expenditure measured with the use of doubly labeled water (kilocalories per day). ICC, intraclass correlation coefficient; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RI, reliability index; WLVS, Women’s Lifestyle Validation Study.
In KSS participants who provided two 24-h urine samples mostly within 1 mo, ICCs for the total urine volume were comparable to those observed in WLVS participants (Table 3). In contrast, ICCs for the urinary excretion of creatinine, sodium, and potassium were higher in the KSS than in the WLVS. For example, in all participants, ranges of ICCs were 0.63–0.66 for creatinine, 0.40–0.55 for sodium, and 0.60–0.65 for potassium in 3 individual cohorts. For these urinary markers, there was no appreciable trend in ICCs by various time intervals between sample collections. ICCs for residuals of potassium were similar to ICCs that were based on original values although the ICCs for sodium residuals were slightly weaker for the same comparison. RIs by various sample-collection intervals ranged from 0.70 to 0.90 for sodium excretion in 3 cohorts, and this range was 0.80–0.92 for potassium excretion.
TABLE 3.
ICCs and corresponding RIs for sodium and potassium by time intervals between 2 collections in KSS participants1
| HPFS (n = 698), d |
NHS (n = 818), d |
NHSII (n = 923), d |
|||||||||||||
| Overall | 1–7 | 8–14 | 15–29 | ≥30 | Overall | 1–7 | 8–14 | 15–29 | ≥30 | Overall | 1–7 | 8–14 | 15–29 | ≥30 | |
| n | 698 | 379 | 168 | 102 | 49 | 818 | 433 | 210 | 91 | 84 | 923 | 403 | 228 | 152 | 140 |
| ICC | |||||||||||||||
| Total volume, L/d | 0.64 | 0.66 | 0.61 | 0.67 | 0.57 | 0.68 | 0.71 | 0.72 | 0.57 | 0.56 | 0.66 | 0.69 | 0.64 | 0.67 | 0.60 |
| Creatinine, mg/d | 0.66 | 0.63 | 0.66 | 0.63 | 0.75 | 0.66 | 0.65 | 0.67 | 0.70 | 0.68 | 0.63 | 0.62 | 0.61 | 0.72 | 0.60 |
| Sodium, mg/d | 0.48 | 0.45 | 0.47 | 0.45 | 0.68 | 0.55 | 0.57 | 0.53 | 0.39 | 0.67 | 0.40 | 0.41 | 0.33 | 0.46 | 0.43 |
| Residual 1 | 0.43 | 0.43 | 0.41 | 0.40 | 0.61 | 0.50 | 0.53 | 0.46 | 0.34 | 0.63 | 0.32 | 0.32 | 0.24 | 0.38 | 0.37 |
| Residual 2 | 0.43 | 0.43 | 0.40 | 0.41 | 0.61 | 0.49 | 0.52 | 0.42 | 0.33 | 0.61 | 0.30 | 0.30 | 0.24 | 0.36 | 0.37 |
| Potassium, mg/d | 0.62 | 0.63 | 0.61 | 0.62 | 0.59 | 0.65 | 0.70 | 0.56 | 0.58 | 0.63 | 0.60 | 0.66 | 0.48 | 0.60 | 0.56 |
| Residual 1 | 0.64 | 0.65 | 0.58 | 0.68 | 0.63 | 0.68 | 0.74 | 0.58 | 0.63 | 0.66 | 0.61 | 0.66 | 0.50 | 0.60 | 0.60 |
| Residual 2 | 0.63 | 0.65 | 0.55 | 0.67 | 0.62 | 0.67 | 0.73 | 0.57 | 0.63 | 0.64 | 0.60 | 0.65 | 0.49 | 0.61 | 0.58 |
| RI | |||||||||||||||
| Sodium, mg/d | 0.81 | 0.79 | 0.80 | 0.79 | 0.90 | 0.84 | 0.85 | 0.83 | 0.75 | 0.89 | 0.76 | 0.76 | 0.70 | 0.80 | 0.78 |
| Residual 1 | 0.78 | 0.77 | 0.76 | 0.76 | 0.87 | 0.82 | 0.83 | 0.79 | 0.72 | 0.88 | 0.70 | 0.70 | 0.63 | 0.74 | 0.73 |
| Residual 2 | 0.78 | 0.77 | 0.76 | 0.76 | 0.87 | 0.81 | 0.82 | 0.77 | 0.70 | 0.87 | 0.68 | 0.68 | 0.62 | 0.72 | 0.73 |
| Potassium, mg/d | 0.87 | 0.88 | 0.87 | 0.87 | 0.86 | 0.89 | 0.91 | 0.85 | 0.85 | 0.88 | 0.87 | 0.89 | 0.80 | 0.86 | 0.84 |
| Residual 1 | 0.88 | 0.89 | 0.86 | 0.90 | 0.88 | 0.90 | 0.92 | 0.86 | 0.88 | 0.89 | 0.87 | 0.89 | 0.81 | 0.87 | 0.86 |
| Residual 2 | 0.88 | 0.89 | 0.84 | 0.89 | 0.87 | 0.90 | 0.92 | 0.85 | 0.88 | 0.88 | 0.87 | 0.89 | 0.81 | 0.87 | 0.86 |
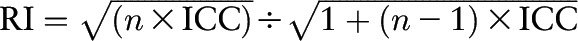 . Original values of urinary markers and total volume were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). HPFS, Health Professionals Follow-Up Study; ICC, intraclass correlation coefficient; KSS, Kidney Stone Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RI, reliability index.
. Original values of urinary markers and total volume were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). HPFS, Health Professionals Follow-Up Study; ICC, intraclass correlation coefficient; KSS, Kidney Stone Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RI, reliability index.
Estimates of ICCs and RIs of other urinary markers in the KSS are shown in Table 4. Of all markers, the urinary excretion of magnesium, calcium, and citrate was the most stable; ICCs for all participants were generally ≥0.70. ICCs for urinary phosphate, sulfate, uric acid, and oxalate were all <0.70 with a range of 0.42 (uric acid in the NHSII) to 0.67 (sulfate in the NHS). Similar to the results for urinary sodium and potassium, sample-collection intervals and the use of residuals did not change the strength of the ICCs materially. For all markers, RIs were ≥0.80 except for uric acid excretion, which had RIs in a range from 0.69 to 0.89.
TABLE 4.
ICCs and corresponding RIs for select urinary markers by time intervals between 2 collections in KSS participants1
| HPFS (n = 698), d |
NHS (n = 818), d |
NHSII (n = 923), d |
|||||||||||||
| Overall | 1–7 | 8–14 | 15–29 | ≥30 | Overall | 1–7 | 8–14 | 15–29 | ≥30 | Overall | 1–7 | 8–14 | 15–29 | ≥30 | |
| n | 698 | 379 | 168 | 102 | 49 | 818 | 433 | 210 | 91 | 84 | 923 | 403 | 228 | 152 | 140 |
| ICC | |||||||||||||||
| Magnesium, mg/d | 0.73 | 0.67 | 0.82 | 0.67 | 0.74 | 0.76 | 0.77 | 0.70 | 0.78 | 0.76 | 0.70 | 0.73 | 0.71 | 0.63 | 0.66 |
| Residual 1 | 0.77 | 0.72 | 0.86 | 0.73 | 0.76 | 0.77 | 0.79 | 0.71 | 0.79 | 0.75 | 0.71 | 0.74 | 0.72 | 0.60 | 0.66 |
| Residual 2 | 0.77 | 0.72 | 0.86 | 0.73 | 0.76 | 0.77 | 0.79 | 0.71 | 0.78 | 0.74 | 0.71 | 0.74 | 0.72 | 0.60 | 0.65 |
| Phosphate, mg/d | 0.58 | 0.55 | 0.62 | 0.58 | 0.65 | 0.58 | 0.56 | 0.62 | 0.55 | 0.55 | 0.56 | 0.58 | 0.50 | 0.63 | 0.53 |
| Residual 1 | 0.51 | 0.56 | 0.43 | 0.47 | 0.54 | 0.54 | 0.55 | 0.59 | 0.50 | 0.45 | 0.48 | 0.53 | 0.40 | 0.48 | 0.43 |
| Residual 2 | 0.52 | 0.57 | 0.42 | 0.48 | 0.53 | 0.55 | 0.55 | 0.59 | 0.51 | 0.45 | 0.47 | 0.53 | 0.40 | 0.47 | 0.44 |
| Sulfate, mg/d | 0.64 | 0.64 | 0.63 | 0.67 | 0.62 | 0.67 | 0.67 | 0.67 | 0.63 | 0.71 | 0.55 | 0.59 | 0.56 | 0.52 | 0.47 |
| Residual 1 | 0.55 | 0.60 | 0.50 | 0.46 | 0.47 | 0.65 | 0.66 | 0.63 | 0.59 | 0.66 | 0.50 | 0.57 | 0.51 | 0.41 | 0.34 |
| Residual 2 | 0.54 | 0.60 | 0.48 | 0.45 | 0.46 | 0.65 | 0.66 | 0.63 | 0.60 | 0.66 | 0.50 | 0.57 | 0.51 | 0.40 | 0.33 |
| Uric acid, mg/d | 0.49 | 0.53 | 0.48 | 0.36 | 0.42 | 0.59 | 0.59 | 0.67 | 0.62 | 0.46 | 0.42 | 0.44 | 0.31 | 0.48 | 0.44 |
| Residual 1 | 0.48 | 0.52 | 0.43 | 0.44 | 0.37 | 0.55 | 0.53 | 0.66 | 0.61 | 0.38 | 0.32 | 0.34 | 0.27 | 0.30 | 0.36 |
| Residual 2 | 0.48 | 0.53 | 0.44 | 0.46 | 0.37 | 0.55 | 0.54 | 0.66 | 0.62 | 0.38 | 0.33 | 0.35 | 0.28 | 0.31 | 0.35 |
| Calcium, mg/d | 0.79 | 0.79 | 0.80 | 0.80 | 0.71 | 0.82 | 0.85 | 0.80 | 0.80 | 0.75 | 0.74 | 0.75 | 0.73 | 0.76 | 0.72 |
| Residual 1 | 0.81 | 0.80 | 0.83 | 0.81 | 0.70 | 0.83 | 0.86 | 0.82 | 0.80 | 0.74 | 0.75 | 0.76 | 0.73 | 0.76 | 0.75 |
| Residual 2 | 0.81 | 0.81 | 0.84 | 0.81 | 0.67 | 0.83 | 0.86 | 0.82 | 0.80 | 0.73 | 0.76 | 0.77 | 0.75 | 0.77 | 0.76 |
| Citrate, mg/d | 0.82 | 0.82 | 0.85 | 0.74 | 0.79 | 0.88 | 0.89 | 0.87 | 0.81 | 0.86 | 0.74 | 0.78 | 0.71 | 0.77 | 0.67 |
| Residual 1 | 0.86 | 0.88 | 0.86 | 0.81 | 0.81 | 0.89 | 0.91 | 0.88 | 0.83 | 0.87 | 0.78 | 0.82 | 0.76 | 0.80 | 0.69 |
| Residual 2 | 0.86 | 0.89 | 0.87 | 0.81 | 0.80 | 0.89 | 0.91 | 0.87 | 0.83 | 0.86 | 0.78 | 0.82 | 0.77 | 0.80 | 0.69 |
| Oxalate, mg/d | 0.55 | 0.54 | 0.63 | 0.46 | 0.53 | 0.56 | 0.59 | 0.58 | 0.54 | 0.45 | 0.56 | 0.55 | 0.58 | 0.56 | 0.56 |
| Residual 1 | 0.55 | 0.57 | 0.60 | 0.39 | 0.56 | 0.57 | 0.59 | 0.55 | 0.60 | 0.45 | 0.55 | 0.53 | 0.61 | 0.55 | 0.52 |
| Residual 2 | 0.56 | 0.57 | 0.61 | 0.38 | 0.54 | 0.55 | 0.58 | 0.54 | 0.59 | 0.44 | 0.54 | 0.51 | 0.60 | 0.53 | 0.50 |
| RI | |||||||||||||||
| Magnesium, mg/d | 0.92 | 0.90 | 0.95 | 0.89 | 0.92 | 0.93 | 0.93 | 0.91 | 0.94 | 0.93 | 0.91 | 0.92 | 0.91 | 0.88 | 0.89 |
| Residual 1 | 0.93 | 0.91 | 0.96 | 0.92 | 0.93 | 0.93 | 0.94 | 0.91 | 0.94 | 0.92 | 0.91 | 0.92 | 0.92 | 0.87 | 0.89 |
| Residual 2 | 0.93 | 0.92 | 0.96 | 0.92 | 0.93 | 0.93 | 0.94 | 0.91 | 0.94 | 0.92 | 0.91 | 0.92 | 0.91 | 0.87 | 0.89 |
| Phosphate, mg/d | 0.86 | 0.84 | 0.87 | 0.86 | 0.89 | 0.85 | 0.85 | 0.88 | 0.84 | 0.84 | 0.85 | 0.86 | 0.81 | 0.88 | 0.83 |
| Residual 1 | 0.82 | 0.85 | 0.78 | 0.80 | 0.84 | 0.84 | 0.84 | 0.86 | 0.82 | 0.79 | 0.81 | 0.83 | 0.76 | 0.81 | 0.78 |
| Residual 2 | 0.83 | 0.85 | 0.77 | 0.81 | 0.83 | 0.84 | 0.84 | 0.86 | 0.82 | 0.79 | 0.80 | 0.83 | 0.76 | 0.80 | 0.78 |
| Sulfate, mg/d | 0.88 | 0.88 | 0.88 | 0.90 | 0.87 | 0.90 | 0.90 | 0.90 | 0.88 | 0.91 | 0.84 | 0.86 | 0.85 | 0.83 | 0.80 |
| Residual 1 | 0.84 | 0.87 | 0.82 | 0.79 | 0.80 | 0.89 | 0.89 | 0.88 | 0.86 | 0.89 | 0.82 | 0.85 | 0.82 | 0.76 | 0.71 |
| Residual 2 | 0.84 | 0.87 | 0.81 | 0.79 | 0.79 | 0.89 | 0.89 | 0.88 | 0.86 | 0.89 | 0.82 | 0.85 | 0.82 | 0.76 | 0.71 |
| Uric acid, mg/d | 0.81 | 0.83 | 0.81 | 0.73 | 0.77 | 0.86 | 0.86 | 0.89 | 0.87 | 0.79 | 0.77 | 0.78 | 0.69 | 0.81 | 0.78 |
| Residual 1 | 0.81 | 0.83 | 0.78 | 0.78 | 0.74 | 0.84 | 0.83 | 0.89 | 0.87 | 0.74 | 0.70 | 0.71 | 0.65 | 0.68 | 0.73 |
| Residual 2 | 0.81 | 0.83 | 0.78 | 0.79 | 0.74 | 0.84 | 0.84 | 0.89 | 0.87 | 0.74 | 0.70 | 0.72 | 0.66 | 0.69 | 0.72 |
| Calcium, mg/d | 0.94 | 0.94 | 0.94 | 0.94 | 0.91 | 0.95 | 0.96 | 0.94 | 0.94 | 0.93 | 0.92 | 0.93 | 0.92 | 0.93 | 0.92 |
| Residual 1 | 0.95 | 0.94 | 0.95 | 0.94 | 0.91 | 0.95 | 0.96 | 0.95 | 0.94 | 0.92 | 0.93 | 0.93 | 0.92 | 0.93 | 0.93 |
| Residual 2 | 0.95 | 0.95 | 0.96 | 0.95 | 0.90 | 0.95 | 0.96 | 0.95 | 0.94 | 0.92 | 0.93 | 0.93 | 0.92 | 0.93 | 0.93 |
| Citrate, mg/d | 0.95 | 0.95 | 0.96 | 0.92 | 0.94 | 0.97 | 0.97 | 0.96 | 0.95 | 0.96 | 0.92 | 0.94 | 0.91 | 0.93 | 0.89 |
| Residual 1 | 0.96 | 0.97 | 0.96 | 0.94 | 0.95 | 0.97 | 0.98 | 0.97 | 0.95 | 0.96 | 0.94 | 0.95 | 0.93 | 0.94 | 0.90 |
| Residual 2 | 0.96 | 0.97 | 0.96 | 0.95 | 0.94 | 0.97 | 0.98 | 0.97 | 0.95 | 0.96 | 0.94 | 0.95 | 0.93 | 0.94 | 0.90 |
| Oxalate, mg/d | 0.84 | 0.84 | 0.88 | 0.79 | 0.83 | 0.85 | 0.86 | 0.86 | 0.84 | 0.79 | 0.85 | 0.84 | 0.86 | 0.85 | 0.85 |
| Residual 1 | 0.84 | 0.85 | 0.87 | 0.75 | 0.85 | 0.85 | 0.86 | 0.84 | 0.87 | 0.79 | 0.84 | 0.83 | 0.87 | 0.84 | 0.83 |
| Residual 2 | 0.85 | 0.85 | 0.87 | 0.74 | 0.84 | 0.84 | 0.86 | 0.84 | 0.86 | 0.78 | 0.84 | 0.82 | 0.87 | 0.83 | 0.82 |
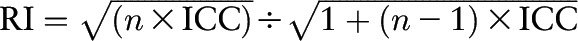 . Original values of urinary markers were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). HPFS, Health Professionals Follow-Up Study; ICC, intraclass correlation coefficient; KSS, Kidney Stone Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RI, reliability index.
. Original values of urinary markers were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). HPFS, Health Professionals Follow-Up Study; ICC, intraclass correlation coefficient; KSS, Kidney Stone Study; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RI, reliability index.
Table 5 presents ICCs and RIs for a variety of polyphenols, phthalates, and BPA that were measured in two 24-h urine samples in 47 WLVS/NHSII participants. A wide range of ICCs was observed in polyphenol metabolites. For instance, urinary enterolactone, enterodiol, resveratrol, ferulic acid, and caffeic acid showed the highest ICCs (≥0.63); urinary epicatechin, daidzein, dihydrodaidzein, and kaempferol showed fair ICCs (≥0.42); and the rest of polyphenol metabolites had ICCs ≥0.32–0.41 except for catechin, which had an ICC of 0.15. ICCs for phthalate metabolites were generally <0.30 except for MBzP (ICC: 0.55). Note that ICCs for MEHHP, MECPP, and MEOHP were 0 because of little between-person variation, and Spearman correlation coefficients were also close to 0 (|r|=0.01) for these biomarkers. Last, the ICC for BPA was 0.39. Consistent with the results for the previously mentioned urinary markers, the use of residuals did not substantially change these observations.
TABLE 5.
ICCs and corresponding RIs for select urinary markers between the first 2 collections in WLVS participants (n = 47)1
| ICC |
RI |
|||||||
| Value | Residual 1 | Residual 2 | Residual 3 | Value | Residual 1 | Residual 2 | Residual 3 | |
| Polyphenols, nmol/d | ||||||||
| Enterolactone | 0.75 | 0.75 | 0.68 | 0.67 | 0.93 | 0.93 | 0.90 | 0.89 |
| Enterodiol | 0.63 | 0.63 | 0.57 | 0.55 | 0.88 | 0.88 | 0.85 | 0.84 |
| Resveratrol | 0.66 | 0.66 | 0.65 | 0.65 | 0.89 | 0.89 | 0.89 | 0.89 |
| Caffeic acid | 0.65 | 0.67 | 0.65 | 0.65 | 0.89 | 0.90 | 0.89 | 0.89 |
| Ferulic acid | 0.74 | 0.77 | 0.76 | 0.75 | 0.92 | 0.93 | 0.93 | 0.93 |
| Catechin | 0.15 | 0.16 | 0.15 | 0.14 | 0.52 | 0.52 | 0.51 | 0.49 |
| Epicatechin | 0.48 | 0.48 | 0.47 | 0.47 | 0.81 | 0.81 | 0.80 | 0.80 |
| Daidzein | 0.42 | 0.42 | 0.40 | 0.41 | 0.77 | 0.77 | 0.76 | 0.76 |
| Dihydrodaidzein | 0.51 | 0.50 | 0.49 | 0.50 | 0.82 | 0.82 | 0.81 | 0.82 |
| O-Desmethylangolensin | 0.38 | 0.38 | 0.39 | 0.40 | 0.74 | 0.74 | 0.75 | 0.75 |
| Genistein | 0.36 | 0.33 | 0.28 | 0.28 | 0.73 | 0.70 | 0.67 | 0.67 |
| Dihydrogenistein | 0.36 | 0.37 | 0.35 | 0.33 | 0.73 | 0.74 | 0.72 | 0.71 |
| Kaempferol | 0.48 | 0.47 | 0.48 | 0.49 | 0.80 | 0.80 | 0.80 | 0.81 |
| Quercetin | 0.38 | 0.39 | 0.41 | 0.40 | 0.74 | 0.75 | 0.76 | 0.76 |
| Isorhamnetin | 0.35 | 0.36 | 0.35 | 0.35 | 0.72 | 0.73 | 0.72 | 0.72 |
| Naringenin | 0.32 | 0.27 | 0.28 | 0.28 | 0.70 | 0.65 | 0.67 | 0.67 |
| Hesperetin | 0.37 | 0.35 | 0.28 | 0.26 | 0.74 | 0.72 | 0.66 | 0.64 |
| Pollutants, μg/d | ||||||||
| MBzP | 0.55 | 0.64 | 0.67 | 0.67 | 0.84 | 0.88 | 0.89 | 0.90 |
| MEHHP | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 |
| MECPP | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.18 | 0.00 | 0.00 |
| MEOHP | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PA | 0.23 | 0.28 | 0.28 | 0.18 | 0.61 | 0.67 | 0.66 | 0.56 |
| MEP | 0.12 | 0.10 | 0.11 | 0.11 | 0.46 | 0.43 | 0.44 | 0.44 |
| MBP | 0.14 | 0.18 | 0.08 | 0.07 | 0.50 | 0.55 | 0.38 | 0.36 |
| MEHP | 0.26 | 0.30 | 0.28 | 0.28 | 0.65 | 0.68 | 0.67 | 0.67 |
| BPA | 0.39 | 0.42 | 0.40 | 0.40 | 0.75 | 0.77 | 0.76 | 0.75 |
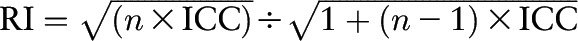 . Original values of urinary markers were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). Residual 3 was adjusted as for residual 2 and for total energy expenditure (kilocalories per day). BPA, bisphenol A; ICC, intraclass correlation coefficient; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono-(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; PA, phthalic acid; RI, reliability index; WLVS, Women’s Lifestyle Validation Study.
. Original values of urinary markers were log transformed to improve normality. Residual 1 was adjusted for the urinary excretion of creatinine (milligrams per day). Residual 2 was adjusted as for residual 1 and for body weight (kilograms), height (meters), total calories (kilocalories), and total physical activities (metabolic equivalent task hours per week). Residual 3 was adjusted as for residual 2 and for total energy expenditure (kilocalories per day). BPA, bisphenol A; ICC, intraclass correlation coefficient; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono-(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; PA, phthalic acid; RI, reliability index; WLVS, Women’s Lifestyle Validation Study.
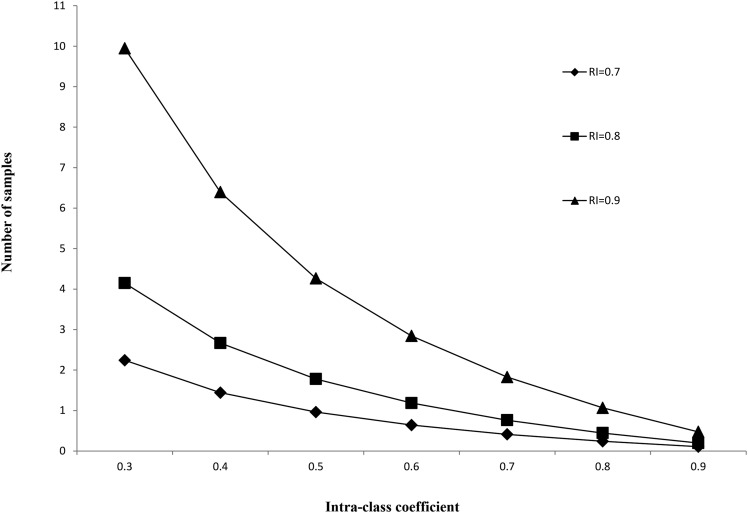
We further estimated the number of samples that were needed to achieve a certain RI by different ICCs (Figure 1). To achieve an RI of 0.8, ≥3 samples were needed for an ICC of 0.37. When ICCs were ≥0.47, two samples were sufficient to have an RI of 0.80. Thus, with three 24-h urine samples, all RIs would have generally been ≥0.80 except for phthalates and certain polyphenol metabolites, especially catechin.
FIGURE 1.
Number of samples needed to achieve a given reliability index by a range of intraclass correlation coefficients. RI, reliability index.
DISCUSSION
In these middle-aged and older US men and women, we examined the reproducibility of various biomarkers that were measured in 24-h urine samples. The different study designs used in the WLVS and KSS enabled us to evaluate the impact of the sample-collection frequency and time frame on the reproducibility. Overall, within a given period, the reproducibility was robust to the number of 24-h urine samples that were collected or to the time intervals between collections although the biomarker concentrations varied less in samples collected within 1 mo compared with those collected at intervals of 3 mo to >1 y. We showed a wide range of reproducibility in individual biomarkers although we estimated that, for the large majority of biomarkers, 2 to three 24-h urine samples were sufficient to obtain a stable measurement of long-term concentrations with the exception of phthalates and a few polyphenol metabolites. Repeating samples at an interval of at least several months would have been desirable to incorporate a better longer-term variation.
Existing data are most abundant for the reproducibility of sodium excretion in 24-h urine samples. In one of the first studies on this topic, Liu et al. (18) examined sodium excretion in 7 consecutive 24-h urine samples that were collected in 73 US children and calculated ICCs of 0.31 for sodium excretion in boys and 0.37 in girls, which were generally similar to the ICCs observed in the WLVS. On the basis of these estimates, the authors further estimated that three 24-h urine samples were needed to achieve an RI of 0.80 or to reduce the misclassification of extreme quintiles of sodium excretion to 1%, although to further improve the RI to 0.90, eight samples would be needed (18). Other studies with various sample-collection intervals documented a similar or somewhat higher reproducibility of sodium excretion in 24-h urine samples in largely healthy individuals (20–25). For example, in the INTERSALT Study that was conducted in 805 adults with two 24-h urine samples that were collected, on average, 2 wk apart, the ICC of sodium excretion ranged from 0.37 to 0.40 (25). In addition, an ICC of 0.53 was observed for sodium excretion in 133 individuals with 2 samples that were collected 4–11 d apart (22). In contrast, in 116 US men, an ICC of 0.24 was observed for sodium excretion in four 24-h urine samples that were collected, on average, within 3 mo (26). In 28 Dutch boys, the same ICC was observed for sodium excretion in 7 consecutive samples (27). The reason for the observations of a greater variability of sodium excretion in these studies is unknown, although the results could also have reflected a lower between-person variation that contributed to the ICC.
Our analysis showed that the urinary excretion of other minerals or electrolytes, including potassium, calcium, phosphate, and magnesium, was more reproducible than for sodium in the WLVS and KSS studies. This result is in line with most previous studies that examined these biomarkers in 24-h urine samples (21, 22, 24, 25, 27, 28), although in 2 relatively small studies, the reproducibility of potassium was comparable (23) or weaker (24) than for sodium. Overall, a wide range of reproducibility was observed for these electrolytes; ranges of ICCs were 0.29–0.71 for potassium (21–25, 27, 28), 0.64–0.77 for calcium (24, 25, 27), and 0.49–0.56 for magnesium (25, 27) in these previous studies. Reports regarding other urinary markers such as iodine, chloride, and selenium have been sporadic, and none of these urinary markers showed an ICC <0.4 (21, 22, 29, 30), below which indicates poor reproducibility (31). The current analysis, for the first time to our knowledge, further illustrated that the excretion of sulfate, uric acid, and citrate had fair to excellent reproducibility in 24-h urine samples (31).
Besides minerals and electrolytes, we also explored the reproducibility of some novel urinary biomarkers including dietary polyphenol metabolites and nonpersistent environmental pollutants, which are known to have poor reproducibility in spot urine samples (1–3, 32–35). For most polyphenol metabolites and BPA, the reproducibility was significantly improved in 24-h urine samples than in spot urine samples that were collected in NHS and NHSII participants (2, 3, 35) because of the better time integration of 24-h urine samples. For example, the ICC of urinary excretion of BPA was improved from 0.14 in spot urine samples (3) to 0.39 in the current analysis. Unlike the minerals or electrolytes that are regulated by homeostasis mechanisms, the reproducibility of the urinary excretion of polyphenols and pollutants primarily depends on the variability of exposure status and half-lives of metabolites (determined according to human metabolism) within the human body. For instance, lignan intake is more stable than flavonoid intake, because lignans widely exist in plant-based foods rather than in a few specific foods (e.g., in soy food for isoflavones). Moreover, plant lignans are subject to microbiota metabolism in the colon to produce enterodiol and enterolactone (36), which subsequently undergo significant enterohepatic circulation after absorption (37). These factors may explain the wide range in reproducibility within the polyphenol metabolites. The lower ICCs observed for most phthalates in 24-h urine samples than in spot urine samples (≤0.26, except 0.55 for MBzP) (3) were unexpected. Whether these values were due to changes in phthalate exposure in the US population over time (38) will be explored in future studies.
Multiple sources of error can contribute to the inaccuracy of quantifying long-term intake or exposure status with the use of biomarkers in 24-h urine samples. These sources include the completeness of sample collection ≤24 h, changes of diet or behavior because of the sample-collection efforts, errors in volume estimation, laboratory measurement errors of biomarkers, other factors that may introduce extraneous variability, and the half-lives of biomarkers in human body. In the current analysis, we showed that the reproducibility of urinary biomarkers was quite robust to the frequency of sample collection, the time intervals between sample collections, and the effects of anthropometric, lifestyle, and dietary factors that may have introduced an additional variability in marker concentrations. Moreover, we showed that, even for urinary markers with an ICC in the range from 0.3 to 0.4, three 24-h urine samples would have been sufficient to achieve an RI of 0.8. In most epidemiologic studies that have compared high exposure status with low exposure status, the use of three 24-h urine samples would be sufficient to substantially reduce the misclassification between contrasting exposure categories (18). The collection of more samples would have only marginally reduce the measurement error and could have led to increased effort and reduced participation or compliance by study members.
The strengths of the current analysis include the examination of multiple urinary biomarkers with various excretion properties in 24-h urine samples, a large sample size, and the use of different study designs in the WLVS and KSS to facilitate the evaluation of sample-collection variables on reproducibility estimates. Our study participants were largely free of major chronic conditions that may have modified the excretion of urinary markers. Whether the results can be generalized to patients with chronic diseases, such as renal insufficiency (21), is unknown. Similarly, because of our participants were primarily of European ancestry, whether our results can be generalized to other ethnic groups will be examined in future investigations, although the limited evidence suggests that the impact of race on urinary marker reproducibility is probably minimal (22). More important sources of differences in ICCs are likely to be true differences in the distributions of dietary and environmental exposures.
In conclusion, we evaluated the reproducibility of a wide variety of urinary biomarkers in 24-h urine samples in ∼3000 US men and women. The majority of urinary biomarkers show fair-to-excellent reproducibility in 24-h urine samples, which did not vary substantially by sample collection interval, frequency, and other variables. These data provide evidence of the reproducibility of some novel biomarkers in 24-h urine samples and also show that, for most of these urinary biomarkers, 2 to three 24-h urine samples are sufficient to achieve an RI ≥0.8. The time integration, versatility, and feasibility of 24-h urine samples make these biospecimens potentially useful for the assessment of various nutritional and environmental exposures in epidemiologic studies.
Acknowledgments
The authors’ responsibilities were as follows—QS: analyzed the data and drafted the manuscript; QS and WCW: had primary responsibility for the final content of the manuscript; KAB and BR: contributed to the biomarker data processing and statistical analyses; AAF: measured the urinary excretion of polyphenols and the environmental pollutants in urine samples; GCC: designed the research for the KSS; WCW: designed the research for the WLVS; GCC and WCE: collected 24-h urine samples and contributed to the assessments of urinary biomarkers; and all authors: contributed to the design of analytical strategies, interpretation of results, and critical revision of the manuscript and read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BPA, bisphenol A; DLW, doubly labeled water; HPFS, Health Professionals Follow-Up Study; ICC, intraclass correlation coefficient; KSS, Kidney Stone Study; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono-(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono-(2-ethyl-5-oxohexyl) phthalate; NHS, Nurses’ Health Study; NHSII, Nurses’ Health Study II; RI, reliability index; SFFQ, semiquantitative food-frequency questionnaire; TEE, total energy expenditure; WLVS, Women’s Lifestyle Validation Study.
REFERENCES
- 1.Nepomnaschy PA, Baird DD, Weinberg CR, Hoppin JA, Longnecker MP, Wilcox AJ. Within-person variability in urinary bisphenol A concentrations: measurements from specimens after long-term frozen storage. Environ Res 2009;109:734–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Q, Wedick NM, Tworoger SS, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM. Urinary excretion of select dietary polyphenol metabolites is associated with a lower risk of type 2 diabetes in proximate but not remote follow-up in a prospective investigation in 2 cohorts of US women. J Nutr 2015;145:1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health 2013;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dam RM, Hunter D. Biochemical indicators of dietary intake. In: Willett WC, editor. Nutritional epidemiology. New York: Oxford University Press; 2013. p. 150–212.
- 5.Greenblatt DJ, Ransil BJ, Harmatz JS, Smith TW, Duhme DW, Koch-Weser J. Variability of 24-hour urinary creatinine excretion by normal subjects. J Clin Pharmacol 1976;16:321–8. [DOI] [PubMed] [Google Scholar]
- 6.James GD, Sealey JE, Alderman M, Ljungman S, Mueller FB, Pecker MS, Laragh JH. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens 1988;1:124–31. [DOI] [PubMed] [Google Scholar]
- 7.Ji C, Sykes L, Paul C, Dary O, Legetic B, Campbell NR, Cappuccio FP. Systematic review of studies comparing 24-hour and spot urine collections for estimating population salt intake. Rev Panam Salud Publica 2012;32:307–15. [DOI] [PubMed] [Google Scholar]
- 8.Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet 2016;388:465–75. [DOI] [PubMed] [Google Scholar]
- 9.Mills KT, Chen J, Yang W, Appel LJ, Kusek JW, Alper A, Delafontaine P, Keane MG, Mohler E, Ojo A, et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA 2016;315:2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willett WC. Nutritional epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 11.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int 2008;73:489–96. [DOI] [PubMed] [Google Scholar]
- 12.Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect 2014;122:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong T, Bull F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health (Bangkok) 2006;14:66–70. [Google Scholar]
- 14.Schoeller DA, Colligan AS, Shriver T, Avak H, Bartok-Olson C. Use of an automated chromium reduction system for hydrogen isotope ratio analysis of physiological fluids applied to doubly labeled water analysis. J Mass Spectrom 2000;35:1128–32. [DOI] [PubMed] [Google Scholar]
- 15.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 16.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC. Implications of total energy intake for epidemiologic analyses. In: Willett WC, editor. Nutritional epidemiology. New York: Oxford University Press; 2013. p. 260–86. [Google Scholar]
- 18.Liu K, Cooper R, Soltero I, Stamler J. Variability in 24-hour urine sodium excretion in children. Hypertension 1979;1:631–6. [DOI] [PubMed] [Google Scholar]
- 19.Webb NM, Shavelson RJ, Haertel EH. 4 reliability coefficients and generalizability theory. In: Rao CR, Sinharay S, editors. Handbook of statistics. Amsterdam: Elsevier; 2006. p. 81–124.
- 20.Liu LS, Zheng DY, Lai SH, Wang GQ, Zhang YL. Variability in 24-hour urine sodium excretion in Chinese adults. Chin Med J (Engl) 1986;99:424–6. [PubMed] [Google Scholar]
- 21.Luft FC, Aronoff GR, Sloan RS, Fineberg NS. Intra- and interindividual variability in sodium intake in normal subjects and in patients with renal insufficiency. Am J Kidney Dis 1986;7:375–80. [DOI] [PubMed] [Google Scholar]
- 22.Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, et al. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J Nutr 2013;143:1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu LS, Zheng DY, Jin L, Liao YL, Liu K, Stamler J. Variability of urinary sodium and potassium excretion in north Chinese men. J Hypertens 1987;5:331–5. [DOI] [PubMed] [Google Scholar]
- 24.Siani A, Iacoviello L, Giorgione N, Iacone R, Strazzullo P. Comparison of variability of urinary sodium, potassium, and calcium in free-living men. Hypertension 1989;13:38–42. [DOI] [PubMed] [Google Scholar]
- 25.Dyer AR, Shipley M, Elliott P. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. I. Estimates of reliability. The INTERSALT Cooperative Research Group. Am J Epidemiol 1994;139:927–39. [DOI] [PubMed] [Google Scholar]
- 26.Liu K, Cooper R, McKeever J, McKeever P, Byington R, Soltero I, Stamler R, Gosch F, Stevens E, Stamler J. Assessment of the association between habitual salt intake and high blood pressure: methodological problems. Am J Epidemiol 1979;110:219–26. [DOI] [PubMed] [Google Scholar]
- 27.Knuiman JT, van Poppel G, Burema J, van der Heijden L, Hautvast JG. Multiple overnight urine collections may be used for estimating the excretion of electrolytes and creatinine. Clin Chem 1988;34:135–8. [PubMed] [Google Scholar]
- 28.Tasevska N, Runswick SA, Bingham SA. Urinary potassium is as reliable as urinary nitrogen for use as a recovery biomarker in dietary studies of free living individuals. J Nutr 2006;136:1334–40. [DOI] [PubMed] [Google Scholar]
- 29.Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, McAdam PA, Patterson KY, Holden JM, Morris JS, et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology 1996;7:384–90. [DOI] [PubMed] [Google Scholar]
- 30.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011;141:2049–54. [DOI] [PubMed] [Google Scholar]
- 31.Rosner B. Multisample inference. In: Rosner B, editor. Fundamentals of biostatistics. Pacific Grove (CA): Duxbury; 2000. p. 511–76.
- 32.Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect 2008;116:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valvi D, Monfort N, Ventura R, Casas M, Casas L, Sunyer J, Vrijheid M. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int J Hyg Environ Health 2015;218:220–31. [DOI] [PubMed] [Google Scholar]
- 34.Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, Braun JM. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol 2014;48:8881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Q, Wedick NM, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of U.S. women. Diabetes Care 2014;37:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clavel T, Henderson G, Alpert CA, Philippe C, Rigottier-Gois L, Dore J, Blaut M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol 2005;71:6077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach Knudsen KE, Serena A, Kjaer AK, Tetens I, Heinonen SM, Nurmi T, Adlercreutz H. Rye bread in the diet of pigs enhances the formation of enterolactone and increases its levels in plasma, urine and feces. J Nutr 2003;133:1368–75. [DOI] [PubMed] [Google Scholar]
- 38.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect 2014;122:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]