Abstract
The CIII protein of lambdoid bacteriophages promotes lysogeny by stabilizing the phage-encoded CII protein, a transcriptional activator of the repressor and integrase genes. We have isolated a set of missense mutations in the cIII gene of phage lambda and of phage HK022 that yield inactive CIII proteins. All the mutations are located in the relatively conserved central region of the protein. A comparative analysis of the CIII protein sequence in lambda, HK022, and the lambdoid bacteriophage P22 leads us to suggest that this central region assumes an amphipathic alpha-helical structure. This part of the lambda cIII gene was cloned within a fragment of the lacZ gene (the alpha-complementing fragment). The resulting fusion protein displays CIII activity. Mutations that yield a nonfunctional fusion protein map within its CIII moiety. These results indicate that the central portion of the CIII protein is both necessary and sufficient for CIII activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altuvia S., Oppenheim A. B. Translational regulatory signals within the coding region of the bacteriophage lambda cIII gene. J Bacteriol. 1986 Jul;167(1):415–419. doi: 10.1128/jb.167.1.415-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
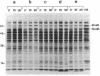
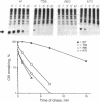
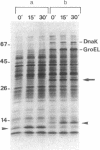
- Bahl H., Echols H., Straus D. B., Court D., Crowl R., Georgopoulos C. P. Induction of the heat shock response of E. coli through stabilization of sigma 32 by the phage lambda cIII protein. Genes Dev. 1987 Mar;1(1):57–64. doi: 10.1101/gad.1.1.57. [DOI] [PubMed] [Google Scholar]
- Belfort M., Wulff D. The roles of the lambda c3 gene and the Escherichia coli catabolite gene activation system in the establishment of lysogeny by bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Mar;71(3):779–782. doi: 10.1073/pnas.71.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. H., Muhlrad P. J., Hoyt M. A., Echols H. Cleavage of the cII protein of phage lambda by purified HflA protease: control of the switch between lysis and lysogeny. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7882–7886. doi: 10.1073/pnas.85.21.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Genetics of proteolysis in Escherichia coli*. Annu Rev Genet. 1989;23:163–198. doi: 10.1146/annurev.ge.23.120189.001115. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Kornitzer D., Altuvia S., Oppenheim A. B. Genetic analysis of the cIII gene of bacteriophage HK022. J Bacteriol. 1991 Jan;173(2):810–815. doi: 10.1128/jb.173.2.810-815.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornitzer D., Teff D., Altuvia S., Oppenheim A. B. Genetic analysis of bacteriophage lambda cIII gene: mRNA structural requirements for translation initiation. J Bacteriol. 1989 May;171(5):2563–2572. doi: 10.1128/jb.171.5.2563-2572.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Oberto J., Weisberg R. A., Gottesman M. E. Structure and function of the nun gene and the immunity region of the lambdoid phage HK022. J Mol Biol. 1989 Jun 20;207(4):675–693. doi: 10.1016/0022-2836(89)90237-4. [DOI] [PubMed] [Google Scholar]
- Palau J., Puigdoménech P. The structural code for proteins: zonal distribution of amino acid residues and stabilization of helices by hydrophobic triplets. J Mol Biol. 1974 Sep 15;88(2):457–469. doi: 10.1016/0022-2836(74)90495-1. [DOI] [PubMed] [Google Scholar]
- Semerjian A. V., Malloy D. C., Poteete A. R. Genetic structure of the bacteriophage P22 PL operon. J Mol Biol. 1989 May 5;207(1):1–13. doi: 10.1016/0022-2836(89)90437-3. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]