Abstract
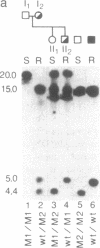
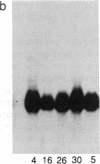
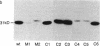
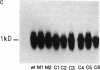
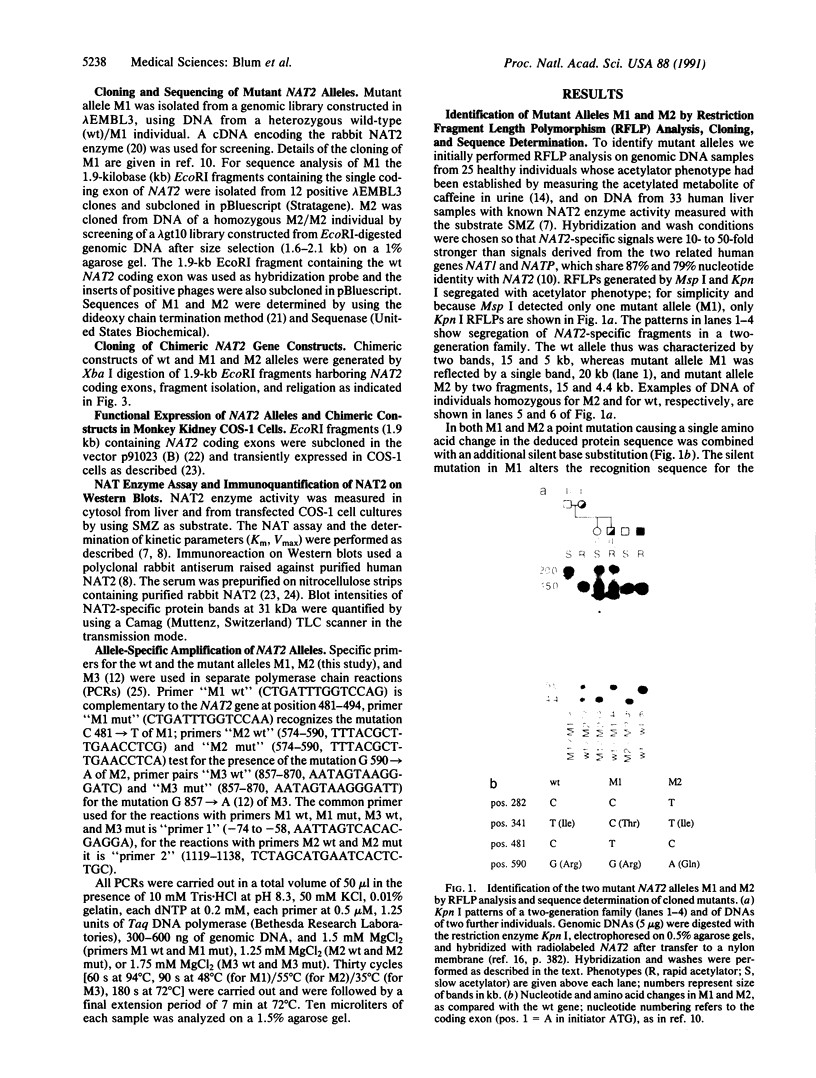
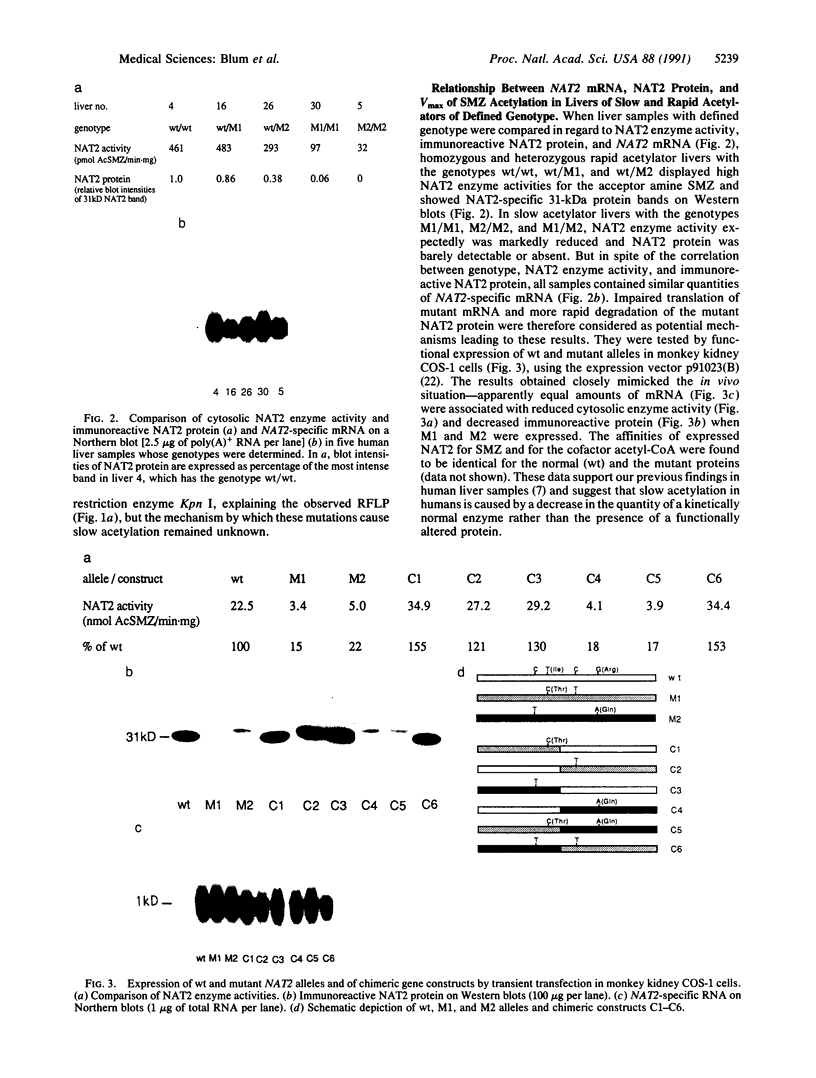
The acetylation polymorphism is one of the most common genetic variations in the transformation of drugs and chemicals. More than 50% of individuals in Caucasian populations are homozygous for a recessive trait and are of the "slow acetylator" phenotype. They are less efficient than "rapid acetylators" in the metabolism of numerous drugs and environmental and industrial chemicals. The acetylation polymorphism is associated with an increased risk of drug toxicity and with an increased frequency of certain cancers. We report the identification of the primary mutations in two alleles of the gene for the N-acetyltransferase (NAT; acetyl-CoA:arylamine N-acetyltransferase, EC 2.3.1.5) isozyme NAT2 associated with slow acetylation. These alleles, M1 and M2, account for more than 90% of slow acetylator alleles in the European population we have studied. M1 and M2 were identified by restriction fragment length polymorphisms with Kpn I and Msp I and subsequently cloned and sequenced. M1 and M2 each are characterized by a combination of two different point mutations, one causing an amino acid substitution (Ile-113----Thr in M1, Arg-197----Gln in M2), the other being silent (C 481----T in M1, C 282----T in M2). Functional expression of M1 and M2 and of chimeric gene constructs between mutant and wild-type NAT2 in COS-1 cells suggests that M1 causes a decrease of NAT2 protein in the liver by defective translation, whereas M2 produces an unstable enzyme. On the basis of the mutations described here and a rare mutant allele (M3) reported recently, we have developed a simple DNA amplification assay that allows the predictive genotyping of more than 95% of slow and rapid acetylator alleles and the identification of individuals at risk.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Grant D. M., Demierre A., Meyer U. A. N-acetylation pharmacogenetics: a gene deletion causes absence of arylamine N-acetyltransferase in liver of slow acetylator rabbits. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9554–9557. doi: 10.1073/pnas.86.23.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Grant D. M., Demierre A., Meyer U. A. Nucleotide sequence of a full-length cDNA for arylamine N-acetyltransferase from rabbit liver. Nucleic Acids Res. 1989 May 11;17(9):3589–3589. doi: 10.1093/nar/17.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Grant D. M., McBride W., Heim M., Meyer U. A. Human arylamine N-acetyltransferase genes: isolation, chromosomal localization, and functional expression. DNA Cell Biol. 1990 Apr;9(3):193–203. doi: 10.1089/dna.1990.9.193. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Mashimo M., Suzuki T. Correlation between acetylator phenotypes and genotypes of polymorphic arylamine N-acetyltransferase in human liver. J Biol Chem. 1990 Aug 5;265(22):12757–12760. [PubMed] [Google Scholar]
- Evans D. A. N-acetyltransferase. Pharmacol Ther. 1989;42(2):157–234. doi: 10.1016/0163-7258(89)90036-3. [DOI] [PubMed] [Google Scholar]
- FRYMOYER J. W., JACOX R. F. INVESTIGATION OF THE GENETIC CONTROL OF SULFADIAZINE AND ISONIAZID METABOLISM IN THE RABBIT. J Lab Clin Med. 1963 Dec;62:891–904. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Blum M., Beer M., Meyer U. A. Monomorphic and polymorphic human arylamine N-acetyltransferases: a comparison of liver isozymes and expressed products of two cloned genes. Mol Pharmacol. 1991 Feb;39(2):184–191. [PubMed] [Google Scholar]
- Grant D. M., Blum M., Demierre A., Meyer U. A. Nucleotide sequence of an intronless gene for a human arylamine N-acetyltransferase related to polymorphic drug acetylation. Nucleic Acids Res. 1989 May 25;17(10):3978–3978. doi: 10.1093/nar/17.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Lottspeich F., Meyer U. A. Evidence for two closely related isozymes of arylamine N-acetyltransferase in human liver. FEBS Lett. 1989 Feb 13;244(1):203–207. doi: 10.1016/0014-5793(89)81193-7. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Mörike K., Eichelbaum M., Meyer U. A. Acetylation pharmacogenetics. The slow acetylator phenotype is caused by decreased or absent arylamine N-acetyltransferase in human liver. J Clin Invest. 1990 Mar;85(3):968–972. doi: 10.1172/JCI114527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Kalow W. A simple test for acetylator phenotype using caffeine. Br J Clin Pharmacol. 1984 Apr;17(4):459–464. doi: 10.1111/j.1365-2125.1984.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann B. G., Frischauf A. M. Isolation of genomic DNA. Methods Enzymol. 1987;152:180–183. doi: 10.1016/0076-6879(87)52018-3. [DOI] [PubMed] [Google Scholar]
- Jennne J. W. Partial purification and properties of the isoniazid transacetylase in human liver. Its relationship to the acetylation of p-aminosalicylic acid. J Clin Invest. 1965 Dec;44(12):1992–2002. doi: 10.1172/JCI105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohsako S., Deguchi T. Cloning and expression of cDNAs for polymorphic and monomorphic arylamine N-acetyltransferases from human liver. J Biol Chem. 1990 Mar 15;265(8):4630–4634. [PubMed] [Google Scholar]
- Patterson E., Radtke H. E., Weber W. W. Immunochemical studies of rabbit N-acetyltransferases. Mol Pharmacol. 1980 May;17(3):367–373. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W. W., Hein D. W. N-acetylation pharmacogenetics. Pharmacol Rev. 1985 Mar;37(1):25–79. [PubMed] [Google Scholar]
- Wohlleb J. C., Hunter C. F., Blass B., Kadlubar F. F., Chu D. Z., Lang N. P. Aromatic amine acetyltransferase as a marker for colorectal cancer: environmental and demographic associations. Int J Cancer. 1990 Jul 15;46(1):22–30. doi: 10.1002/ijc.2910460107. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]