Abstract
Exercise has been shown to be the best intervention in the treatment of many diseases. Many of the benefits of exercise are mediated by adaptions induced in skeletal muscle. The peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family of transcriptional coactivators has emerged as being key mediators of the exercise response and is considered to be essential for many of the adaptions seen in skeletal muscle. However, the contribution of the PGC-1s in skeletal muscle has been evaluated by the use of either whole body or congenital skeletal muscle-specific deletion. In these models, PGC-1s were never present, thereby opening the possibility to developmental compensation. Therefore, we generated an inducible muscle-specific deletion of PGC-1α and -1β (iMyo-PGC-1DKO), in which both PGC-1α and -β can be deleted specifically in adult skeletal muscle. These iMyo-PGC-1DKO animals were used to assess the role of both PGC-1α and -1β in adult skeletal muscle and their contribution to the exercise training response. Untrained iMyo-PGC-1DKO animals exhibited a time-dependent decrease in exercise performance 8 wk postdeletion, similar to what was observed in the congenital muscle-specific PGC-1DKOs. However, after 4 wk of voluntary training, the iMyo-PGC-1DKOs exhibited an increase in exercise performance with a similar adaptive response compared with control animals. This increase was associated with an increase in electron transport complex (ETC) expression and activity in the absence of PGC-1α and -1β expression. Taken together these data suggest that PGC-1α and -1β expression are not required for training-induced exercise performance, highlighting the contribution of PGC-1-independent mechanisms.
Keywords: PGC-1, exercise, skeletal muscle, electron transport chain
mitochondrial dysfunction in skeletal muscle is associated with the development of many metabolic diseases (34, 51). This decreased mitochondrial function is associated with decreased exercise performance and exercise intolerance (1, 20). However, exercise has been proven in many cases to be the best medicine in the treatment of many of these metabolic diseases (63). Many of the benefits of exercise are mediated via skeletal muscle’s ability to be an endocrine organ capable of secreting factors to potentiate the benefits of exercise in other organs (71). In addition, exercise is able to induce molecular changes in skeletal muscle including fiber-type switch, increasing mitochondrial biogenesis, angiogenesis, and oxidative capacity (22). Therefore, understanding the mechanisms by which mitochondria function in skeletal muscle is regulated in response to exercise training is critically important.
The three members of the peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family of transcriptional coactivators (PGC-1α, PGC-1β, and PRC) have been identified as key regulators of mitochondrial function (82). PGC-1α, first identified by its ability to interact with the PPARγ transcription factor (57), has emerged as a pivotal regulator of exercise-induced adaptions in skeletal muscle (4). PGC-1α has been shown to be induced in skeletal muscle in response to exercise in both humans and rodents (5, 29, 50, 55, 65, 77). The other PGC-1 family members are less studied in response to exercise in skeletal muscle.
Muscle-specific overexpression of PGC-1α has been shown to increase oxidative capacity and exercise capacity with decreased skeletal muscle fatigability (11, 43). Conversely, congenital loss of function of PGC-1α in skeletal muscle results in a mild decrease in oxidative capacity (31). While PGC-1β has not been reported to directly respond to exercise, overexpression of PGC-1β in skeletal muscle is sufficient to improve exercise capacity (3). Similarly muscle-specific loss of function of PGC-1β reportedly has a negative effect on exercise capacity (25, 85). Recent studies have also reported that mice with congenital deletion of both PGC-1s in skeletal muscle have significant decreased exercise capacity (61, 85). However, the effect of exercise training in the absence of both PGC-1s has not been assessed.
In this current study, we sought to investigate the contribution of PGC-1α and -1β in adult skeletal muscle on training performance by taking advantage of an inducible muscle-specific PGC-1α/β knockout (iMyo-PGC-1DKO). Our results reveal that in the absence of both PGC-1α and -1β in skeletal muscle, these animals are still able to respond to 4 wk of voluntary training. Moreover, these animals are able to increase exercise performance without either PGC-1α or -1β. Taken together these data highlight the presence of exercise-sensitive PGC-1-independent mechanisms in skeletal muscle that are able to increase exercise capacity.
METHODS
Animal studies.
All animal experiments were performed according to procedures approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC). Twelve- to 14-wk-old male and female mice on a C57BL/6 background were used for indicated experiments. To generate inducible muscle-specific PGC-1 double knockout animals (iMyo-PGC-1DKO), PGC-1α floxed mice (44) were crossed with PGC-1β floxed mice (39) to generate doubly PGC-1α/β floxed mice (61), and were intercrossed with the tamoxifen inducible Cre recombinase protein under the control of human skeletal actin promoter (HSA-MerCreMer) (48). To induce deletion, animals were administered tamoxifen in sesame oil (2 mg/day) by intraperitoneal injections for 5 consecutive days as previously described (73).
Exercise studies.
For the treadmill stress test, animals were acclimated to treadmill running for 2 days before endurance stress tests for 5 min at 10 m/min with a 10% grade. Endurance tests were performed at 10 m/min with a 10% grade for 5 min and then subsequently increased by 2 m/min every 2 min subsequently until mice reached exhaustion or a maximum speed of 36 m/min. Time to exhaustion was recorded, and distance, power, and work were calculated as previously described (31, 61). For voluntary training exercise, animals were subjected to 4 wk of in-cage voluntary running wheel endurance exercise. Running performance was measured using an electronic monitoring system with the total distance recorded. Animals were removed from the wheels around Zeitgeber Time (ZT0) on the day of the harvest, and all tissues were collected around ZT6. All mice were housed individually and had access to food and water ad libitum.
Real-time qPCR.
Total RNA was isolated from snap-frozen tissue using the TRIzol method (Invitrogen). Samples for real-time PCR analyses were reverse transcribed (Thermo Fisher), and quantitative real-time PCRs were performed on the cDNAs in the presence of fluorescent dye SYBR green (Bio-Rad). Relative expression levels were determined using the comparative cycle threshold method as previously described (60, 61).
Western blotting analysis.
Total protein was isolated from skeletal muscle using modified RIPA buffer supplemented with protease and phosphatase inhibitors. Protein extracts (~30 µg) were subjected to electrophoresis on 4–20% TGX gels (Bio-Rad) and transferred to nitrocellulose membranes (Bio-Rad) for Western blot analysis. Total OXPHOS Rodent Western blot cocktail antibodies (Thermo Fisher) was used to detect electron transport chain complex protein expression. Bands were detected by chemiluminescence and imaged on a digital imager. Densitometry analysis was performed using ImageJ software.
Electron transport complex activity.
Skeletal muscle was isolated and snap-frozen in liquid nitrogen until ready to measure complex activity. Complex I activity was measured using 2,6-dichloroindophenol (DCIP) as the terminal electron acceptor at 600 nm with the oxidation of NADH reducing artificial substrates Coenzyme Q10 that then reduces DCIP (37). Complex II activity was measured by reduction of dichloroindophenol at 600 nm with succinate as the substrate, and complex II/III was measured as the reduction of cytochrome c at 550 nm with succinate as the substrate (75). Complex III activity was measured by reduction of cytochrome c measured at 550 nm at 30°C. Cell lysate (30–60 µg) was added to the reaction mixture with and without 10 µM antimycin to determine the antimycin-sensitive activity. The reaction was initiated by adding 75 µM reduced decylubiquinone (DUBQH2), and the increase in absorbance to 550 nm was measured for 3 min. The sample rate was calculated from the linear portion of the trace and the specific complex III activity calculated (75). Complex IV activity was measured by the oxidation of cytochrome c at 550 nm and presented as the reaction rate constant k per second per milligram (79). ATP synthase (Complex V) activity was measured by the continuous spectrophotometric monitoring of the oxidation of NADH in an enzyme-linked ATP-regenerating assay using ATP, phosphoenolpyruvate, pyruvate kinase, and lactate dehydrogenase to determine the ATPase activity (NADH loss) in micromoles per minute per milligram protein (23, 58).
Transmission electron micrographs.
Skeletal muscle from the recruited portion of the quadriceps was dissected, trimmed (1 mm × 2 mm), and placed into fixative. Samples were processed for transmission electron micrographs (TEM) in the UAB High Resolution Imaging Facility using standard procedures. Images were acquired from random fields using a digital camera connected to a FEI-Tecnai T12 Spirit 20–120 kv. Mitochondrial area per total section area was quantified computationally using ImageJ software. All quantifications were performed blindly.
Statistical analysis.
The data are presented as means ± standard error of the mean (SE) or standard deviation (as indicated). P values were calculated by two-tailed Student’s t test. P values of < 0.05 were considered statistically significant. For multiple pairwise comparisons, two-way ANOVAs were performed, followed by a Tukey adjustment. The statistical package within Prism 6 was used for all calculations.
RESULTS
Generation of an adult inducible muscle-specific double PGC-1 knockout.
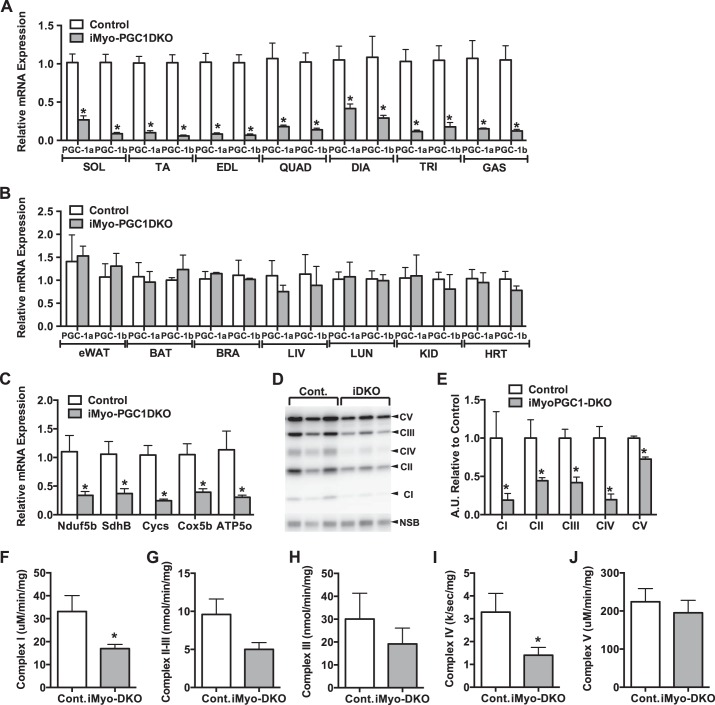
We have previously reported that PGC-1α in skeletal muscle is not required for exercise-induced mitochondrial biogenesis (59). Furthermore, we have shown that muscle-specific deletion of both PGC-1α and -1β is able to disassociate mitochondrial biogenesis from oxidative capacity (61). Here, we sought to determine the role of PGC-1α and -1β in adult skeletal muscle in the exercise response. We therefore generated an inducible skeletal muscle-specific PGC-1α/β double knockout (iMyo-PGC-1DKO) mouse model, in which PGC-1s can be deleted in adult skeletal muscle. To accomplish this, mice were injected with tamoxifen for 5 days and assessed for the effect on PGC-1α and -1β expression 4 wk postinjection. We observed a 60–90% reduction in the expression of both PGC-1α and -1β depending on the skeletal muscle bed tested (Fig. 1A); the remaining PGC-1α/β expression is most likely due to PGC-1 expression in nonmyocytes. This is supported by the observation that we did not detect a significant decrease in PGC-1α and -1β expression in nonmuscle tissue (Fig. 1B), showing the specificity of this inducible Cre promoter to skeletal muscle as previously described by others (25, 33, 48). The mRNA expression of electron transport chain (ETC) subunits was decreased 60% to 75% compared with control animals (Fig. 1C). This decrease in mRNA expression was associated with a decrease in protein expression representing the various components of the ETC (Fig. 1D). Complex I through V subunits exhibited reduced expression levels ranging from 25% to 90% compared with control animals (Fig. 1E). We also measured the individual complex enzymatic activities 4 wk postdeletion of PGC-1α and -1β. We observed a reduction in Complex I through V activity (Fig. 1, F–J), with only Complex I and IV reaching statistical significance (Fig. 1F and 1I). These observations were similar to what was previously reported in the congenital muscle-specific PGC-1 DKO animals (61).
Fig. 1.
Adult deletion of both PGC-1α and -1β decreases baseline ETC expression. A: PGC-1α and -1β mRNA expression in different skeletal muscle beds. Sol, soleus; TA, tibialis anterior; EDL, extensor digitorum longus; QUAD, quadriceps; DIA, diaphragm, TRI, triceps; GAS, gastrocnemius. B: PGC-1α and -1β mRNA expression in different non-skeletal muscle tissues. eWAT, epididymal white adipose tissue; BAT, brown adipose tissue; LIV, liver; LUN, lung; KID, kidney; BRA, brain; HRT, heart. C: ETC subunit mRNA expression. D: ETC protein expression. E: densitometry quantification of ETC protein expression normalized to nonspecific band (NSB) and relative to control. F: NADH dehydrogenase (Complex I) activity. G: succinate dehydrogenase/cytochrome-c reductase (Complex II-III) activity. H: cytochrome-c reductase (Complex III) activity. I: cytochrome-c oxidase (Complex IV) activity. J: ATP synthase (Complex V) activity from gastrocnemius (GAS) muscle of iMyo-PGC-1DKO and control littermates. Data are presented as means ± SE; n = 3–4 per group; *P < 0.05 compared with control as determined by unpaired t-test.
Exercise response in iMyo-PGC-1DKO.
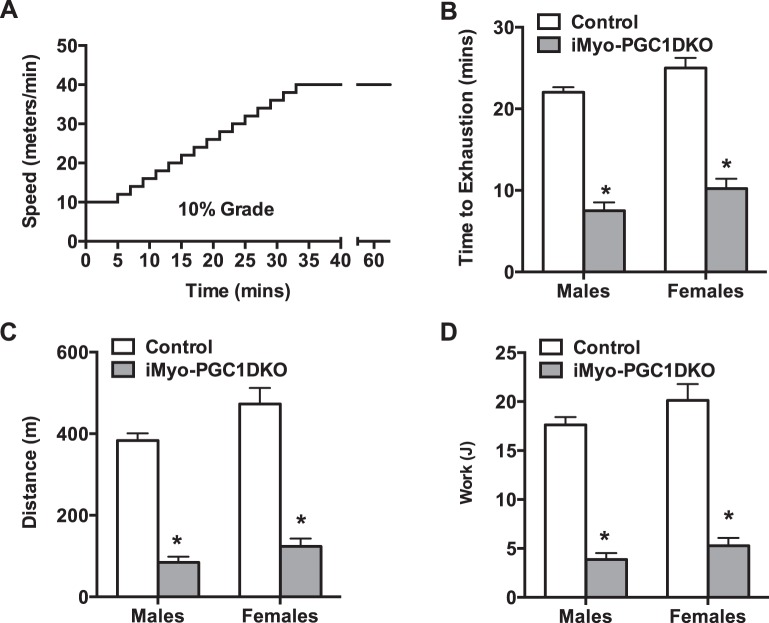
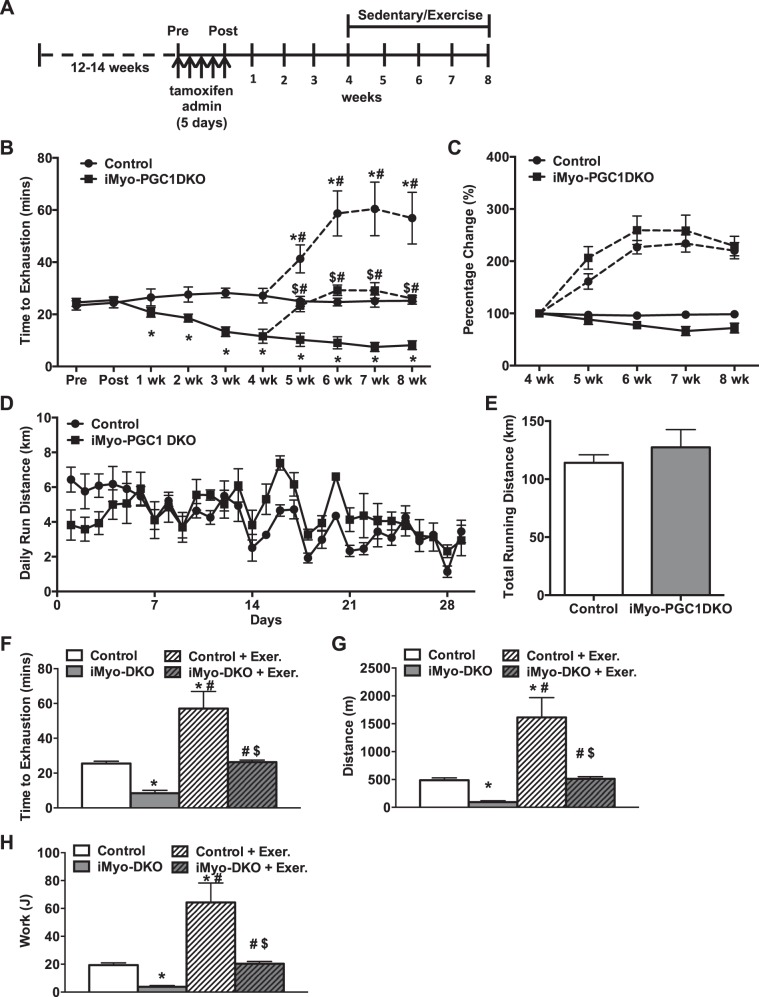
We next sought to determine whether loss of PGC-1α and -1β expression in adult skeletal muscle would affect exercise capacity. We induced the deletion of PGC-1s as described above in either 12- to 14-wk-old female or male mice and then subjected them to a progressive endurance exercise stress test protocol (Fig. 2A). The iMyo-PGC-1DKO mice exhibited a significant decrease in time to exhaustion compared with control animals (Fig. 2B). This decrease in time corresponded to a reduced distance run as well as work performed (Fig. 2, C and D), which was similar to what was previously reported in the congenital muscle-specific PGC-1 DKO animals (61). We did not observe any phenotypic difference between male and female iMyo-PGC-1DKOs; however, for the remainder of the study we chose to use female animals, based on our experience, female mice tend to perform better in these exercise studies. We next sought to investigate whether PGC-1s in adult skeletal muscle were necessary for training-induced increased exercise performance. In addition, given that we could control the induction of the deletion, we also determined in a temporal manner how long it took to reach the low point with regard to exercise capacity. To achieve this, mice were assessed for time to exhaustion before administration of tamoxifen (Pre), immediately after the last injection on day 5 (Post), and at subsequent time points for 4 wk (Fig. 3A). At the 4-wk time point, iMyo-PGC-1DKO and control mice were split into 2 groups (sedentary or exercised). The exercised mice were given access to voluntary running wheels to exercise for an additional 4 wk. Exercise exhaustion times were assessed for all 4 groups of mice every week for the remaining 4 wk (Fig. 3A). The iMyo-PGC-1DKO showed no difference in exhaustion time before or immediately after tamoxifen administration but exhibited a time-dependent decrease in exhaustion when compared with their control counterparts for the first 4 wk (Fig. 3B). The iMyo-PGC-1DKO ultimately plateaued at ~7 wk (Fig. 3B) to levels observed previously with congenital PGC-1α/β deletion in skeletal muscle (61, 85). When subjected to voluntary wheel exercise, the iMyo-PGC-1DKO animals exhibited a significant increase in response to exercise to similar percent change when compared with their sedentary counterparts (Fig. 3C). The iMyo-PGC-1DKO ran similar daily averages (Fig. 3D) and total distance (Fig. 3E) on the running wheels as the control mice for the total duration of the training period. Detailed analysis of the 8-wk time point highlights the extent of the exercise response in the iMyo-PGC-1DKO (Fig. 3, F–H). The iMyo-PGC-1DKO exercised animals exhibited a twofold increase in exhaustion time when compared with sedentary iMyo-PGC-1DKO animals (Fig. 3F); a similar twofold increase in response to exercise training was observed in the control animals. This increase in time to exhaustion is also reflected in an increase in total distance run on treadmill (Fig. 3G) as well as work performed (Fig. 3H), overall reflecting a longer time to reach exhaustion. Altogether, these observations suggest that increases in exercise performance with training do not require PGC-1α and -1β in adult skeletal muscle.
Fig. 2.
Adult deletion of PGC-1s in skeletal muscle decreases exercise performance. A: progressive endurance exercise exhaustion protocol. B: baseline time to exhaustion. C: total distance run. D: calculated total work in male and female iMyo-PGC-1DKO and control littermates. Data are presented as means ± SE; n = 5–7 per group; *P < 0.05 compared with control groups as determined by 2-way ANOVA with pairwise comparisons (Tukey adjustment).
Fig. 3.
Improvements in exercise performance with 4 wk of voluntary endurance training are PGC-1 independent. A: schematic of study design. B: longitudinal exercise stress test. C: percentage change relative to 4-wk time point for each genotype (circles, control animals; squares, iMyo-PGC1DKO animals; dashed lines, exercised groups). D: average day to day running distance. E: total running distance while on voluntary wheels for 4 wk training period. F: time to exhaustion. G: total distance run. H: calculated total work. Data are presented as means ± SE; n = 5–10 per group; *P < 0.05 compared with sedentary control; $P < 0.05 compared with exercised controls; #P < 0.05 compared with sedentary iMyo-PGC-1DKO animals as determined by 2-way ANOVA with pairwise comparisons (Tukey adjustment) for B, F–H.
Increased electron transport chain components in the absence of skeletal muscle PGC-1s.
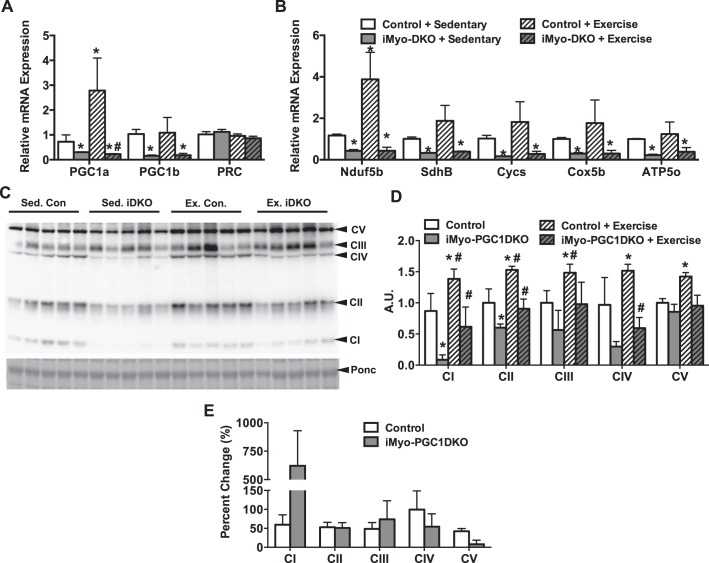
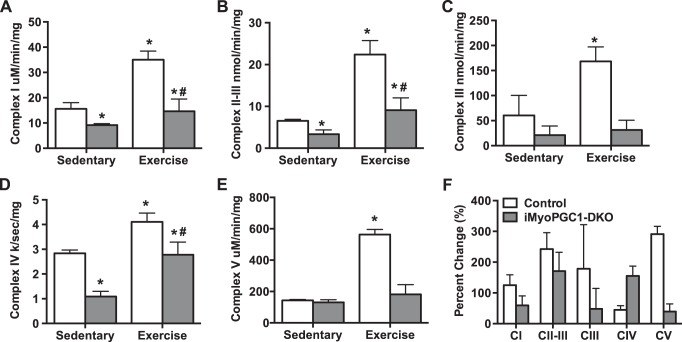
We next sought to determine what could be responsible for this increase in exercise performance. We first confirmed that PGC-1α and -1β were still absent in response to exercise. Real-time qPCR analysis of the gastrocnemius (GAS) revealed that PGC-1α mRNA was increased in response to exercise in the control animals, but no increase in PGC-1α was observed in the iMyo-PGC-1DKO animals (Fig. 4A). PGC-1β, which is not thought to respond positively to exercise, was not affected in the control animals in response to exercise and was absent in the iMyo-PGC-1DKOs (Fig. 4A). We did not observe any difference in the level of PRC mRNA expression in either sedentary or exercised animals (Fig. 4A). Moreover, we observed an increase in ETC mRNA in the control animals in response to exercise; this was not seen in the iMyo-PGC-1DKO animals (Fig. 4B). We next looked at the protein expression of ETC complex subunits in the GAS of iMyo-PGC-1DKO animals and control animals in response to exercise (Fig. 4C). In response to exercise, the control animals exhibited a 50% increase for all ETC protein complex expression levels (Fig. 4D). However, the iMyo-PGC-1DKO also exhibited a significant increase in Complex I, II, and IV with a trend towards an increase in Complex III compared with sedentary iMyo-PGC-1DKOs (Fig. 4D). Overall, both iMyo-PGC-1DKOs and control animals responded positively to exercise training with increased protein expression of ETC complexes (Fig. 4E). We next asked whether these changes in protein expression corresponded to changes in ETC enzymatic complex activity, as this could help explain the increase in exercise performance. We observed a significant increase in all ETC complex activities tested in control animals in response to exercise, ranging from 0.5-fold to 5-fold (Fig. 5, A–E). The iMyo-PGC-1DKO animals exhibited a significant increase in Complexes I, II+III, and IV in response to exercise training (Fig. 5, A–E), with Complex IV showing the greatest response to training in the iMyo-PGC-DKO animals (Fig. 5F). Taken together, these data suggest that in the absence of PGC-1α and -1β in skeletal muscle, exercise is able to increase ETC complex protein and enzymatic activity.
Fig. 4.
Deletion of PGC-1s decreases ETC subunit gene expression but not protein expression. A: PGC-1 family mRNA expression. B: ETC subunit mRNA expression. C: representative Western blot of ETC protein expression. D: densitometry quantification of ETC protein expression normalized to Ponceau S (Ponc) staining from gastrocnemius (GAS) muscle of iMyo-PGC-1DKO and control littermates, exercised and sedentary. E: percent change in complex activity with exercise from sedentary animals. Data are presented as means ± SE; n = 5 per group; *P < 0.05 compared with sedentary control; #P < 0.05 compared with sedentary iMyo-PGC-1DKO animals as determined by 2-way ANOVA with pairwise comparisons (Tukey adjustment) for A, B–D.
Fig. 5.
ETC complex activity increases with exercise training independent of PGC-1 expression. A: NADH dehydrogenase (Complex I) activity. B: succinate dehydrogenase/cytochrome-c reductase (Complex II-III) activity. C: cytochrome-c reductase (Complex III) activity. D: cytochrome-c oxidase (Complex IV) activity. E: ATP synthase (Complex V) activity from gastrocnemius (GAS) muscle of iMyo-PGC-1DKO and control littermates, exercised and sedentary. F: percentage change from sedentary controls. Data are presented as means ± SE; n = 4–5 per group; *P < 0.05 compared with sedentary control; #P < 0.05 compared with sedentary iMyo-PGC-1DKO animals. P values were determined by 2-way ANOVA with pairwise comparisons (Tukey adjustment) for A–E.
PGC-1s are needed for exercise-induced mitochondrial biogenesis.
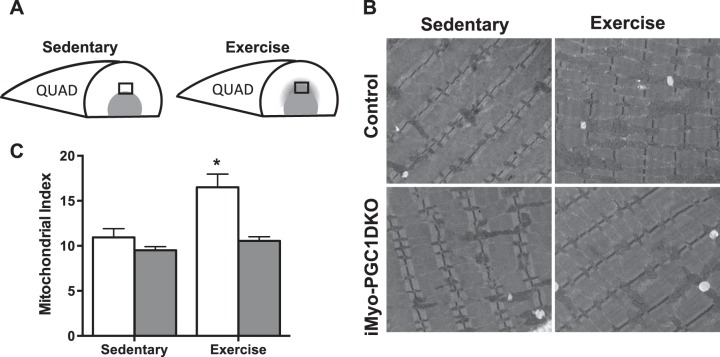
We have previously reported that PGC-1α was not required for exercise-induced mitochondrial biogenesis (59). We asked next whether mitochondrial biogenesis was affected in adult skeletal muscle with deletion of PGC-1α and -1β with exercise training. We processed the portion of the quadriceps (QUAD) skeletal muscle that has been shown to be recruited in response to exercise (Fig. 6A), for transmission electron micrograph (TEM). Blinded analysis of the micrographs revealed an increase in the intramyofibral mitochondria (Fig. 6B) in the control animals; this was not observed in the iMyo-PGC-1DKO animals. This increase in mitochondria was confirmed by blinded morphometric quantification of the TEMs, which showed a 50% increase in mitochondrial density (Fig. 6C). Taken together, these data suggest that PGC-1β may play a role in the exercise-induced mitochondrial biogenesis.
Fig. 6.
PGC-1s may be necessary for exercise-induced mitochondrial biogenesis in adult skeletal muscle. A: schematic of recruited portion of quadriceps (QUAD) during exercise. B: representative electron micrograph images. C: quantification of mitochondrial index from electron micrographs. Data are presented as means ± SE; n = 5 animals per group; 20 images per animal; *P < 0.05 compared with sedentary control as determined by 2-way ANOVA with pairwise comparisons (Tukey adjustment).
DISCUSSION
Exercise training has been shown to induce a multitude of cellular, molecular, and biochemical adaptions in skeletal muscle (22, 35). In particular, endurance-based exercise training induces increased mitochondrial biogenesis and increased oxidative capacity, which results in an overall increased endurance capacity (22). The PGC-1 family of transcriptional coactivators, particularly PGC-1α, is thought to be largely responsible for mediating these changes on mitochondrial biogenesis and oxidative capacity in skeletal muscle. These conclusions are largely predicated on observations in gain-of-function rodent models and that PGC-1α is induced in response to exercise in both rodents and humans (5, 29, 50, 55, 65, 77). However, some recent studies using loss-of-function rodent models have suggested that PGC-1α might be dispensable for some of these benefits (26, 41, 59). These studies, however, relied on congenital deletion of PGC-1s and therefore cannot rule out the possibility of developmental compensation or functional redundancy by other PGC-1 isoforms or family members. Here, we generated adult inducible muscle-specific PGC-1α and -1β double knockout mice, allowing us to remove the two main PGC-1s in adult skeletal muscle and assess the role they play in the exercise response. We conclude that 1) PGC-1α and -1β in adult skeletal muscle are not required for training-induced increases in exercise performance, 2) in the absence of PGC-1α and -1β, exercise training is still able to increase ETC protein expression and enzymatic activity, and 3) in the absence of both PGC-1α and -1β, exercise failed to increase mitochondrial content, suggesting a possible role of PGC-1β in controlling mitochondrial biogenesis.
Our data reveal that in less than 8 wk postdeletion of both PGC-1α and -1β in adult skeletal muscle we observed a significant reduction in ETC genes, proteins, and exercise performance (Figs. 1 and 2), similar to a level that was previously reported in the congenital deletion of PGC-1α and -1β (61). These data suggest that PGC-1s play an important role in maintaining mitochondrial ETC activity in adult skeletal muscle. It is also interesting that in the iMyo-PGC-1DKOs we did not observe any decrease in baseline mitochondrial density (Fig. 6); this was similar to what was previously reported in the congenital muscle-specific PGC-1 double knockouts (61). These observations confirm that PGC-1α and -1β are able to separate ETC activity from mitochondrial biogenesis under baseline conditions. Moreover, we show that after 4 wk of voluntary training the iMyo-PGC-1DKO animals were able to mount a difference in exercise performance of similar magnitude to that of control animals (Fig. 3C). The increase in exercise performance appears to be associated with an increase in ETC protein expression and enzymatic activity. However, in the absence of both PGC-1α and -1β in skeletal muscle, it is not fully understood how the iMyo-PGC-1DKOs are still able to respond positively to exercise training.
Increasing attention has been placed on alternatively spliced forms of PGC-1α (including NT-PGC-1α and PGC-1α-4) and the role they play in the exercise response (49, 64, 74, 83, 84). However, the targeting strategy used to generate the null allele for PGC-1α in skeletal muscle removes exons 3 through 5 (44), which encode the majority of the activation domain and part of the repression domain of PGC-1α (46). We cannot exclude the possibility of alternative splicing around the deleted exons such as from one of the exon 1s or exon 2 to exon 6. However, based on our current understanding of PGC-1α structure, these alternative spliced forms would not give rise to any known functional form of PGC1α. Whether PGC-1β is subjected to alternative splicing like PGC-1α is currently unknown. However the targeting strategy employed here would remove exons 4 through 6 of PGC-1β (39), which encodes 2 of the LXXLL binding motifs required for PGC-1β to interact with nuclear receptors (17, 36). Therefore, compensation by a truncated, alternatively spliced isoform of PGC-1 isoforms in the iMyo-PGC1DKO animals is unlikely.
Another possibility is that the third member of the PGC-1 family, PGC-1-related coactivator (PRC), might be contributing to this increase in exercise performance, as PRC mRNA has also been reported to be induced in response to acute bouts of exercise (21, 66). In addition, PRC mRNA expression is increased with electrical stimulated contraction of C2C12 myotubes in vitro in as little as 3 h poststimulation (54). However, the specific role PRC plays in the exercise response has yet to be tested experimentally due to absence of a good genetic model, as germline deleted PRC mice exhibit peri-implantation lethality (32). We have previously reported that deletion of PGC-1α and -1β in skeletal muscle did not increase the expression of PRC mRNA (61), which we also observed in iMyo-PGC-1DKO animals (Fig. 4A). PRC has been shown to control the expression of genes involved in ETC activity, similar to that of PGC-1α and -1β (69, 70). It is therefore possible that PRC is not causing a direct transcriptional response by increasing the level of expression of the ETC mRNA subunits, but PRC is able to contribute to the maintenance of baseline ETC mRNA transcript levels. This separation between transcription and translation of ETC components would be sufficient to contribute increased ETC protein and activity similar to what was recently described (16). In addition, PRC has been shown to regulate mitochondrial biogenesis, as loss of PRC results in a decrease in mitochondrial content (81). It is not known whether in skeletal muscle PRC performs such a role, but could explain why in the absence of both PGC-1α and -1β we do not observe any decrease in mitochondrial content in the iMyo-PGC-1DKO animals.
Other transcriptional regulators, including nuclear receptors and other coactivators, have also been reported to be involved in the exercise response and have been shown to increase exercise performance (53). These include members of the estrogen-related receptor (ESRR) and peroxisome proliferator-activated receptor (PPAR) family of transcription factors; however, these nuclear receptors are direct transcriptional targets of PGC-1s and require them for transcriptional transactivation (24, 72, 80). Similarly, other pathways, such as the Sirtuin 1 (SIRT1) or 5′-AMP-activated protein kinase (AMPK) pathways (30, 45) have also been shown to respond to exercise and energy status. However, these pathways are thought to act at least in part via PGC-1α (13, 14, 40, 42, 76).
As stated above, exercise training induces many metabolic adaptions in skeletal muscle, including changes in capillary density, fiber composition, and mitochondrial biogenesis (22, 63). Overexpression of either PGC-1α or -1β in skeletal muscle can induce a very robust angiogenic response (2, 15, 60, 62). However, we and others have shown that exercise-induced effects on capillary density are PGC-1α dependent, and that loss of PGC-1α alone in skeletal muscle is sufficient to block exercise-induced angiogenesis (15, 26). Moreover, we did not observe any increases in expression of the proangiogenic marker vascular endothelial growth factor-a (VEGFa) or platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), a marker of endothelial cells in the muscles of the iMyo-PGC-1DKO animals. It is therefore unlikely that changes in capillary densities could explain the benefits of exercise seen in the iMyo-PGC-1DKO animals. The effect of exercise training on mitochondrial content through biogenesis does not require PGC-1α as its deletion does not prevent exercise-induced mitochondrial biogenesis (41, 59). Nonetheless, after 4 wk of voluntary training, we did not observe any increases in mitochondrial biogenesis in the iMyo-PGC-1DKO animals (Fig. 6) making increased mitochondrial content not responsible for the observed increased exercise performance.
One possible explanation for the increased performance in the iMyo-PGC-1DKOs is that exercise training is able to stabilize the existing ETC machinery, thereby providing sufficient complex activity to positively affect performance. Endurance training has also been shown to induce the expression of heat shock proteins which have been shown to increase the components of Complex II and Complex IV expression and activity (68). Muscle contractile activity has also been shown to affect the half-life of mitochondrial proteins (9, 10). However, this alone does not explain the increased exercise performance, as stability would only prevent the time-dependent decrease observed in the sedentary iMyo-PGC-1DKO animals. A recent report has suggested a separation of transcriptional regulation and translation of the ETC during mitochondrial biogenesis in yeast as a result of metabolic needs (16). There is evidence for such a mechanism in response to exercise, where the activity of Complex IV does not correspond to mRNA expression in trained rats (78). However, this could explain, in part, the apparent absence in changes of mRNA gene expression and protein expression of the ETC components observed in the iMyo-PGC1-DKO animals in response to exercise (Fig. 3). Overall, the increase in Complex IV activity in the trained iMyo-PGC1-DKO mice could be sufficient to explain the increase in exercise performance. Whether the changes we observe in the iMyo-PGC-1DKO animals is the result of posttranslational modifications or regulation at the translational level will need to be further investigated.
We cannot exclude the contribution of non-PGC-1 mechanisms that explain the increase in exercise performance in the iMyo-PGC-1DKO animals such as activation of Akt and its downstream substrates or activation of the TP53 tumor suppression protein (p53) (6, 19). The activation of Akt has been primarily reported to be involved in resistance-based exercise and the activation of protein synthesis and muscle hypertrophic response (7, 8, 38), but has also been shown to respond to endurance exercise (12, 18). Moreover, Akt hyperactivation has been shown to increase ETC complex expression and activity independent of PGC-1α (27). p53, which also responds to exercise and is activated by AMPK and p38 MAPK, may be another PGC-1-independent mechanism which may contribute to ETC complex activity and expression (6). p53 has been shown to facilitate transcription of factors involved in mitochondrial biogenesis and ETC expression during endurance-based exercise (47, 52, 67). This is further bolstered by the fact rodents lacking p53 exhibit significant decreases in exercise capacity and mitochondrial number in skeletal muscle (47, 52). We have not directly tested the contribution of Akt or p53 activation in the absence of PGC-1s in the current study, and therefore cannot exclude their contribution.
Last, our model takes advantage of muscle-specific knockouts for PGC-1α and PGC-1β and therefore the possibility exists that the training effect observed in the iMyo-PGC-1DKOs is due to other adaptions within the cardiovascular system, such as the heart and other hemodynamic changes. It is well established that in response to endurance-based training the heart undergoes physiological hypertrophy, increased vasodilation and perfusion, and overall increased cardiac output and stroke volume (56). This increase in cardiac function and output would potentially increase maximal aerobic capacity (V̇o2max), thus increasing exercise performance (28). We did not measure cardiac function or V̇o2max in this current study, but presumably these would be increased in the iMyo-PGC-1DKO animals in response to exercise training. However, it is unclear how these changes alone would explain the increase in ETC protein expression and activity observed in the skeletal muscle of the iMyo-PGC-1DKOs in response to training.
In summary, the current study demonstrates that exercise training-induced increased exercise capacity does not require PGC-1α and PGC-1β in skeletal muscle. Exercise is still the best known intervention for the treatment of many conditions, and many of these benefits have been attributed to PGC-1 function. Therefore, identifying these PGC-1-independent pathways will be extremely important in understanding the molecular mechanisms that contribute to the benefits of exercise.
GRANTS
We are grateful to the UAB DRC BARB Core, supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-079626, for help with electron transport complex activity assays, as well as the University of Alabama-Birmingham Neuroscience Behavioral Assessment Core, supported by National Institute of Neurological Disorders and Stroke Grant P30-NS-47466. This work was supported in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-062128 to G.C.R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.B., Y.T., Z.B., and G.C.R. performed experiments; C.B., Y.T., Z.B., and G.C.R. analyzed data; C.B. and G.C.R. prepared figures; C.B. drafted manuscript; Y.T., Z.B., and G.C.R. interpreted results of experiments; Y.T., Z.B., and G.C.R. edited and revised manuscript; G.C.R. conception and design of research; G.C.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the University of Kentucky Center for Muscle Biology and Dr. Karyn Esser for kindly providing us with the HSA-MerCreMer mouse line.
REFERENCES
- 1.Andreu AL, Hanna MG, Reichmann H, Bruno C, Penn AS, Tanji K, Pallotti F, Iwata S, Bonilla E, Lach B, Morgan-Hughes J, DiMauro S. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N Engl J Med 341: 1037–1044, 1999. doi: 10.1056/NEJM199909303411404. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 3.Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Baar K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc Nutr Soc 63: 269–273, 2004. doi: 10.1079/PNS2004334. [DOI] [PubMed] [Google Scholar]
- 5.Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16: 1879–1886, 2002. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett JD, Close GL, Drust B, Morton JP. The emerging role of p53 in exercise metabolism. Sports Med 44: 303–309, 2014. doi: 10.1007/s40279-013-0127-9. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc 38: 1950–1957, 2006. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 8.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 9.Booth F. Effects of endurance exercise on cytochrome C turnover in skeletal muscle. Ann N Y Acad Sci 301: 431–439, 1977. doi: 10.1111/j.1749-6632.1977.tb38219.x. [DOI] [PubMed] [Google Scholar]
- 10.Booth FW, Holloszy JO. Cytochrome c turnover in rat skeletal muscles. J Biol Chem 252: 416–419, 1977. [PubMed] [Google Scholar]
- 11.Calvo JA, Daniels TG, Wang X, Paul A, Lin J, Spiegelman BM, Stevenson SC, Rangwala SM. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol (1985) 104: 1304–1312, 2008. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 12.Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42: 1843–1852, 2010. doi: 10.1249/MSS.0b013e3181d964e4. [DOI] [PubMed] [Google Scholar]
- 13.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11: 213–219, 2010. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci USA 106: 21401–21406, 2009. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couvillion MT, Soto IC, Shipkovenska G, Churchman LS. Synchronized mitochondrial and cytosolic translation programs. Nature 533: 499–503, 2016. doi: 10.1038/nature18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delerive P, Wu Y, Burris TP, Chin WW, Suen CS. PGC-1 functions as a transcriptional coactivator for the retinoid X receptors. J Biol Chem 277: 3913–3917, 2002. doi: 10.1074/jbc.M109409200. [DOI] [PubMed] [Google Scholar]
- 18.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes 55: 1776–1782, 2006. doi: 10.2337/db05-1419. [DOI] [PubMed] [Google Scholar]
- 19.Deshmukh AS, Hawley JA, Zierath JR. Exercise-induced phospho-proteins in skeletal muscle. Int J Obes 32, Suppl 4: S18–S23, 2008. doi: 10.1038/ijo.2008.118. [DOI] [PubMed] [Google Scholar]
- 20.DiMauro S, Andreu AL. Mutations in mitochondrial DNA as a cause of exercise intolerance. Ann Med 33: 472–476, 2001. doi: 10.3109/07853890109002096. [DOI] [PubMed] [Google Scholar]
- 21.Egan B, O’Connor PL, Zierath JR, O’Gorman DJ. Time course analysis reveals gene-specific transcript and protein kinetics of adaptation to short-term aerobic exercise training in human skeletal muscle. PLoS One 8: e74098, 2013. doi: 10.1371/journal.pone.0074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Feniouk BA, Suzuki T, Yoshida M. Regulatory interplay between proton motive force, ADP, phosphate, and subunit epsilon in bacterial ATP synthase. J Biol Chem 282: 764–772, 2007. doi: 10.1074/jbc.M606321200. [DOI] [PubMed] [Google Scholar]
- 24.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116: 615–622, 2006. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gali Ramamoorthy T, Laverny G, Schlagowski AI, Zoll J, Messaddeq N, Bornert JM, Panza S, Ferry A, Geny B, Metzger D. The transcriptional coregulator PGC-1β controls mitochondrial function and anti-oxidant defence in skeletal muscles. Nat Commun 6: 10210, 2015. doi: 10.1038/ncomms10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng T, Li P, Okutsu M, Yin X, Kwek J, Zhang M, Yan Z. PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol 298: C572–C579, 2010. doi: 10.1152/ajpcell.00481.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goo CK, Lim HY, Ho QS, Too HP, Clement MV, Wong KP. PTEN/Akt signaling controls mitochondrial respiratory capacity through 4E-BP1. PLoS One 7: e45806, 2012. doi: 10.1371/journal.pone.0045806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman JM, Liu PP, Green HJ. Left ventricular adaptations following short-term endurance training. J Appl Physiol (1985) 98: 454–460, 2005. doi: 10.1152/japplphysiol.00258.2004. [DOI] [PubMed] [Google Scholar]
- 29.Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274: 350–354, 2000. doi: 10.1006/bbrc.2000.3134. [DOI] [PubMed] [Google Scholar]
- 30.Gurd BJ. Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis. Appl Physiol Nutr Metab 36: 589–597, 2011. doi: 10.1139/h11-070. [DOI] [PubMed] [Google Scholar]
- 31.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282: 30014–30021, 2007. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 32.He X, Sun C, Wang F, Shan A, Guo T, Gu W, Cui B, Ning G. Peri-implantation lethality in mice lacking the PGC-1-related coactivator protein. Dev Dyn 241: 975–983, 2012. doi: 10.1002/dvdy.23769. [DOI] [PubMed] [Google Scholar]
- 33.Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, Schroder EA, Esser KA. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle 5: 17, 2015. doi: 10.1186/s13395-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Højlund K, Mogensen M, Sahlin K, Beck-Nielsen H. Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinol Metab Clin North Am 37: 713–731, 2008. doi: 10.1016/j.ecl.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol 56: 831–838, 1984. [DOI] [PubMed] [Google Scholar]
- 36.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem 277: 40265–40274, 2002. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 37.Janssen AJ, Trijbels FJ, Sengers RC, Smeitink JA, van den Heuvel LP, Wintjes LT, Stoltenborg-Hogenkamp BJ, Rodenburg RJ. Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin Chem 53: 729–734, 2007. doi: 10.1373/clinchem.2006.078873. [DOI] [PubMed] [Google Scholar]
- 38.Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol 24: 9295–9304, 2004. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev 22: 1948–1961, 2008. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, Pehmøller C, Sanz N, Sakakibara I, Saint-Amand E, Rimbaud S, Maire P, Marette A, Ventura-Clapier R, Ferry A, Wojtaszewski JF, Foretz M, Viollet B. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J 28: 3211–3224, 2014. doi: 10.1096/fj.14-250449. [DOI] [PubMed] [Google Scholar]
- 41.Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab 294: E463–E474, 2008. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Mühlfeld C, Niemann B, Pan R, Li R, Hilfiker-Kleiner D, Chen Y, Rohrbach S. Mitochondrial biogenesis and PGC-1α deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol 106: 1221–1234, 2011. doi: 10.1007/s00395-011-0213-9. [DOI] [PubMed] [Google Scholar]
- 43.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jäger S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119: 121–135, 2004. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Marcinko K, Steinberg GR. The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise. Exp Physiol 99: 1581–1585, 2014. doi: 10.1113/expphysiol.2014.082255. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Redondo V, Pettersson AT, Ruas JL. The hitchhiker’s guide to PGC-1α isoform structure and biological functions. Diabetologia 58: 1969–1977, 2015. doi: 10.1007/s00125-015-3671-z. [DOI] [PubMed] [Google Scholar]
- 47.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science 312: 1650–1653, 2006. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy JJ, Srikuea R, Kirby TJ, Peterson CA, Esser KA. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet Muscle 2: 8, 2012. doi: 10.1186/2044-5040-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norrbom J, Sällstedt EK, Fischer H, Sundberg CJ, Rundqvist H, Gustafsson T. Alternative splice variant PGC-1α-b is strongly induced by exercise in human skeletal muscle. Am J Physiol Endocrinol Metab 301: E1092–E1098, 2011. doi: 10.1152/ajpendo.00119.2011. [DOI] [PubMed] [Google Scholar]
- 50.Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol (1985) 96: 189–194, 2004. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- 51.Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev 31: 25–51, 2010. doi: 10.1210/er.2009-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park JY, Wang PY, Matsumoto T, Sung HJ, Ma W, Choi JW, Anderson SA, Leary SC, Balaban RS, Kang JG, Hwang PM. p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ Res 105: 705–712, 2009. doi: 10.1161/CIRCRESAHA.109.205310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Schindler J, Philp A. Regulation of skeletal muscle mitochondrial function by nuclear receptors: implications for health and disease. Clin Sci (Lond) 129: 589–599, 2015. doi: 10.1042/CS20150246. [DOI] [PubMed] [Google Scholar]
- 54.Philp A, Belew MY, Evans A, Pham D, Sivia I, Chen A, Schenk S, Baar K. The PGC-1α-related coactivator promotes mitochondrial and myogenic adaptations in C2C12 myotubes. Am J Physiol Regul Integr Comp Physiol 301: R864–R872, 2011. doi: 10.1152/ajpregu.00232.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platt C, Houstis N, Rosenzweig A. Using exercise to measure and modify cardiac function. Cell Metab 21: 227–236, 2015. doi: 10.1016/j.cmet.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 58.ME, Penefsky HS, Datta A, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem 235: 3322–3329, 1960. [PubMed] [Google Scholar]
- 59.Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One 7: e41817, 2012. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowe GC, Jang C, Patten IS, Arany Z. PGC-1β regulates angiogenesis in skeletal muscle. Am J Physiol Endocrinol Metab 301: E155–E163, 2011. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowe GC, Patten IS, Zsengeller ZK, El-Khoury R, Okutsu M, Bampoh S, Koulisis N, Farrell C, Hirshman MF, Yan Z, Goodyear LJ, Rustin P, Arany Z. Disconnecting mitochondrial content from respiratory chain capacity in PGC-1-deficient skeletal muscle. Cell Reports 3: 1449–1456, 2013. doi: 10.1016/j.celrep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rowe GC, Raghuram S, Jang C, Nagy JA, Patten IS, Goyal A, Chan MC, Liu LX, Jiang A, Spokes KC, Beeler D, Dvorak H, Aird WC, Arany Z. PGC-1α induces SPP1 to activate macrophages and orchestrate functional angiogenesis in skeletal muscle. Circ Res 115: 504–517, 2014. doi: 10.1161/CIRCRESAHA.115.303829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rowe GC, Safdar A, Arany Z. Running forward: new frontiers in endurance exercise biology. Circulation 129: 798–810, 2014. doi: 10.1161/CIRCULATIONAHA.113.001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151: 1319–1331, 2012. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Dériaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 52: 2874–2881, 2003. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 66.Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J 19: 986–988, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Saleem A, Adhihetty PJ, Hood DA. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol Genomics 37: 58–66, 2009. doi: 10.1152/physiolgenomics.90346.2008. [DOI] [PubMed] [Google Scholar]
- 68.Samelman TR, Shiry LJ, Cameron DF. Endurance training increases the expression of mitochondrial and nuclear encoded cytochrome c oxidase subunits and heat shock proteins in rat skeletal muscle. Eur J Appl Physiol 83: 22–27, 2000. doi: 10.1007/s004210000241. [DOI] [PubMed] [Google Scholar]
- 69.Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann N Y Acad Sci 1147: 321–334, 2008. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scarpulla RC, Vega RB, Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23: 459–466, 2012. doi: 10.1016/j.tem.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 80: 115–125, 2015. doi: 10.1016/j.bone.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci USA 101: 6472–6477, 2004. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schroder EA, Harfmann BD, Zhang X, Srikuea R, England JH, Hodge BA, Wen Y, Riley LA, Yu Q, Christie A, Smith JD, Seward T, Wolf Horrell EM, Mula J, Peterson CA, Butterfield TA, Esser KA. Intrinsic muscle clock is necessary for musculoskeletal health. J Physiol 593: 5387–5404, 2015. doi: 10.1113/JP271436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silvennoinen M, Ahtiainen JP, Hulmi JJ, Pekkala S, Taipale RS, Nindl BC, Laine T, Häkkinen K, Selänne H, Kyröläinen H, Kainulainen H. PGC-1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol Rep 3: e12563, 2015. doi: 10.14814/phy2.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc 7: 1235–1246, 2012. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 76.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism 57: 986–998, 2008. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 296: 350–354, 2002. doi: 10.1016/S0006-291X(02)00881-1. [DOI] [PubMed] [Google Scholar]
- 78.Town GP, Essig DA. Cytochrome oxidase in muscle of endurance-trained rats: subunit mRNA contents and heme synthesis. J Appl Physiol (1985) 74: 192–196, 1993. [DOI] [PubMed] [Google Scholar]
- 79.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264: 484–509, 1996. doi: 10.1016/S0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 80.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20: 1868–1876, 2000. doi: 10.1128/MCB.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vercauteren K, Gleyzer N, Scarpulla RC. Short hairpin RNA-mediated silencing of PRC (PGC-1-related coactivator) results in a severe respiratory chain deficiency associated with the proliferation of aberrant mitochondria. J Biol Chem 284: 2307–2319, 2009. doi: 10.1074/jbc.M806434200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villena JA. New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond. FEBS J 282: 647–672, 2015. doi: 10.1111/febs.13175. [DOI] [PubMed] [Google Scholar]
- 83.Wen X, Wu J, Chang JS, Zhang P, Wang J, Zhang Y, Gettys TW, Zhang Y. Effect of exercise intensity on isoform-specific expressions of NT-PGC-1 α mRNA in mouse skeletal muscle. BioMed Res Int 2014: 402175, 2014. doi: 10.1155/2014/402175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ydfors M, Fischer H, Mascher H, Blomstrand E, Norrbom J, Gustafsson T. The truncated splice variants, NT-PGC-1α and PGC-1α4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep 1: e00140, 2013. doi: 10.1002/phy2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, Kelly DP. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab 12: 633–642, 2010. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]