Abstract
Background: Observational associations between red meat intake and cardiovascular disease (CVD) are inconsistent. There are limited comprehensive analyses of randomized controlled trials (RCTs) that investigate the effects of red meat consumption on CVD risk factors.
Objective: The purpose of this systematically searched meta-analysis was to assess the effects of consuming ≥0.5 or <0.5 servings of total red meat/d on CVD risk factors [blood total cholesterol (TC), LDL cholesterol, HDL cholesterol, triglycerides, ratio of TC to HDL cholesterol (TC:HDL), and systolic and diastolic blood pressures (SBP and DBP, respectively)]. We hypothesized that the consumption of ≥0.5 servings of total red meat/d would have a negative effect on these CVD risk factors.
Design: Two researchers independently screened 945 studies from PubMed, Cochrane Library, and Scopus databases and extracted data from 24 qualified RCTs. Inclusion criteria were 1) RCT, 2) subjects aged ≥19 y, 3) consumption of ≥0.5 or <0.5 total red meat servings/d [35 g (1.25 ounces)], and 4) reporting ≥1 CVD risk factor. We performed an adjusted 2-factor nested ANOVA mixed-effects model procedure on the postintervention values of TC, LDL cholesterol, HDL cholesterol, TC:HDL cholesterol, triglycerides, SBP, and DBP; calculated overall effect sizes of change values; and used a repeated-measures ANOVA to assess pre- to postintervention changes.
Results: Red meat intake did not affect lipid-lipoprotein profiles or blood pressure values postintervention (P > 0.05) or changes over time [weighted mean difference (95% CI): −0.01 mmol/L (−0.08, 0.06 mmol/L), 0.02 mmol/L (−0.05, 0.08 mmol/L), 0.03 mmol/L (−0.01, 0.07 mmol/L), and 0.04 mmol/L (−0.02, 0.10 mmol/L); −0.08 mm Hg (−0.26, 0.11 mm Hg); and −1.0 mm Hg (−2.4, 0.78 mm Hg) and 0.1 mm Hg (−1.2, 1.5 mm Hg) for TC, LDL cholesterol, HDL cholesterol, triglycerides, TC:HDL cholesterol, SBP, and DBP, respectively]. Among all subjects, TC, LDL cholesterol, HDL cholesterol, TC:HDL cholesterol, triglycerides, and DBP, but not SBP, decreased over time (P < 0.05).
Conclusions: The results from this systematically searched meta-analysis of RCTs support the idea that the consumption of ≥0.5 servings of total red meat/d does not influence blood lipids and lipoproteins or blood pressures.
Keywords: dietary guidance, blood lipids, blood lipoproteins, blood pressure, animal flesh, meat products, diet, meat
INTRODUCTION
The effects of red meat consumption on cardiovascular disease (CVD)3 are inconsistent throughout the literature. CVD has been the leading cause of death in the United States since the 1950s and is currently attributable to 610,000 US deaths each year (1). Historically, epidemiologic cohort data support associations between high red meat intake and CVD-related events (2, 3) and mortality (4–6). This notion is currently being challenged due to data collection methods that group red meat with processed meat and/or inconsistent nomenclature and classification of red meat throughout the literature (7, 8). Regardless of contradicting evidence, an observational study design is unable to show causality such as with a randomized controlled trial (RCT). There is a paucity of literature that systematically and comprehensively assesses the effects of total red meat consumption amounts on CVD risk with data from RCTs (9).
The purpose of this meta-analysis was to systematically search the literature to assess the effects of total red meat consumption on indexes of CVD risk. The search included studies with an RCT design that measured blood lipids, lipoproteins, and/or blood pressures. We hypothesized that the consumption of ≥0.5 servings of red meat/d (or ∼3.5 servings/wk) would negatively affect blood lipids, lipoproteins, and blood pressures. Our hypothesis was based on a current prospective cohort analysis that estimated that 8.6% and 12.2% of CVD-related deaths in men and women, respectively, would be preventable if participants consumed <0.5 servings of total red meat/d (5).
METHODS
Search strategy and data extraction
We followed the same systematic search protocol as the 2015 Dietary Guidelines Advisory Committee from the Nutrition Evidence Library (10). The PICOS (Population, Intervention, Comparator, Outcome, Study design) criteria used to define our research question are listed in Table 1. Inclusion criteria consisted of the following: 1) use of an RCT study design, 2) subjects aged ≥19 y, 3) an intervention group or phase with consumption of ≥0.5 servings of total red meat/d compared with a control group or phase with consumption of <0.5 servings of total red meat/d, and 4) reporting of ≥1 CVD risk factor as a dependent variable [i.e., blood total cholesterol (TC), LDL cholesterol, HDL cholesterol, TC-to-HDL-cholesterol ratio (TC:HDL), triglycerides, systolic blood pressure (SBP), and diastolic blood pressure (DBP)]. Our meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (11).
TABLE 1.
Description of PICOS criteria for a systematically searched meta-analysis assessing the effects of consuming ≥0.5 or <0.5 servings of total red meat/d on blood lipids, lipoproteins, and blood pressures1
| Variable | Description |
| Population | Adults aged ≥19 y |
| Intervention | Groups who consumed ≥0.5 servings (35 g or 1.25 ounces) of total red meat/d |
| Comparator | Groups who consumed <0.5 servings of total red meat/d |
| Outcome | Changes in modifiable traditional cardiovascular disease risk factors, specifically blood lipids, lipoproteins, and blood pressures |
| Study design | Randomized controlled trials |
| Research question | What is the effect of consuming ≥0.5 servings of total red meat/d on blood lipids, lipoproteins, and blood pressure in adults? |
PICOS, Population, Intervention, Comparator Outcome, Study design.
The original search took place in May 2015 but was updated in May 2016. We identified studies via a computerized search of 3 databases: 1) PubMed (http://www.ncbi.nlm.nih.gov/pubmed), 2) Cochrane Library (http://www.cochranelibrary.com), and 3) Scopus (http://www.scopus.com). We reviewed reference lists of the identified studies and found 10 additional potential studies. Search terms and results are identified in Table 2. All of the database searches were completed independently by the primary author (LEO) and the secondary author (JEK). A research librarian assisted both reviewers (see Acknowledgments) in database and search term selection to optimize the search process and to reduce the chance of bias.
TABLE 2.
Search terms and results for a systematically searched meta-analysis assessing the effects of consuming ≥0.5 or <0.5 servings of total red meat/d on blood lipids, lipoproteins, and blood pressures1
| Source | Search terms | Filters | Results yielded |
| PubMed database | (“Meat”[MESH] OR “Meat Products”[MESH] OR “red meat” OR “beef” OR “pork”) AND (“hypertension”[MESH] OR “Cholesterol, LDL”[MESH] OR “Cholesterol, HDL”[MESH] OR “Blood Pressure”[MESH] OR “lipoproteins”[MESH]) | Humans, aged ≥19 y, English | 332 |
| Scopus database | Meat AND (blood pressure OR lipoprotein) | English, human, humans, source type journals, limit to article and conference paper; exclude physical sciences, social sciences, humanities, agriculture, immunology, chemistry, environmental sciences, neuroscience, chemical engineering, engineering, computer science, psychology, arts and humanities, mathematics, veterinary and multidisciplinary | 426 |
| Cochrane Central database | Meat AND (blood pressure OR lipoprotein) | Trials | 177 |
| Reference lists of identified studies | N/A | N/A | 10 |
| Total | — | — | 945 |
MESH, Medical Subject Heading; N/A, not applicable.
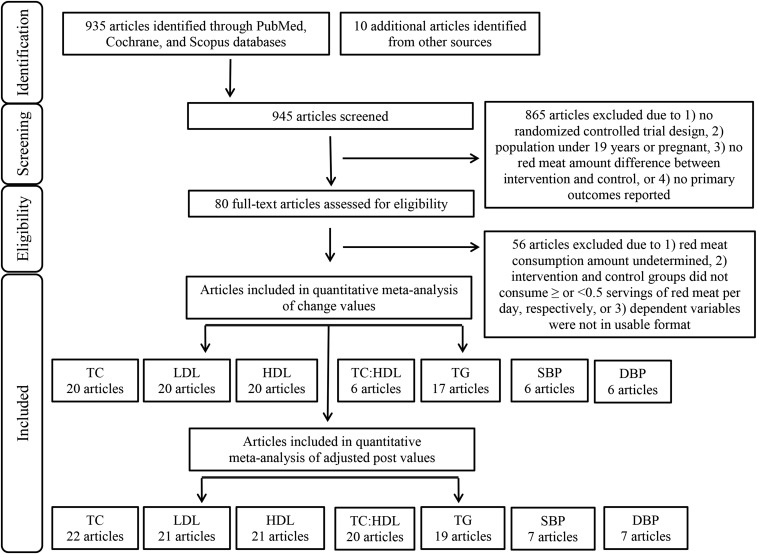
We excluded 865 of 945 studies from our search for the following reasons: 1) the study design was not an RCT, 2) the population was <19 y of age or pregnant, 3) the control and intervention diets did not differ in total red meat consumption amounts, or 4) the researchers did not report the dependent variables of interest (see Figure 1). The primary and secondary authors independently read 80 potentially eligible studies to further assess inclusion criteria and to avoid selection bias. We contacted corresponding authors when clarification or unpublished data were needed. We excluded 56 of the 80 studies from the analysis for the following reasons: 1) we were unable to determine the amount of red meat consumed, 2) the control and intervention diets did not meet our requirements of ≥0.5 or <0.5 servings/d or ≥3.5 or <3.5 servings/wk of total red meat, or 3) we were unable to obtain the dependent variables of interest in a usable data format. The primary and secondary authors independently extracted data from the final 24 studies including the following: 1) author name, 2) publication year, 3) population size and description, 4) intervention duration, 5) protein source comparison consumed by the control group, and 6) the amount of total red meat intake, dietary patterns, method of diet administration, assessment of dietary compliance, and pre- and postintervention values and net changes in blood TC, LDL cholesterol, HDL cholesterol, TC:HDL, triglycerides, SBP, and DBP for both the control and intervention groups.
FIGURE 1.
PRISMA flowchart. DBP, diastolic blood pressure; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Definitions
For this meta-analysis, we used the 2015–2020 Dietary Guidelines for Americans (DGA) glossary definition of red meat (or “meat”) and processed meat: “all forms of beef, pork, lamb, veal, goat, and non-bird games (e.g. venison, bison, elk)” and “preserved by smoking, curing, salting, and/or the addition of chemical preservatives,” respectively (12). Unprocessed meat refers to meat that is preserved by refrigeration or freezing only (13). However, all meat available for purchase is processed to an extent (e.g., slaughtering and packaging) so the term “minimally processed” will be used in this meta-analysis to further describe the red meat consumed by research subjects. Blood TC, LDL cholesterol, HDL cholesterol, TC:HDL, triglycerides, SBP, and DBP are common modifiable biomarkers of CVD risk regularly assessed by physicians and therefore are the dependent variables assessed in this meta-analysis.
Calculations, bias assessment, and statistical analyses
We obtained or calculated the amount of red meat consumed by each group from the dietary data available in the study and contacted authors for clarification or raw data when needed. According to the American Heart Association, a serving size of cooked meat is 2–3 ounces (14); therefore, we considered 1 serving and 0.5 servings of red meat to be equivalent to 2.5 and 1.25 ounces, respectively. With the use of ProNutra software version 3.3 (Viocare, Inc.), we calculated that 1.25 ounces of red meat was equivalent to 35 g. The cutoff of 0.5 servings/d is supported by a 2012 prospective cohort analysis that estimated that 8.6% and 12.2% of CVD-related deaths in men and women, respectively, were preventable if subjects consumed <0.5 servings of total red meat/d (5).
We converted all blood lipid and lipoprotein data to mmol/L [TC, LDL-cholesterol, and HDL-cholesterol conversion: mg/dL ÷ 38.67; triglycerides conversion: mg/dL ÷ 88.57 (15)]. We extracted pre- and postintervention means, SDs, change values, and SDs of the change values from the studies when available. If not available, we calculated values, when appropriate, either from raw data obtained from the researchers or from information that was provided in the study and calculated change-value SDs by using a correlation factor representative of the change-value SDs that were available from the other studies (16). We evaluated the risk of selection, performance, and detection biases by using the modified Cochrane risk-of-bias assessment tool (17).
When discussing “studies” throughout this meta-analysis, we are referring to the entirety of each publication. Some studies contained >1 intervention or control group or phase. In this case, these interventions are presented separately and treated as independent trials to account for within-study differences (18). Crossover trials were included in this meta-analysis; the present results and figures show crossover trial means and SDs incorporated into the data set as if they were parallel designs (19). This approach uses a correlational factor of 0 for all trial SDs. We recognize that this approach is conservative and causes crossover studies to be underweighted; therefore, we conducted secondary analyses to approximate a paired analysis for each variable by imputing missing SDs with the use of a correlational factor of 0.99 for all crossover design studies (20).
With the use of SAS version 9.4 (SAS Institute), we performed a repeated-measures ANOVA to assess pre- to postintervention changes in TC, LDL cholesterol, HDL cholesterol, TC:HDL, triglycerides, SBP, and DBP. We performed a 2-factor, nested ANOVA mixed-effects model procedure on the postintervention values of each dependent variable after adjustment for baseline values, age, sex, BMI, length of intervention, and whether energy restriction was or was not included in the protocol (21). These results are reported as adjusted least-squares means. We analyzed the change values by using STATA/IC 14 (StataCorp) and calculated the overall effect size by using the metaan function (intervention group or phase change value minus control group or phase change value). We used a random-effects model when heterogeneity was indicated by a significant chi-square test; otherwise, a fixed-effects model was used (22, 23). These results are reported as weighted mean differences and 95% CIs. Studies in Figures 2–8 are organized in descending order from smallest to largest amounts of total red meat consumed per day by the intervention group or phase. Significance was set at P < 0.05. A statistical consultant approved all calculations and analyses (see Acknowledgments).
FIGURE 2.
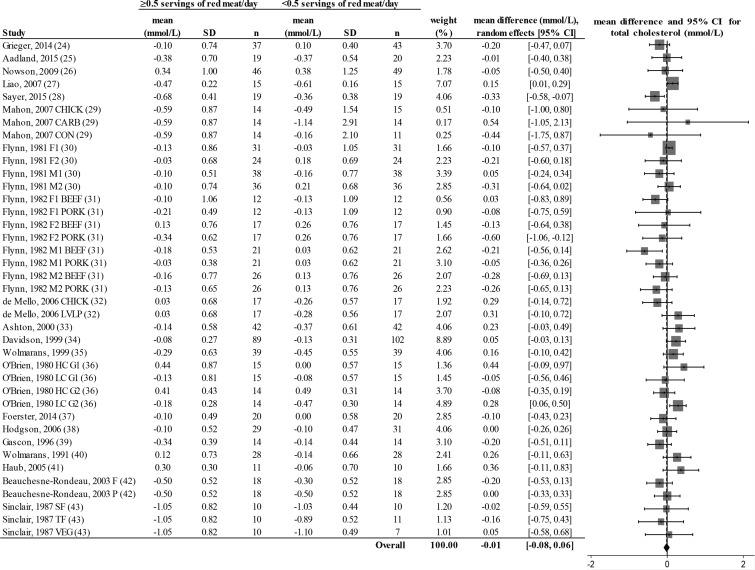
Random-effects model meta-analysis for changes in total blood cholesterol concentrations from randomized controlled trials comparing ≥0.5 or <0.5 servings of total red meat/d. Heterogeneity: τ2 = 0.011, χ2 = 1.48, df = 38 (P = 0.028), I2 = 32%. Data are shown in descending order from smallest to largest amounts of red meat consumed by the intervention group or phase. CARB, carbohydrate control diet; CHICK, chicken control diet; CON, habitual control diet; F, lean fish control diet; F1, first female group; F1 BEEF, first female group consuming beef diet; F1 PORK, first female group consuming pork diet; F2, second female group; F2 BEEF, second female group consuming beef diet; F2 PORK, second female group consuming pork diet; HC G1, first group consuming high-cholesterol diet; HC G2, second group consuming high-cholesterol diet; LC G1, first group consuming low-cholesterol diet; LC G2, second group consuming low-cholesterol diet; LVLP, lactovegetarian low-protein control diet; M1, first male group; M1 BEEF, first male group consuming beef diet; M1 PORK, first male group consuming pork diet; M2, second male group; M2 BEEF, second male group consuming beef diet; M2 PORK, second male group consuming pork diet; P, poultry control diet; SF, southern fish control diet; TF, tropical fish control diet; VEG, vegetarian control diet.
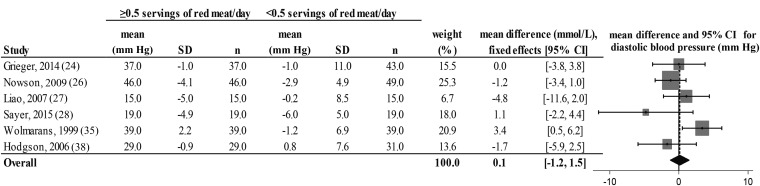
FIGURE 8.
Fixed-effects model meta-analysis for changes in diastolic blood pressure from randomized controlled trials comparing ≥0.5 or <0.5 servings of total red meat/d. Heterogeneity: τ2 = 0.662, χ2 = 4.42, df = 5 (P = 0.097), I2 = 46%. Data are shown in descending order from smallest to largest amounts of red meat consumed by the intervention group or phase.
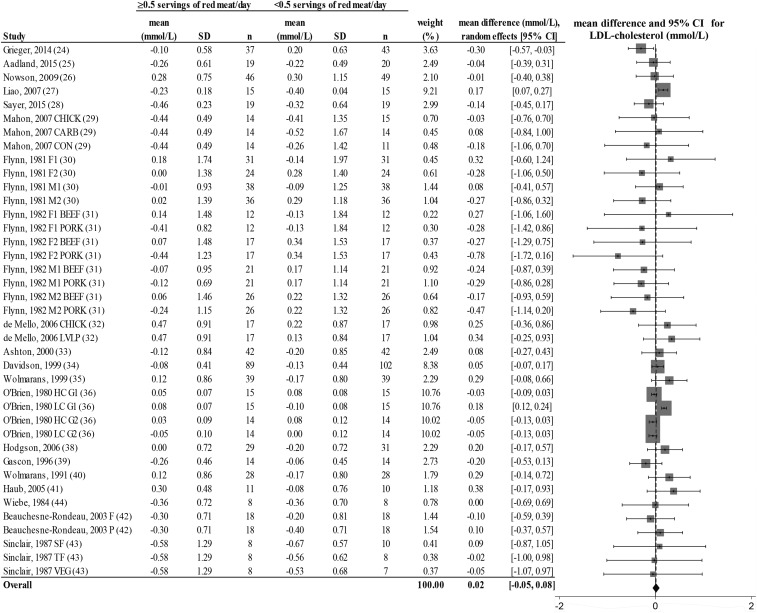
FIGURE 3.
Random-effects model meta-analysis for changes in blood LDL-cholesterol concentrations from randomized controlled trials comparing ≥0.5 or <0.5 servings of total red meat/d. Heterogeneity: τ2 = 0.011, χ2 = 6.62, df = 38 (P = 0.001), I2 = 85%. Data are shown in descending order from smallest to largest amounts of red meat consumed by the intervention group or phase. CARB, carbohydrate control diet; CHICK, chicken control diet; CON, habitual control diet; F, lean fish control diet; F1, first female group; F1 BEEF, first female group consuming beef diet; F1 PORK, first female group consuming pork diet; F2, second female group; F2 BEEF, second female group consuming beef diet; F2 PORK, second female group consuming pork diet; HC G1, first group consuming high-cholesterol diet; HC G2, second group consuming high-cholesterol diet; LC G1, first group consuming low-cholesterol diet; LC G2, second group consuming low-cholesterol diet; LVLP, lactovegetarian low-protein control diet; M1, first male group; M1 BEEF, first male group consuming beef diet; M1 PORK, first male group consuming pork diet; M2, second male group; M2 BEEF, second male group consuming beef diet; M2 PORK, second male group consuming pork diet; P, poultry control diet; SF, southern fish control diet; TF, tropical fish control diet; VEG, vegetarian control diet.
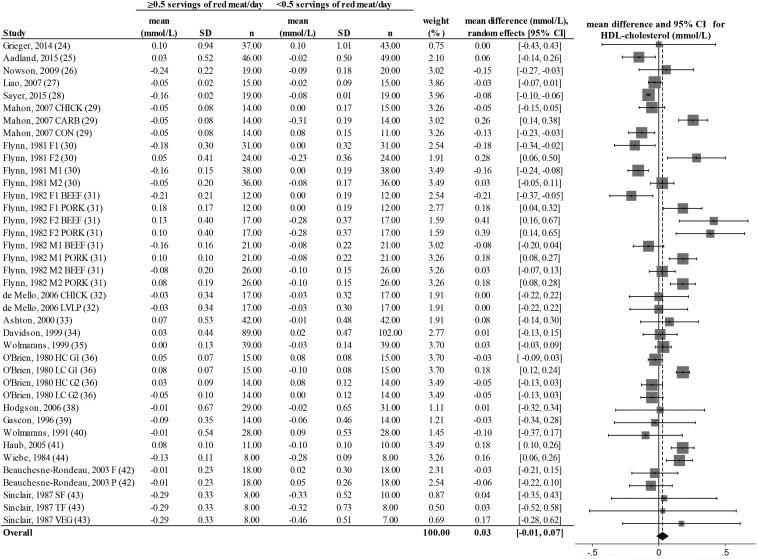
FIGURE 4.
Random-effects model meta-analysis for changes in blood HDL-cholesterol concentrations from randomized controlled trials comparing ≥0.5 or <0.5 servings of total red meat/d. Heterogeneity: τ2 = 0.011, χ2 = 6.62, df = 38 (P = 0.001), I2 = 85%. Data are shown in descending order from smallest to largest amounts of red meat consumed by the intervention group or phase. CARB, carbohydrate control diet; CHICK, chicken control diet; CON, habitual control diet; F, lean fish control diet; F1, first female group; F1 BEEF, first female group consuming beef diet; F1 PORK, first female group consuming pork diet; F2, second female group; F2 BEEF, second female group consuming beef diet; F2 PORK, second female group consuming pork diet; HC G1, first group consuming high-cholesterol diet; HC G2, second group consuming high-cholesterol diet; LC G1, first group consuming low-cholesterol diet; LC G2, second group consuming low-cholesterol diet; LVLP, lactovegetarian low-protein control diet; M1, first male group; M1 BEEF, first male group consuming beef diet; M1 PORK, first male group consuming pork diet; M2, second male group; M2 BEEF, second male group consuming beef diet; M2 PORK, second male group consuming pork diet; P, poultry control diet; SF, southern fish control diet; TF, tropical fish control diet; VEG, vegetarian control diet.
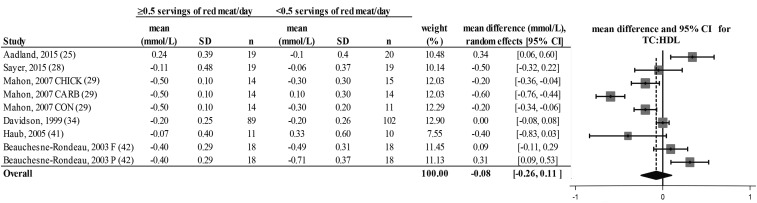
FIGURE 5.
Random-effects model meta-analysis for changes in blood TC:HDL from randomized controlled trials comparing ≥0.5 or <0.5 servings of total red meat/d. Heterogeneity: τ2 = 0.064, χ2 = 9.93, df = 8 (P = 0.001), I2 = 90%. Data are shown in descending order from smallest to largest amounts of red meat consumed by the intervention group or phase. CARB, carbohydrate control diet; CHICK, chicken control diet; CON, habitual control diet; F, lean fish control diet; P, poultry control diet; TC:HDL, ratio of total cholesterol to HDL cholesterol.
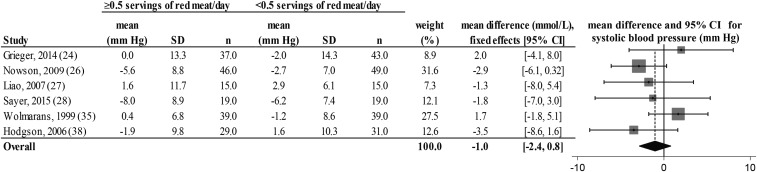
FIGURE 7.
Fixed-effects model meta-analysis for changes in systolic blood pressure from randomized controlled trials comparing ≥0.5 or <0.5 servings of total red meat/d. Heterogeneity: τ2 = 0.662, χ2 = 4.42, df = 5 (P = 0.346), I2 = 11%. Data are shown in descending order from smallest to largest amounts of red meat consumed by the intervention group or phase.
We performed traditional sensitivity analyses by removing 1 study or trial at a time and reconducting the analyses. We performed additional sensitivity analyses by removing clusters of studies containing design features that had the potential to confound results, including weight-loss diets (27, 29), heart-healthy diets (25, 26, 28, 34, 35, 39, 42, 43), diseased populations [hypertensive (26, 28, 38), hypercholesterolemic (34, 35, 42), and/or diabetic (32)], studies that resulted in significant weight loss (25, 27–29, 35), inclusion of processed meat (45), studies that did not specify the degree of meat processing (24, 25, 27, 32, 36, 40, 43, 46, 47), and studies that used different amounts of protein intake in the control and intervention group or phase (29, 32, 38, 43). We also performed post hoc analyses by dividing the studies into specific quantities of red meat consumption [1.0–1.9 servings of red meat/d (24–29), 2.0–2.9 servings of red meat/d (30–37), or ≥3.0 servings of red meat/d (38–43)] and reconducted the analyses in STATA.
RESULTS
Study features and subject characteristics
Twenty-four studies were included in the statistical analyses (see Figure 1); some contained >1 control group or phase (29–32, 36, 42, 43) and are reported as separate studies. Details of each study are shown in Table 3. The median total red meat servings per day in the control and intervention groups were 0 servings/d (range: 0–0.4 servings/d or 0–30 g/d) and 2 servings/d (or 140 g/d; range 1.0–7.1 servings/d or 68–500 g), respectively. Two of the selected studies included a weight-loss diet (27, 29), 8 studies included a heart-healthy dietary pattern (25, 26, 28, 34, 35, 39, 42, 43), the subjects self-selected their diet similar to their habitual intake in 9 studies (24, 30, 31, 36–38, 46, 40, 41), and 5 of the selected studies were unclear about the diet other than the predominant protein source (32, 33, 44, 45, 47). Only minimally processed meats were consumed in 15 studies (25, 26, 28, 29, 30, 31, 33–35, 37–39, 41, 42, 44), highly processed meats were consumed in 1 study (45), and the extent of meat processing was unclear in the remaining 8 studies (24, 27, 32, 36, 40, 43, 46, 47). Intervention lengths varied from 2 to 32 wk.
TABLE 3.
Study design of randomized controlled trials assessing the effects of consuming ≥0.5 or <0.5 servings of total red meat/d on blood lipids, lipoproteins, and blood pressures1
| First author, year (ref) | Total red meat servings in intervention (g/d); type of red meat; degree of meat processing | Total red meat servings in control (g/d); comparison protein source | Dietary pattern; diet administration method; dietary compliance assessment | Intervention length in weeks; study design | Population size and description; mean age in years; mean BMI in kg/m2 |
| Grieger, 2014 (24) | 1.0 (68); beef, lamb, ham, pork; N/A | 0; fatty fish | Habitual diet; protein source and some other foods provided; verbal motivation and interviews every 2 wk | 8; 2-arm parallel | 80 generally healthy men and women; 69.6; 26.4 |
| Prescott, 1988 (45) | 1.0 (72); meat supplement containing sausage, beef, lamb, pork; processed | 0; nonmeat supplement | N/A; 2 meals/d and protein source provided; urinary 3-methyl histidine | 12; 2-arm parallel | 64 generally healthy men and women; N/A; N/A |
| Aadland, 2015 (25) | 1.1 (77); pork and lean beef; minimally processed | 0; lean seafood (cod, pollock, saithe, and scallops) | Norwegian nutritional recommendations; some food provided; daily oral questionnaire and regular weigh-ins | 20; 2-phase crossover | 20 generally healthy men and women; 50.6; 25.6 |
| Nowson, 2009 (26) | 1.2 (86); raw lean beef, lamb, veal, or combination; minimally processed | ≤0.4 (28.6); combination control | DASH; protein source and some other food provided with dietary counseling; 24-h urinary electrolyte excretion and food records | 14; 2-arm parallel | 95 normal-hypertensive postmenopausal women; 59.2; 29.6 |
| Liao, 2007 (27) | 1.5 (105); N/A; N/A | 0; soy | Weight-loss diet (1200 kcal); partial food provided for soy group but none for intervention group with dietary counseling and education; N/A | 8; 2-arm parallel | 30 generally healthy men and women; 33.4; 29.8 |
| Navas-Carretero, 2009 (46) | 1.6 (113); red meat; N/A | 0.3 (22.3); oily fish | Habitual diet; daily 24-h dietary recalls and monthly 72-h detailed intake report; daily menu forms and weekly interviews | 8; 2-phase crossover | 25 iron-deficient women; 18–30; 22.1 |
| Sayer, 2015 (28) | 1.7 (121); pork tenderloin, uncured ham, beef tenderloin; minimally processed | 0.2 (10.7); chicken or fish | DASH; protein source provided; daily food logs and 24-h urinary urea nitrogen | 6; 2-phase crossover | 19 prehypertensive or hypertensive men and women; 61; 31.2 |
| Mahon, 2007 (29) | 1.7 (121); cooked beef tenderloin; minimally processed | 0; chicken, carbohydrate, or habitual control | Weight-loss diet (1250 kcal); protein source provided with dietary counseling, written instructions, menus, and shopping lists; biweekly dietary counseling sessions | 9; 4-arm parallel | 43 generally healthy postmenopausal women; 58; 29.6 |
| Flynn, 1981 (30) | 2.0 (140); raw beef; minimally processed | 0; fish or poultry | Habitual diet; protein source provided; daily food logs and 4-d food records | 12; 2-phase crossover | 129 generally healthy men and women; N/A; N/A |
| Flynn, 1982 (31) | 2.0 (140); raw beef or pork; minimally processed | 0; fish or poultry | Habitual diet; protein source provided; 4-d food records | 12; 2-phase crossover | 76 generally healthy men and women; N/A; N/A |
| de Mello, 2006 (32) | 2.0 (141); beef; N/A | 0; chicken or lactoovo low-protein | N/A; no food provided; 2-d weighed food records and 24-h urinary urea nitrogen output | 4; 2-phase crossover | 17 men with type 2 diabetes and macroalbuminuria; 59; 26.2 |
| Ashton, 2000 (33) | 2.1 (150); lean, raw red meat; minimally processed | 0; tofu | N/A; tofu provided, dietary counseling for meat selection; N/A | 4; 2-phase crossover | 42 generally healthy men; 45.8; 26.2 |
| Davidson, 1999 (34) | 2.2 (159); lean beef, veal, pork, or lamb; minimally processed | 21.9; lean white meat (poultry and fish) | National Cholesterol Education Program Step I diet; no food provided, dietary counseling; food logs | 32; 2-arm parallel | 165 hypercholesterolemic men and women; 55.8; 27.3 |
| Wolmarans, 1999 (35) | 2.4 (165); lean beef and lean mutton; minimally processed | 0; chicken or fish | Prudent; pre and post 7-d weighed food records; postquestionnaires to assess compliance | 6; 2-phase crossover | 39 hypercholesterolemic men and women; 33.4; 24.4 |
| O'Brien, 1980 (36) | 2.4 (170); beef, pork, or lamb; N/A | 0; fish or poultry with varying dietary cholesterol prescriptions | Varying cholesterol prescriptions but otherwise habitual diet; N/A; diet records | 6; 4-phase crossover | 29 generally healthy men; 43; N/A |
| Horrocks, 1999 (47) | 2.9 (200); pork; N/A | 0; chicken | N/A; N/A; N/A | 4; 2-phase crossover | 20 generally healthy women; N/A; N/A |
| Foerster, 2014 (37) | 2.9 (200); fresh pork cutlet and beef steak; minimally processed | <0.4 (30); whole-grain products | Habitual diet; protein sources provided; regular check-ins with research staff | 10; 2-phase crossover | 20 generally healthy men and women; 40.1; 24.4 |
| Hodgson, 2006 (38) | 3.1 (215); lean, raw red meat; minimally processed | 0; plant protein | Habitual diet; protein source provided with dietary counseling; pre and post 3-d weighed food diary | 8; 2-arm parallel | 60 hypertensive men and women; 58.7; 27.7 |
| Gascon, 1996 (39) | 3.3 (230); lean beef, pork, veal; minimally processed | 0; lean white fish | American Heart Association prudent diet; partial meals provided; verbal interview every 2 d | ∼4 (1 menstrual cycle); 2-phase crossover | 14 generally healthy women; 22.4; 22 |
| Wolmarans, 1991 (40) | 3.5 (246); beef and mutton; N/A | 0; fatty fish | Habitual diet; N/A ; pre and post 7-d dietary records | 6; 2-phase crossover | 28 generally healthy men and women; 33.8; N/A |
| Haub, 2005 (41) | 3.5 (248); cube steak, ground beef, beef tips; minimally processed | 0; plant protein | Habitual diet; protein source provided; routine interviews to assess compliance | 12; 2-arm parallel | 21 generally healthy men; 65.0; 28.2 |
| Wiebe, 1984 (44) | 3.6 (250); frozen beef patties; minimally processed | 0; plant protein | Controlled but not specified; food provided; N/A | 3; 2-phase crossover | 8 generally healthy men; 20.9, 21.7 |
| Beauchesne-Rondeau, 2003 (42) | 5.4 (380); lean ground beef, exterior round, sirloin top; minimally processed | 0; lean fish or poultry | American Heart Association diet; partial food provided; N/A | 3; 3-phase crossover | 17 hypercholesterolemic men; 50.1; 26.5 |
| Sinclair, 1987 (43) | 7.1 (500); kangaroo; N/A | 0; southern fatty fish, tropical fatty fish, or plant protein | Low-fat (<7% of total energy); protein source provided with dietary counseling; daily food records | 2; 4-phase crossover | 13 generally healthy men and women; 31.3; 21.2 |
DASH, Dietary Approaches to Stop Hypertension; lactoovo, lacto-ovovegetarian; N/A, not applicable; post, postintervention; pre, preintervention.
Quality and bias of selected studies
Due to clear reporting of randomization methods, we deemed 5 studies at low risk of selection bias (24, 25, 29, 38, 46). Researchers disclosed allocation concealment methods in 2 studies (24, 25), but the rest were unclear about allocation methods. Three studies were at low risk of performance bias [2 investigator-blinded studies (34, 38) and 1 double-blind study (45)] but the rest did not report blinding. Detection bias was unclear in all of the studies except for 3 that were blinded for outcome assessment (25, 34, 38) (see Supplemental Table 1). In 16 articles, the researchers provided food to the subjects (mainly protein-rich foods) (24, 26–29, 31, 33, 37–45), but the rest did not provide food or did not specify if they provided food to the subjects. Researchers assessed dietary compliance in numerous ways, which are shown in Table 3, including dietary counseling, interviews, or questionnaires (24–27, 33–35, 37, 39, 41, 43, 46); food records, logs, or menus (26, 28–32, 34–36, 38, 40, 43, 46); and/or urinary markers such as urinary 3-methyl histidine (45), urinary electrolyte excretion (26), and 24-h urinary urea nitrogen output (28, 32). Most studies showed the use of >1 of these methods of dietary compliance.
Results of statistical analyses
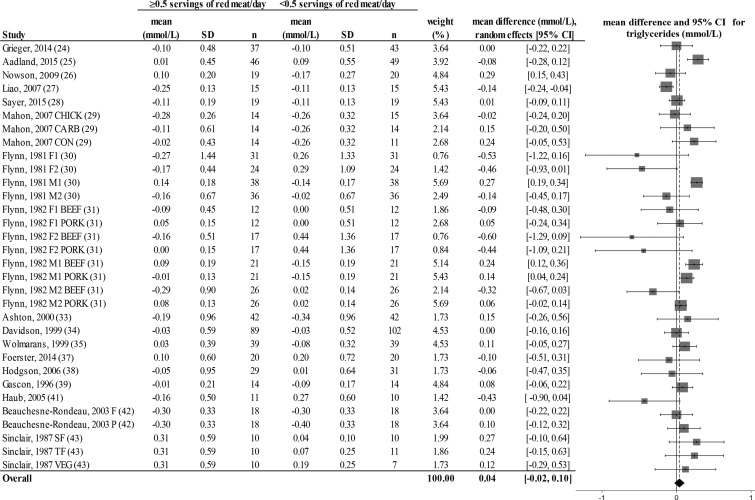
There was a decrease from pre- to postintervention values of TC, LDL cholesterol, HDL cholesterol, TC:HDL, triglycerides, and DBP but not SBP in both groups (repeated-measures ANOVA). The results showed no differences in postintervention values between the groups who consumed ≥0.5 or <0.5 servings of total red meat/d for any of the dependent variables (2-factor nested ANOVA mixed-effects model; P > 0.05 for all variables; see Table 4). Our analysis of the change values suggested no difference in responses over time between the groups who consumed ≥0.5 or <0.5 servings of total red meat/d in TC, LDL cholesterol, HDL cholesterol, TC:HDL, triglycerides, SBP, or DBP (fixed- or random-effects model; see Figures 2–8). There was no indication that consumption of progressively higher red meat amounts influenced these CVD risk factors (see Figures 2–8; the amount of red meat consumed progressively increases from top to bottom of each figure). Results from imputing SDs of crossover designs with 0.99 as the correlational factor did not differ from the original results with the use of 0 as the correlational factor.
TABLE 4.
Analysis of postintervention values of consuming ≥0.5 or <0.5 servings of total red meat/d in randomized controlled trials1
| Dependent variable2 | Number of studies included | ≥0.5 servings of total red meat/d | <0.5 servings of total red meat/d | P |
| Total cholesterol, mmol/L | 22 | 4.93 ± 0.11 | 4.88 ± 0.10 | 0.57 |
| LDL cholesterol, mmol/L | 21 | 3.18 ± 0.08 | 3.13 ± 0.07 | 0.52 |
| HDL cholesterol, mmol/L | 21 | 1.30 ± 0.04 | 1.27 ± 0.03 | 0.41 |
| Triglycerides, mmol/L | 20 | 1.23 ± 0.05 | 1.21 ± 0.05 | 0.83 |
| TC:HDL | 19 | 3.93± 0.07 | 3.98 ± 0.07 | 0.46 |
| Systolic blood pressure, mm Hg | 7 | 121± 10 | 122 ± 11 | 0.51 |
| Diastolic blood pressure, mm Hg | 7 | 64 ± 4 | 63 ± 5 | 0.55 |
Unless otherwise indicated, values are least-squares means ± SEs adjusted for baseline values, age, sex, BMI, length of intervention, and whether energy restriction was or was not included in the protocol. A 2-factor nested ANOVA showed no differences between post values of consuming ≥0.5 or <0.5 servings of total red meat/d. Total cholesterol, LDL-cholesterol, and HDL-cholesterol conversion: mmol/L × 38.67 = mg/dL; triglyceride conversion: mmol/L × 88.57 = mg/dL. TC:HDL, ratio of total cholesterol to HDL cholesterol.
A repeated-measures ANOVA showed that all dependent variables changed over time except for systolic blood pressure (P < 0.05).
More than 99% of the traditional sensitivity analyses showed no significant change in results. No cluster sensitivity analyses significantly changed results when we removed studies that included weight-loss diets, heart-healthy diets, significant weight loss, diseased populations, consumption of processed red meats or no specification of the degree of meat processing, and studies that used different amounts of protein intake in the control and intervention group/phase. Post hoc analyses of red meat consumption amounts showed no differences in change values between the control and intervention group, whether consuming 1.0–1.9, 2.0–2.9, or ≥3.0 servings of red meat/d, with the exception that HDL cholesterol was higher when ≥3.0 servings of red meat/d was consumed (weighted mean difference: 0.10; 95% CI: 0.05, 0.16). (See Supplemental Table 2 for results of all sensitivity analyses.)
DISCUSSION
To the best of our knowledge, this is the first systematically searched meta-analysis to assess the consumption of ≥0.5 servings of total red meat/d on blood lipids, lipoproteins, and blood pressures by using data from RCTs. This serving size is consistent with the dietary patterns recommended by the 2010–2015 DGA and the Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Our results indicate that the consumption of ≥0.5 servings of total red meat/d does not influence these clinically relevant and commonly measured modifiable CVD risk factors. These results do not support our hypothesis, which was based on a 2012 observational cohort study that estimated that the consumption of ≥0.5 servings of total red meat/d would increase CVD mortality (5). Our results align with a previous meta-analysis of 8 studies, which concluded that changes in blood lipids and lipoproteins did not differ when lean, unprocessed beef was consumed compared with poultry or fish (9). Our meta-analysis of 24 studies is more generalizable because it was inclusive of a variety of red meat types and also assessed blood pressure. It is important to emphasize that our conclusions do not support a cardioprotective effect of higher red meat consumption, such as is shown with fatty fish (48), but that the consumption of ≥0.5 servings of total red meat/d does not affect changes in blood lipids, lipoproteins, and blood pressures.
Although the median daily total red meat intake in the intervention group or phase was 2 servings, almost double what the average American consumes [∼1.2 servings/d (49)], the range was large (1.0–7.1 servings/d). There is no visual threshold of total red meat consumption that indicates an apparent negative effect on blood lipids, lipoproteins, and blood pressures, as shown by the nondescript dispersal of the data in Figures 2–8. Although we used the cutoff of 0.5 servings of total red meat/d (5), we performed post hoc analyses to test if the studies with lower red meat consumption were washing out the effects of higher red meat consumption. The highest category of red meat consumption (>3 servings of red meat/d) showed no negative effects on blood lipid and lipoprotein concentrations and blood pressures and resulted in higher HDL concentrations. Because substituting protein for carbohydrate and adopting a “heart healthy” diet are shown to improve blood lipid and lipoprotein concentrations and blood pressure (50–53), we performed cluster sensitivity analyses to assess studies without these characteristics. This did not influence our conclusion that consuming ≥0.5 servings of red meat/d does not affect changes in blood lipid and lipoprotein concentrations and blood pressures. Therefore, this meta-analysis compared protein sources rather than macronutrient compositions within the context of a variety of diets.
The Mediterranean-style and the DASH (Dietary Approaches to Stop Hypertension) dietary patterns are “heart healthy” diets that include <0.5 servings of red meat/d. The Mediterranean-style dietary pattern is predominantly modeled on observational cohort studies (54–57) and 1 large-scale RCT (58) that indicate a lower incidence of CVD-related events, mortality, and lower CVD risk with the consumption of this dietary pattern. However, these studies reported red meat consumption of >0.5 servings of red meat/d [range: ∼2–3.5 servings/1000 kcal; see Figure D1.59 in the Scientific Report of the 2015 Dietary Guidelines Advisory Committee (59) for a graphic summary of these studies, with the exception of our reference 57]. Therefore, it is unclear what studies are supportive of this recommendation for red meat in the context of a Mediterranean-style diet. The DASH diet, by design, limits red meat consumption to <0.5 servings/d (60). However, current RCTs showed that the DASH diet has equivalent effectiveness to reduce blood lipids, lipoproteins, and blood pressures when it contains >0.5 servings of red meat/d [1.6 or 2.2 servings of beef (61, 62) or 1.7 servings of pork (28) daily]. Collectively, these studies suggest that the consumption of >0.5 servings of red meat/d in the context of these recommended dietary patterns does not hinder improvements in CVD risk factors.
The conflicting literature creates ambiguous conclusions in dietary guidance pertaining to red meat consumption amounts. The Scientific Report of the 2015 Dietary Guidelines Advisory Committee concluded that “lean meats” can be incorporated into a healthy diet in relatively small amounts, but there is no specificity to the type or amount of lean meat. Communication to the general public from the 2015–2020 DGA combines red meat with the “meat, eggs, and poultry” recommendation rather than its own food group (12), as done in previous DGAs (63). Dietary recommendations based on the 2010–2015 DGA, with support from the 2015 Advisory Report, suggest that red meat consumption should be limited to ∼0.5–0.7 servings/d or ∼3.5–5 servings/wk (59, 63); this varies because the serving size range is 2–3 ounces. The Dietary Guidelines Advisory Committee search process has strict criteria that limit the inclusion of data from available RCTs (64), so this conclusion is based predominantly on epidemiologic associations (63). This restricts the conclusions to be mainly based on associative conclusions of morbidity and mortality rather than cause and effect of disease risk, both of which need to be considered in determining dietary guidance and public policy.
A strength of this systematically searched meta-analysis is the use of RCT designs, which allows our conclusions to be based on the principle of causation. These RCTs assessed the effects of consumption of total red meat on CVD risk factors for relatively short periods of time (2–32 wk). In contrast, epidemiologic studies have assessed the association between total red meat consumption and CVD-related morbidity and mortality that typically require years or decades of follow-up and are not suitable to determine causality. Thus, results from RCTs support that the consumption of red meat does not influence CVD risk factors, whereas epidemiologic studies support that the consumption of red meat is associated with higher incidences of CVD-related morbidity and mortality. Future efforts and research by academic, industry, and government leaders are needed to improve the scientific foundation and communication to the public about the effects of red meat on diet quality and human health by including evidence from both types of study designs.
Another strength of this meta-analysis is the high external validity because we did not restrict our search to certain dietary patterns, populations, or types of red meat (65). Although this created heterogeneity among data within each blood lipid and lipoprotein variable (indicated by the I2 scores; see Figures 2–6), the extensive sensitivity analyses did not affect overall findings when potential modifiers were excluded. Data from other CVD risk factors, such as endothelial cell function and inflammation, were not collected for this meta-analysis. These factors can progress to CVD when traditional risk factors are unchanged (66) and therefore may be a limitation of this analysis. We did not exclude studies based on the criteria used by the Dietary Guidelines Advisory Committee (64) and recognize that a meta-analysis is only as strong as the empirical evidence included. We raise concern about the unclear bias reporting, which was common in the studies included in this meta-analysis, and urge researchers to comprehensively report study design characteristics. We are also aware that there are other potential human and environmental health risks associated with higher red meat intake, which are beyond the scope of this review, and include but are not limited to cancer (67) and environmental sustainability (68, 69).
FIGURE 6.
Random-effects model meta-analysis for changes in blood triglyceride concentrations from randomized controlled trials comparing ≥0.5 or <0.5 servings of total red meat/d. Heterogeneity: τ2 = 0.017, χ2 = 3.16, df = 31 (P = 0.001), I2 = 68%. Data are shown in descending order from smallest to largest amounts of red meat consumed by the intervention group or phase. CARB, carbohydrate control diet; CHICK, chicken control diet; CON, habitual control diet; F, lean fish control diet; F1, first female group; F1 BEEF, first female group consuming beef diet; F1 PORK, first female group consuming pork diet; F2, second female group; F2 BEEF, second female group consuming beef diet; F2 PORK, second female group consuming pork diet; M1, first male group; M1 BEEF, first male group consuming beef diet; M1 PORK, first male group consuming pork diet; M2, second male group; M2 BEEF, second male group consuming beef diet; M2 PORK, second male group consuming pork diet; P, poultry control diet; SF, southern fish control diet; TF, tropical fish control diet; VEG, vegetarian control diet.
In conclusion, the results from this systematically searched meta-analysis of RCTs support that the consumption of ≥0.5 compared with <0.5 servings of total red meat/d does not influence blood lipids, lipoproteins, and/or blood pressures, which are clinically relevant CVD risk factors. These results are generalizable across a variety of populations, dietary patterns, and types of red meat. These results are inconsistent with much of the observational evidence related to red meat consumption and CVD, which prompts the need for future research to reconcile the apparent disconnect between RCT and observation-based conclusions.
Acknowledgments
Vicki J Killion, an associate professor of Library Science from Purdue’s Health and Life Sciences Library Division, assisted LEO and JEK with database and search term selection. Ningning Chen, a statistical consultant from Purdue’s Department of Statistics, assisted LEO and JEK with the analyses. Jia Lia, a PhD student from Purdue’s Department of Nutrition Science, assisted with calculations.
The authors’ responsibilities were as follows—LEO, JEK, and WWC: designed the research; LEO and JEK: conducted the research; LEO: analyzed the data; and LEO and WWC: wrote the manuscript and have primary responsibility for the final content. During the time this manuscript was being developed and written, WWC received research support from American Egg Board–Egg Nutrition Center, Beef Checkoff, Coca-Cola Foundation, National Dairy Council, National Institutes of Health, Pork Checkoff, and USDA and had a consulting arrangement with Coca-Cola Company. None of these organizations provided support to conduct this meta-analysis. WWC also served on the 2015 Dietary Guidelines Advisory Committee and was a member of the Advisory Council on Nutrition and Healthy Food Choices, Foundation for Food and Agriculture Research. JEK received support from the American Egg Board–Egg Nutrition Center. LEO reported no conflicts of interest.
Footnotes
Abbreviations used: CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; DGA, Dietary Guidelines for Americans; RCT, randomized controlled trial; SBP, systolic blood pressure; TC, total cholesterol; TC:HDL, ratio of total cholesterol to HDL cholesterol.
REFERENCES
- 1.CDC. Heart disease facts. Version current 10 August 2015 [cited 2016 May 9]. Available from: http://www.cdc.gov/heartdisease/facts.htm.
- 2.Kaluza J, Wolk A, Larsson SC. Red meat consumption and risk of stroke: a meta-analysis of prospective studies. Stroke 2012;43:2556–60. [DOI] [PubMed] [Google Scholar]
- 3.Chen GC, Lv DB, Pang Z, Liu QF. Red and processed meat consumption and risk of stroke: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 2013;67:91–5. [DOI] [PubMed] [Google Scholar]
- 4.Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr 2014;112:762–75. [DOI] [PubMed] [Google Scholar]
- 5.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr 2016;19:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence. Curr Atheroscler Rep 2012;14:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maki KC, Van Elswyk ME, Alexander DD, Rains TM, Sohn EL, McNeill S. A meta-analysis of randomized controlled trials that compare the lipid effects of beef versus poultry and/or fish consumption. J Clin Lipidol 2012;6:352–61. [DOI] [PubMed] [Google Scholar]
- 10.Dietary Guidelines Advisory Committee. 2015 Dietary Guidelines Advisory Committee Nutrition Evidence Library methodology. USDA [cited 2016 Oct 1]. Available from: http://www.nel.gov/topic.cfm?cat=3381%202016. (accessed 20 September 2016).
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–41. [DOI] [PubMed] [Google Scholar]
- 12.USDA, US Department of Health and Human Services. 2015-2020 Dietary guidelines for Americans. 8th ed. Washington (DC): US Government Printing Office; 2015. [Google Scholar]
- 13.World Cancer Research Fund; American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington (DC): American Institute for Cancer Research; 2007. [Google Scholar]
- 14.American Heart Association. Meat, poultry and fish. Version current 2016 [cited 2016 Jul 7]. Available from: http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/Nutrition/Meat-Poultry-and-Fish_UCM_306002_Article.jsp#.V37iHmNMLww.
- 15.Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M. Screening and treatment of subclinical hypothyroidism or hyperthyroidism. Rockville (MD): Agency for Healthcare Research and Quality; 2011. Report No.: 11(12)-EHC033-EF. [PubMed] [Google Scholar]
- 16.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Chapter 8: Assessing risk of bias in included studies [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2016 Sep 20]. Available from: www.cochrane-handbook.org.
- 18.DeCoster J. Meta-analysis notes. Tuscaloosa (AL): University of Alabama, Department of Psycology; 2004. [Google Scholar]
- 19.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Chapter 16.4.5: Methods for incorporating cross-over trials into a meta-analysis [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2016 Sep 20]. Available from: http://handbook.cochrane.org/chapter_16/16_4_5_methods_for_incorporating_cross_over_trials_into_a.htm.
- 20.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Chapter 16.4.6.1: Mean differences [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2016 Sep 20]. Available from: http://handbook.cochrane.org/chapter_16/16_4_6_1_mean_differences_a.htm.
- 21.Bland JM, Altman DG. Best (but oft forgotten) practices: testing for treatment effects in randomized trials by separate analyses of changes from baseline in each group is a misleading approach. Am J Clin Nutr 2015;102:991–4. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- 23.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96:1281–98. [DOI] [PubMed] [Google Scholar]
- 24.Grieger JA, Miller MD, Cobiac L. Investigation of the effects of a high fish diet on inflammatory cytokines, blood pressure, and lipids in healthy older Australians. Food Nutr Res 2014;58:20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aadland EK, Lavigne C, Graff IE, Eng O, Paquette M, Holthe A, Mellgren G, Jacques H, Liaset B. Lean-seafood intake reduces cardiovascular lipid risk factors in healthy subjects: results from a randomized controlled trial with a crossover design. Am J Clin Nutr 2015;102:582–92. [DOI] [PubMed] [Google Scholar]
- 26.Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium Dietary Approaches to Stop Hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res 2009;29:8–18. [DOI] [PubMed] [Google Scholar]
- 27.Liao FH, Shieh MJ, Yang SC, Lin SH, Chien YW. Effectiveness of a soy-based compared with a traditional low-calorie diet on weight loss and lipid levels in overweight adults. Nutrition 2007;23:551–6. [DOI] [PubMed] [Google Scholar]
- 28.Sayer RD, Wright AJ, Chen N, Campbell WW. Dietary Approaches to Stop Hypertension diet retains effectiveness to reduce blood pressure when lean pork is substituted for chicken and fish as the predominant source of protein. Am J Clin Nutr 2015;102:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahon AK, Flynn MG, Stewart LK, McFarlin BK, Iglay HB, Mattes RD, Lyle RM, Considine RV, Campbell WW. Protein intake during energy restriction: effects on body composition and markers of metabolic and cardiovascular health in postmenopausal women. J Am Coll Nutr 2007;26:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flynn MA, Heine B, Nolph GB, Naumann HD, Parisi E, Ball D, Krause G, Ellersieck M, Ward SS. Serum lipids in humans fed diets containing beef or fish and poultry. Am J Clin Nutr 1981;34:2734–41. [DOI] [PubMed] [Google Scholar]
- 31.Flynn MA, Naumann HD, Nolph GB, Krause G, Ellersieck M. Dietary “meats” and serum lipids. Am J Clin Nutr 1982;35:935–42. [DOI] [PubMed] [Google Scholar]
- 32.de Mello VD, Zelmanovitz T, Perassolo MS, Azevedo MJ, Gross JL. Withdrawal of red meat from the usual diet reduces albuminuria and improves serum fatty acid profile in type 2 diabetes patients with macroalbuminuria. Am J Clin Nutr 2006;83:1032–8. [DOI] [PubMed] [Google Scholar]
- 33.Ashton E, Ball M. Effects of soy as tofu vs meat on lipoprotein concentrations. Eur J Clin Nutr 2000;54:14–9. [DOI] [PubMed] [Google Scholar]
- 34.Davidson MH, Hunninghake D, Maki KC, Kwiterovich PO Jr, Kafonek S. Comparison of the effects of lean red meat vs lean white meat on serum lipid levels among free-living persons with hypercholesterolemia: a long-term, randomized clinical trial. Arch Intern Med 1999;159:1331–8. [DOI] [PubMed] [Google Scholar]
- 35.Wolmarans P, Laubscher JA, van der Merwe S, Kriek JA, Lombard CJ, Marais M, Vorster HH, Tichelaar HY, Dhansay MA, Benade AJ. Effects of a prudent diet containing either lean beef and mutton or fish and skinless chicken on the plasma lipoproteins and fatty acid composition of triacylglycerol and cholesteryl ester of hypercholesterolemic subjects. J Nutr Biochem 1999;10:598–608. [DOI] [PubMed] [Google Scholar]
- 36.O’Brien BC, Reiser R. Human plasma lipid responses to red meat, poultry, fish, and eggs. Am J Clin Nutr 1980;33:2573–80. [DOI] [PubMed] [Google Scholar]
- 37.Foerster J, Maskarinec G, Reichardt N, Tett A, Narbad A, Blaut M, Boeing H. The influence of whole grain products and red meat on intestinal microbiota composition in normal weight adults: a randomized crossover intervention trial. PLoS One 2014;9:e109606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodgson JM, Burke V, Beilin LJ, Puddey IB. Partial substitution of carbohydrate intake with protein intake from lean red meat lowers blood pressure in hypertensive persons. Am J Clin Nutr 2006;83:780–7. [DOI] [PubMed] [Google Scholar]
- 39.Gascon A, Jacques H, Moorjani S, Deshaies Y, Brun LD, Julien P. Plasma lipoprotein profile and lipolytic activities in response to the substitution of lean white fish for other animal protein sources in premenopausal women. Am J Clin Nutr 1996;63:315–21. [DOI] [PubMed] [Google Scholar]
- 40.Wolmarans P, Benade AJ, Kotze TJ, Daubitzer AK, Marais MP, Laubscher R. Plasma lipoprotein response to substituting fish for red meat in the diet. Am J Clin Nutr 1991;53:1171–6. [DOI] [PubMed] [Google Scholar]
- 41.Haub MD, Wells AM, Campbell WW. Beef and soy-based food supplements differentially affect serum lipoprotein-lipid profiles because of changes in carbohydrate intake and novel nutrient intake ratios in older men who resistive-train. Metabolism 2005;54:769–74. [DOI] [PubMed] [Google Scholar]
- 42.Beauchesne-Rondeau E, Gascon A, Bergeron J, Jacques H. Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. Am J Clin Nutr 2003;77:587–93. [DOI] [PubMed] [Google Scholar]
- 43.Sinclair AJ, O’Dea K, Dunstan G, Ireland PD, Niall M. Effects on plasma lipids and fatty acid composition of very low fat diets enriched with fish or kangaroo meat. Lipids 1987;22:523–9. [DOI] [PubMed] [Google Scholar]
- 44.Wiebe SL, Bruce VM, McDonald BE. A comparison of the effect of diets containing beef protein and plant proteins on blood lipids of healthy young men. Am J Clin Nutr 1984;40:982–9. [DOI] [PubMed] [Google Scholar]
- 45.Prescott SL, Jenner DA, Beilin LJ, Margetts BM, Vandongen R. A randomized controlled trial of the effect on blood pressure of dietary non-meat protein versus meat protein in normotensive omnivores. Clin Sci (Lond) 1988;74:665–72. [DOI] [PubMed] [Google Scholar]
- 46.Navas-Carretero S, Pérez-Granados AM, Schoppen S, Vaquero MP. An oily fish diet increases insulin sensitivity compared to a red meat diet in young iron-deficient women. Br J Nutr 2009;102:546–53. [DOI] [PubMed] [Google Scholar]
- 47.Horrocks LA, Yeo YK. Docosahexaenoic acid-enriched foods: production and effects on blood lipids. Lipids 1999;34(Suppl):S313. [DOI] [PubMed] [Google Scholar]
- 48.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57. [DOI] [PubMed] [Google Scholar]
- 49.Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr 2011;14:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. . A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 51.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER III, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. . Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 52.Rees K, Hartley L, Flowers N, Clarke A, Hooper L, Thorogood M, Stranges S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013(8):CD009825. [DOI] [PubMed] [Google Scholar]
- 53.Sacks FM, Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. . A dietary approach to prevent hypertension: a review of the Dietary Approaches to Stop Hypertension (DASH) Study. Clin Cardiol 1999;22(7 Suppl):III6–10. [DOI] [PubMed] [Google Scholar]
- 54.Buckland G, Agudo A, Travier N, Huerta JM, Cirera L, Tormo MJ, Navarro C, Chirlaque MD, Moreno-Iribas C, Ardanaz E, et al. . Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br J Nutr 2011;106:1581–91. [DOI] [PubMed] [Google Scholar]
- 55.Martínez-González MA, Garcia-Lopez M, Bes-Rastrollo M, Toledo E, Martinez-Lapiscina EH, Delgado-Rodriguez M, Vazquez Z, Benito S, Beunza JJ. Mediterranean diet and the incidence of cardiovascular disease: a Spanish cohort. Nutr Metab Cardiovasc Dis 2011;21:237–44. [DOI] [PubMed] [Google Scholar]
- 56.Núñez-Córdoba JM, Valencia-Serrano F, Toledo E, Alonso A, Martinez-Gonzalez MA. The Mediterranean diet and incidence of hypertension: the Seguimiento Universidad de Navarra (SUN) Study. Am J Epidemiol 2009;169:339–46. [DOI] [PubMed] [Google Scholar]
- 57.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr 2014;17:2769–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 59.US Department of Agriculture and Department of Health and Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee. February 2015. [cited 2016 May 28]. Available from: https://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. [DOI] [PMC free article] [PubMed]
- 60.Karanja NM, Obarzanek E, Lin PH, McCullough ML, Phillips KM, Swain JF, Champagne CM, Hoben KP. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc 1999;99(8 Suppl):S19–27. [DOI] [PubMed] [Google Scholar]
- 61.Roussell MA, Hill AM, Gaugler TL, West SG, Ulbrecht JS, Vanden Heuvel JP, Gillies PJ, Kris-Etherton PM. Effects of a DASH-like diet containing lean beef on vascular health. J Hum Hypertens 2014;28:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roussell MA, Hill AM, Gaugler TL, West SG, Vanden Heuvel JP, Alaupovic P, Gillies PJ, Kris-Etherton PM. Beef in an optimal lean diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr 2012;95:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.USDA, US Department of Health and Human Services. 2010-2015 Dietary guidelines for Americans. 7th ed. Washington (DC): US Government Printing Office; 2010. [Google Scholar]
- 64.2015 Dietary Guidelines Advisory Committee Nutrition Evidence Library methodology. Literature search, screen, and select studies to review. Version current 30 January 2015 [cited 2016 Jul 18]. Available from: http://www.nel.gov/topic.cfm?cat=3383.
- 65.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Table 5.6.a: Some advantages and disadvantages of broad versus narrow review questions [updated March 2011]. The Cochrane Collaboration; 2011. [cited 2016 Sep 20]. Available from: http://handbook.cochrane.org/chapter_5/table_5_6_a_some_advantages_and_disadvantages_of_broad_versus.htm.
- 66.Foo SY, Heller ER, Wykrzykowska J, Sullivan CJ, Manning-Tobin JJ, Moore KJ, Gerszten RE, Rosenzweig A. Vascular effects of a low-carbohydrate high-protein diet. Proc Natl Acad Sci USA 2009;106:15418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol 2015;16:1599–600. [DOI] [PubMed] [Google Scholar]
- 68.de Carvalho AM, Cesar CL, Fisberg RM, Marchioni DM. Excessive meat consumption in Brazil: diet quality and environmental impacts. Public Health Nutr 2013;16:1893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kingston-Smith AH, Edwards JE, Huws SA, Kim EJ, Abberton M. Plant-based strategies towards minimizing ‘livestock’s long shadow’. Proc Nutr Soc 2010;69:613–20. [DOI] [PubMed] [Google Scholar]