IgA pemphigus is a rare variant of pemphigus presented clinically with vesiculopustular skin lesions, and histopathologically neutrophil infiltration and acantholysis in the epidermis. Moreover, circulating IgA autoantibodies target cell surface components of the epidermis.
According to clinical, histopathological and direct immunofluorescence features, IgA pemphigus is subdivided into subcorneal pustular dermatosis (SPD) type reactive with desmocollin 1 (Dsc1) [1] and intraepidermal neutrophilic IgA dermatosis (IEN) type reactive with an unknown antigen. However, in some reported cases of IEN type IgA pemphigus, the IgA anti-cell surface autoantibodies reacted with desmoglein 1 (Dsg1) or Dsg3 [2, 3], which are widely accepted to be the target antigens in pemphigus foliaceus and pemphigus vulgaris, respectively.
Here, we present a case of IgA pemphigus with histopathological features typical of the SPD type and immunological characteristics of the IEN type with anti-Dsg3 antibodies, and a review of the literature.
An 87-year-old male of Polish origin presented erosions in the oral mucosa and in intertriginous areas. The patient had numerous internal disorders, including ischemic cardiomyopathy, type 2 diabetes, abdominal aortic aneurysm, partial stomach resection because of peptic ulcer disease, colitis, diverticular disease, iron deficiency anemia, hemorrhoids and prostatic hypertrophy, and took torasemide, finasteride, acetylsalicylic acid, pantoprazole, ramipril and nebivolol.
The patient was initially treated in the Department of Internal Medicine with topical antibiotics and topical glucocorticosteroids with no improvement. Oral prednisolone in a dose of 10 mg/day for 2 weeks was not effective, either. The unsuccessful treatment and polymorphic skin lesions led to the hypothesis of paraneoplastic disorder. Therefore, abdominal ultrasonography, gastroscopy and chest X-ray were performed and showed no abnormalities. However, the fecal blood test was positive in the patient and colonoscopy evaluation revealed colitis. Histopathological evaluation of the patient skin indicated pyodermitis, whereas the results of direct and indirect immunofluorescence were negative.
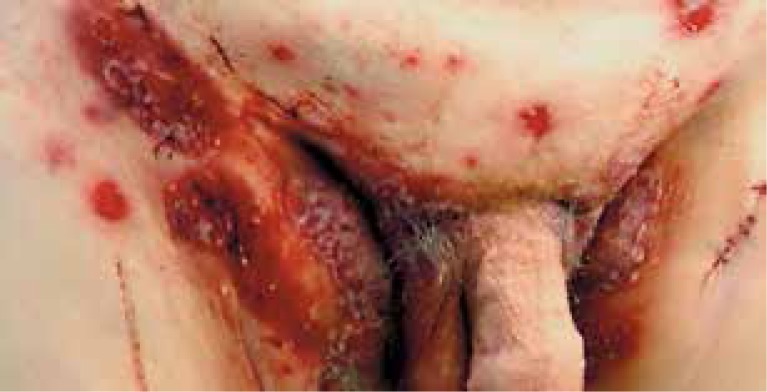
Three months later, the patient was referred to the Department of Dermatology. Physical examination revealed annular erythematous skin lesions with erosions and pustules on the abdomen, groins, buttocks and right armpit. Groins were most severely affected (Figure 1). Erosive lesions of the oral mucosae in the anamnesis were already healed at that time. Laboratory examinations showed microcytic anemia, elevated C-reactive protein (40.91 mg/l; normal < 5 mg/l), low level of sodium (133.8 mmol/l; normal 136 mmol/l) and extremely lowered serum albumins (1.93 g/dl; normal 3.5 g/dl).
Figure 1.
Annular erythematous skin lesions with erosions and pustules within groins
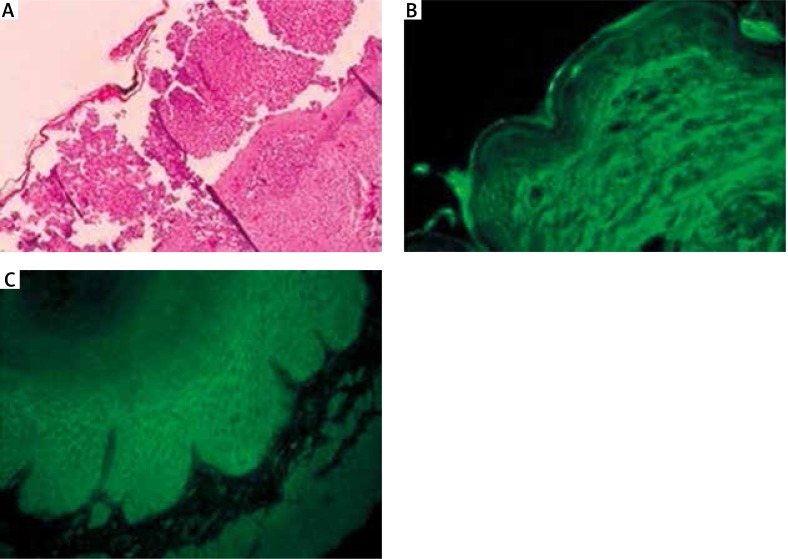
Histopathology of skin lesion revealed extensive neutrophilic pustules in the upper part of the epidermis, with minimum pustules in the middle epidermis (Figure 2 A). Direct immunofluorescence of perilesional skin disclosed IgA deposits on cell surfaces of the entire epidermis (Figure 2 B). Indirect immunofluorescence of monkey esophagus showed IgA, but not IgG, anti-cell surface autoantibodies at a titer of 1 : 160 (Figure 2 C).
Figure 2.
A – Histopathology of the skin lesion. Extensive neutrophilic pustules in the upper part of the epidermis and minimum pustules in the middle epidermis (hematoxylin and eosin stain), B – direct immunofluorescence of perilesional skin. IgA deposits on cell surfaces of the entire epidermis; C – indirect immunofluorescence of monkey esophagus. IgA anti-cell surface autoantibodies
Then, to define the subtype of IgA pemphigus, we performed further immunological studies. Immunoblotting of normal human epidermal extract showed negative results for both IgG and IgA antibodies. IgA ELISAs of recombinant baculoprotein (RPs) of Dsgs revealed positive reactivity with Dsg3 (optical density (OD) = 1.063, cut-off > 0.15), but negative reactivity with Dsg1 (OD = 0.006, cut-off > 0.15). IgG ELISA of Dsg1 and Dsg3 showed negative results. Novel ELISAs of mammalian RPs of Dsc1-3 showed negative results for both IgG and IgA antibodies.
Dapsone of 100 mg/day led to a significant improvement within few days. Although the dosage was reduced to 50 mg/day because of methemoglobinemia (0.50% – 1.20% – 1.80%) and concomitant internal disorders. The patient became asymptomatic in a few weeks.
IgA pemphigus is a heterogeneous variant of pemphigus and must be differentiated from other blistering diseases including dermatitis herpetiformis, pemphigus herpetiformis, pemphigus foliaceus, pemphigus erythematosus, pemphigus vulgaris, pemphigus vegetans, paraneoplastic pemphigus or linear IgA bullous dermatosis. Particularly, IEN type typically shows greater clinical, histopathological and immunological heterogeneity than SPD type. Moreover, discrepancies between clinical, histopathological and immunological features made it difficult to finally establish the subtype of pemphigus IgA in some cases, including our patient.
So far there have been described only four cases fulfilling histological features typical of SPD and immunological ones typical of IEN (Table 1). All of them clinically presented vesiculo-pustular eruptions localized in different areas such as the trunk, extremities, scalp and buttocks, whereas our patient showed superficial pustular lesions predominantly on the intertriginous areas, which were rather characteristic of SPD type IgA pemphigus [4–6]. Interestingly, the patient did not present deeper pustular lesions with sunflower-like configurations on the entire body usually observed in IEN type [4, 5]. However, our patient showed oral mucosal lesions that were not described in the four previously reported patients. In general, oral involvement is more frequent in cases of IEN type [3, 7] than SPD type.
Table 1.
Summary of reported cases of IgA pemphigus with histological features typical of SPD and immunological typical of IEN
| Age, gender | Clinical presentation | Histopathology | Immunofluorescence studies | Target antigens | Treatment | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Features | Localization | Dsg1 | Dsg3 | Dsc1 | Dsc2 | Dsc3 | ||||
| 48-year-old man [13] |
|
Entire integument with predilection sites:
|
|
DIF: IgA deposition on keratinocyte cell surfaces throughout all epidermal layers. IIF: negative (normal human skin, monkey esophagus) |
IgA | – | IgA | – | – | Dapsone |
| 48-year-old man [14] |
|
|
|
DIF: negative. IIF: bound anti-cell-surface IgA antibodies throughout the epidermis (normal human skin) |
– | – | – | – | – | Dapsone |
| 64-year-old woman [15] |
|
|
|
DIF: IgA deposits within the intercellular spaces between keratinocytes. IIF: intercellular deposition of IgA at a titer of 1 : 10 (normal human skin and rat tongue) |
Examination not performed |
|
||||
| 35-year-old woman [16] |
|
|
Subcorneal pustule containing numerous neutrophils | DIF: IgA deposits in the intercellular space throughout the epidermis, more intense in superficial layers. IIF: circulating IgA antibodies at a titer of 1 : 160 (normal human skin) |
– | – | – | – | – | Dapsone |
| 87-year-old-man (present case) |
Annular erythematous skin lesions with pustules and erosions |
|
Extensive neutrophilic pustules in the upper part of the epidermis, with minimum pustules in the middle epidermis | DIF: IgA deposits on cell surfaces of the entire epidermis. IIF: IgA autoantibodies against intercellular spaces at a titer of 1 : 160 (monkey esophagus) |
– | IgA | – | – | – | Dapsone |
Histopathology performed in the four cases disclosed neutrophilic infiltrates in the upper epidermis typically of SPD type [4]. However histopathology in our patient showed extensive pustules in the upper epidermis, but minimum pustules were also seen in the middle epidermis.
Direct immunofluorescence performed in the present case disclosed IgA deposits on cell surfaces of the entire epidermis, typically of IEN type [4, 5].
The discrepancy between histopathology features and DIF results encouraged us to perform antigen detection studies showing only ELISA with recombinant Dsg3 positive with circulating IgA antibodies. This result is along with the localization of IgA deposits in lower epidermis. Since our patient presented neutrophilic infiltrates mainly in the upper epidermis, whereas IgA deposits in the entire epidermis, it is likely that circulating IgA antibodies may be directed not only to Dsg3, but also to other antigens which were not detected in our system. Autoantigen detection studies showed negative results or were not performed in 3 of 4 cases. In one case circulating IgA antibodies reacted with Dsg1 and Dsc1 which differed from Dsg3.
In both subtypes of IgA pemphigus dapsone has been proved to be the first choice therapy [6, 8] usually at an effective dose of 100 mg/day. Four previously reported cases and the present case were successfully treated with dapsone alone. However, various types of treatment have also been reported in IgA pemphigus like retinoids [9], systemic corticosteroids at moderate doses alone or in combination with immunosuppressive agents [6]. Rarely, azithromycin [10] or mycophenolate mofetil [11] may be used in intractable cases.
Recently Hashimoto et al. has proposed the term “intercellular IgA dermatosis (IAD)” for IgA pemphigus and classification dividing the disease into 6 subtypes [12]. Diagnosis of SPD-type and IEN-type was established for cases, which showed typical clinical and histopathological features. IgA-pemphigus vegetans (IgA-PVeg) was characterized by specific vegetating lesions with PVeg-like histopathological presentation. If cases could not be categorized to these three subtypes they were determined as unclassified IAD. Finally, diagnoses of IgA-pemphigus foliaceus (IgA-PF) and IgA-pemphigus vulgaris (IgA-PV) were based on immunological reactivity despite clinical and histopathological diagnoses of the 4 IAD subtypes. Cases with positive IgA ELISA results for Dsg1 were diagnosed as IgA-PF and for Dsg3 were diagnosed as IgA-PV. Our patient presented discrepancies as clinical and histopathological features resembled SPD and immunological features were characteristic for IEN, according to the classification presence of anti-Dsg3 antibodies was essential. Eventually we consider our patient as IgA-PV.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Yasuda H, Kobayashi H, Hashimoto T, et al. Subcorneal pustular dermatosis type of IgA pemphigus: demonstration of autoantibodies to desmocollin-1 and clinical review. Br J Dermatol. 2000;143:144–8. doi: 10.1046/j.1365-2133.2000.03604.x. [DOI] [PubMed] [Google Scholar]
- 2.Kárpáti S, Amagai M, Liu WL, et al. Identification of desmoglein 1 as autoantigen in a patient with intraepidermal neutrophilic IgA dermatosis type of IgA pemphigus. Exp Dermatol. 2000;9:224–8. doi: 10.1034/j.1600-0625.2000.009003224.x. [DOI] [PubMed] [Google Scholar]
- 3.Tajima M, Mitsuhashi Y, Irisawa R, et al. IgA pemphigus reacting exclusively to desmoglein 3. Eur J Dermatol. 2010;20:626–9. doi: 10.1684/ejd.2010.1021. [DOI] [PubMed] [Google Scholar]
- 4.Robinson ND, Hashimoto T, Amagai M, Chan LS. The new pemphigus variants. J Am Acad Dermatol. 1999;40:649–71. doi: 10.1016/s0190-9622(99)70145-3. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa T, Hashimoto T, Teraki Y, Ebihara T. The clinical and histopathological spectrum of IgA pemphigus. Clin Exp Dermatol. 1991;16:401–2. doi: 10.1111/j.1365-2230.1991.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 6.Wallach D. Intraepidermal IgA pustulosis. J Am Acad Dermatol. 1992;27:993–1000. doi: 10.1016/0190-9622(92)70301-u. [DOI] [PubMed] [Google Scholar]
- 7.Borradori L, Saada V, Rybojad M, et al. Oral intraepidermal IgA pustulosis and Crohn’s disease. Br J Dermatol. 1992;126:383–6. doi: 10.1111/j.1365-2133.1992.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 8.Raap U, Völker B, Petering H, et al. Successful treatment of subcorneal pustular type of IgA pemphigus. Hautarzt. 2005;56:66–70. doi: 10.1007/s00105-004-0822-4. [DOI] [PubMed] [Google Scholar]
- 9.Gruss C, Zillikens D, Hashimoto T, et al. Rapid response of IgA pemphigus of subcorneal pustular dermatosis type to treatment with isotretinoin. J Am Acad Dermatol. 2000;43:923–6. doi: 10.1067/mjd.2000.104002. [DOI] [PubMed] [Google Scholar]
- 10.Bliziotis I, Rafailidis P, Vergidis P, Falagas ME. Regression of subcorneal pustular dermatosis type of IgA pemphigus lesions with azithromycin. J Infect. 2005;51:E31–4. doi: 10.1016/j.jinf.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Burchardt T, Büchau A, Ruzicka T, Megahed M. IgA pemphigus. Successful treatment with mycophenolate mofetil. Hautarzt. 2004;55:387–9. doi: 10.1007/s00105-004-0713-8. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Teye K, Ishii N. Clinical and immunological studies of 49 cases of various types of intercellular IgA dermatosis and 13 cases of classical subcorneal pustular dermatosis examined at Kurume University. Br J Dermatol. 2016 doi: 10.1111/bjd.14780. in press [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Kopp T, Sitaru C, Pieczkowski F, et al. IgA pemphigus: occurrence of anti-desmocollin 1 and anti-desmoglein 1 antibody reactivity in an individual patient. J Dtsch Dermatol Ges. 2006;4:1045–50. doi: 10.1111/j.1610-0387.2006.06166.x. [DOI] [PubMed] [Google Scholar]
- 14.Iida K, Sueki H, Ohyama B, et al. A unique case of intra-epidermal neutrophilic dermatosis-type IgA pemphigus presenting with subcorneal pustules. Dermatology. 2011;222:15–9. doi: 10.1159/000322839. [DOI] [PubMed] [Google Scholar]
- 15.Kim SC, Won JH, Chung J, Bang DS. IgA pemphigus: report of a case with immunoelectron localization of bound IgA in the skin. J Am Acad Dermatol. 1996;34:852–4. doi: 10.1016/s0190-9622(96)90044-4. [DOI] [PubMed] [Google Scholar]
- 16.Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546–9. doi: 10.1067/mjd.2000.107478. [DOI] [PubMed] [Google Scholar]