Abstract
Background/Aims:
No medications have been approved for managing nonalcoholic fatty liver disease (NAFLD). Lifestyle intervention is the mainstay for its treatment. Hyperferritinemia, which appears to be associated with the severity of liver injury and insulin resistance, is frequently observed in patients with NAFLD.
Patients and Methods:
We conducted a systematic review and meta-analysis of the outcomes of four interventional trials regarding the effect of phlebotomy in patients with NAFLD versus the outcomes of NAFLD patients who did not undergo phlebotomy. Primary outcome was the pooled mean difference (MD) of the homeostasis model assessment of insulin resistance (HOMA-IR). The secondary outcomes were the changes in liver enzymes and the lipid profile.
Results:
Four interventional studies involving 438 participants were included in the meta-analysis. HOMA-IR was lower in patients who underwent phlebotomy, with an MD of 0.84 [95% confidence interval (CI) 0.01 to 1.67, I2 = 72%]. Phlebotomy also significantly reduced the alanine aminotransferase (MD = 10.05, 95% CI 7.19–12.92, I2 = 34%) and triglyceride (MD = 9.89, 95% CI 4.96–14.83, I2 = 22%) levels and increased the high-density cholesterol level (MD = 3.48, 95% CI 2.03–4.92, I2 = 18%).
Conclusion:
Phlebotomy decreased insulin resistance and liver transaminase levels in patients with NAFLD. In addition, it improved their lipid profile.
Keywords: Insulin resistance, meta-analysis, nonalcoholic fatty liver disease, phlebotomy
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of disorders, ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), that are characterized by histologic evidence of progressive hepatocellular injury. It is also known as hepatic manifestation of metabolic syndrome.[1,2] Along with the growing epidemic of obesity and diabetes mellitus (DM), the prevalence of NAFLD is rapidly increasing worldwide.[3]
Because NASH has a tendency to progress to cirrhosis and hepatocellular carcinoma, it is a major cause of liver-related morbidity and mortality.[4] Based on the 2013 United Network for Organ Sharing and Organ Procurement Transplant Network registry, NASH became the second most common cause of liver pathology among adults awaiting liver transplantation in the United States. The frequency of NASH has increased by almost 170% during the last decade and is on a trajectory to becoming the most common cause in the near future.[5]
Serum ferritin has been shown to correlate with hepatic iron deposition in patients with chronic liver disease[6] and was frequently observed in patients with NAFLD (frequently NASH).[7,8,9,10] Approximately one-third of patients with NAFLD have hyperferritinemia. This high ferritin level was associated with advanced hepatic fibrosis and a high NAFLD activity score, which might be caused by increased oxidative stress.[11,12] High body iron levels negatively affect insulin sensitivity by modulating the genetic expression that increases insulin resistance.[13] This hepatic condition, termed insulin-resistance hepatic iron overload, is characterized by increased ferritin with normal transferrin saturation.[14] Because iron plays a major role in liver damage and insulin resistance, phlebotomy, an iron-depleting therapy, has been extensively studied in recent years. It appears to be a safe and, promising intervention that could ameliorate the harmful effect of iron. The practice of bloodletting, which began during the medieval period, has been shown to decrease liver transaminases in a variety of liver diseases.[15,16]
Few clinical trials have been performed to investigate the effect of phlebotomy on insulin resistance in NAFLD patients,[17,18,19,20] and hence, the effect of phlebotomy on insulin resistance is still controversial. We, therefore, conducted a systematic review with a meta-analysis of all published interventional trials investigating the impact of phlebotomy in NAFLD patients compared with the outcomes of NAFLD patients who did not undergo phlebotomy.
PATIENTS AND METHODS
We followed the established guidelines for meta-analyses of interventional trials.[21,22] We registered our systematic review and meta-analysis in PROSPERO (registration number: CRD42015028079).
Search strategy
Two investigators (V.J. and S.U.) searched for titles of articles in the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, and EMBASE. We performed a search in October 2015 and did not restrict publication dates. The following main search terms were used: Phlebotomy, venesection, NAFLD, NASH and fatty liver. The full search strategy is detailed in Item S1 in the Supplementary Material.
All published RCTs and nonrandomized trials that evaluated any phlebotomy procedures in NAFLD or NASH patients were included. Observational studies−prospective cohort, retrospective cohort, case-control, and cross-sectional studies−were included. Reviews, case reports, letters, commentaries, abstracts, and unpublished studies were not included.
Patients aged 18 years or older who had NAFLD (frequently NASH) were included. Studies that included hereditary hemochromatosis were excluded. Studies that evaluated the iron-depletion intervention using phlebotomy were included as well as those studies that evaluated patients undergoing either usual care or lifestyle modifications (e.g., exercise and dietary changes).
The primary outcome was the level of insulin resistance, which was measured by the homeostasis model assessment of insulin resistance (HOMA-IR) and was compared between the phlebotomy and nonphlebotomy groups of NAFLD (frequently NASH) patients. Secondary outcomes were the changes in the lipid profile and liver enzymes in the two groups.
Data extraction and quality assessment
Two investigators independently extracted the following data: Authors, publication year, country of origin, study design, patients' characteristics, interventions, and outcome measures. We contacted authors of the primary reports to request any unpublished data. Any conflicting opinion on data extraction were resolved by consensus of the investigators.
The Jadad composite scale was used to assess the methodological quality of the RCTs based on randomization, blinding, and withdrawals. The scale ranged from 0 to 5 points. A score of ≤2 was considered low, resulting in exclusion from the meta-analysis.[23,24] The Newcastle − Ottawa Quality Assessment Scale (NOS) was used to assess the quality of the nonrandomized studies based on selection of the study groups, comparability of study groups, and ascertainment of exposure/outcome. Studies with total scores of >6 and <4 were considered to be of high and low quality, respectively. We excluded any studies that the meta-analysis indicated were of poor quality.[25]
Statistical methods
We reported the pooled mean difference (MD) of a change in HOMA-IR, serum alanine aminotransferase (ALT), high-density lipoprotein cholesterol (HDL-C), or triglyceride (TG) between phlebotomized patients versus nonphlebotomized (control) patients who had NAFLD (frequently NASH). The inconsistency index (I2) was used to measure heterogeneity, with values of I2 > 50% indicating substantial heterogeneity.[21] We reported the pooled effect estimate of a change in outcome using a fixed effects model if I2< 50% and a random effects model if I2 ≥ 50%. Regression meta-analysis (random effects model: within-study variance estimated with the unrestricted maximum-likelihood method) was performed if the number of studies was adequate to assess the influence of study-related factors on outcomes and analysis of heterogeneity. We planned for, but did not assess, publication bias as we found fewer than 10 studies. All analyses were performed using Comprehensive Meta-analysis 3.3 software.
RESULTS
Description of included studies
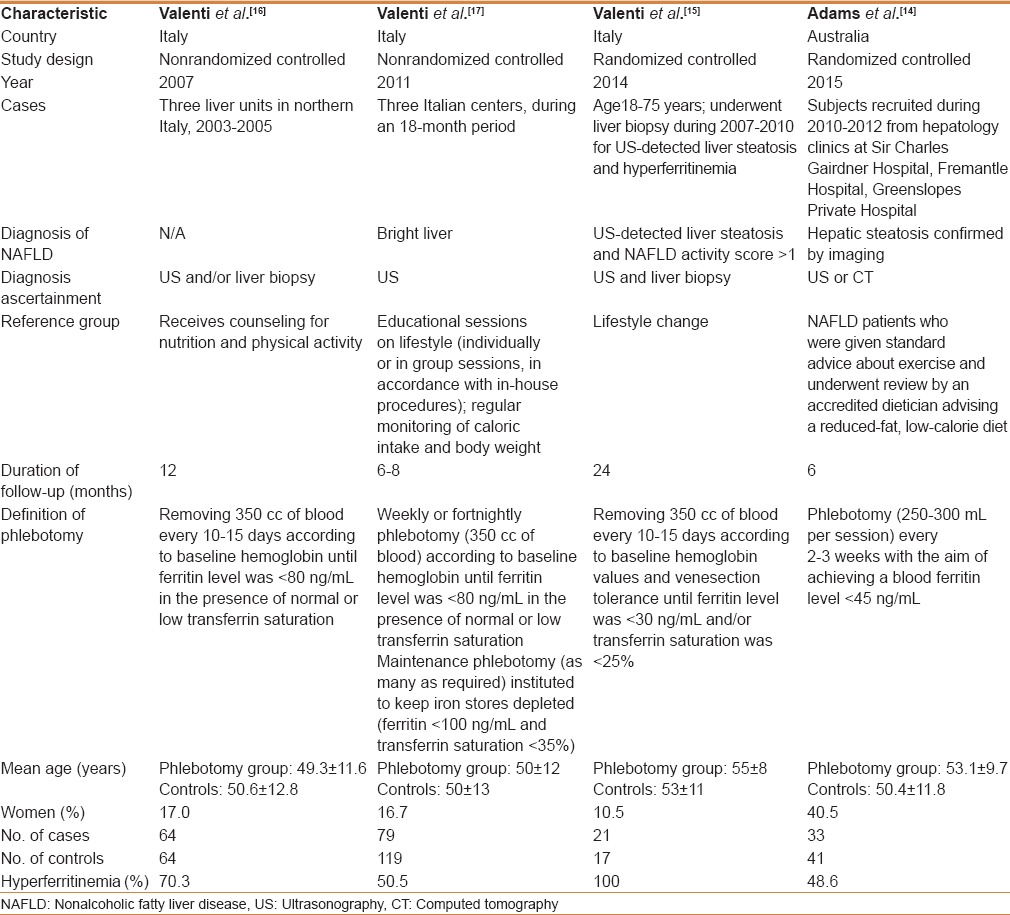
The initial search yielded 248 articles, 221 of which were excluded because they did not have a control group, were not conducted in patients with NAFLD (frequently NASH), did not perform phlebotomy, or were in a language other than English. A total of 10 articles underwent full-length review. Six of them were excluded because they did not have a control group or did not have an eligible study population. Finally, we included four studies: Two RCTs[17,19] and two prospective controlled cohort studies.[18,20] They included 438 patients with NAFLD, 197 of whom had undergone phlebotomy and 241 had not (controls). Figure 1 outlines our search methodology and selection process. Table 1 describes the characteristics of the extracted studies. The total sample size range was 38-198 patients. The mean age range was 49-55 years. The follow-up duration ranged from 6 to 24 months. NAFLD was diagnosed by ultrasonography in all four studies. Two studies performed biopsy to confirm the diagnosis.[18,19] Phlebotomy consisted of removing 250-350 cc of blood every 1-3 weeks until the ferritin level was <30-80 ng/ml.
Figure 1.
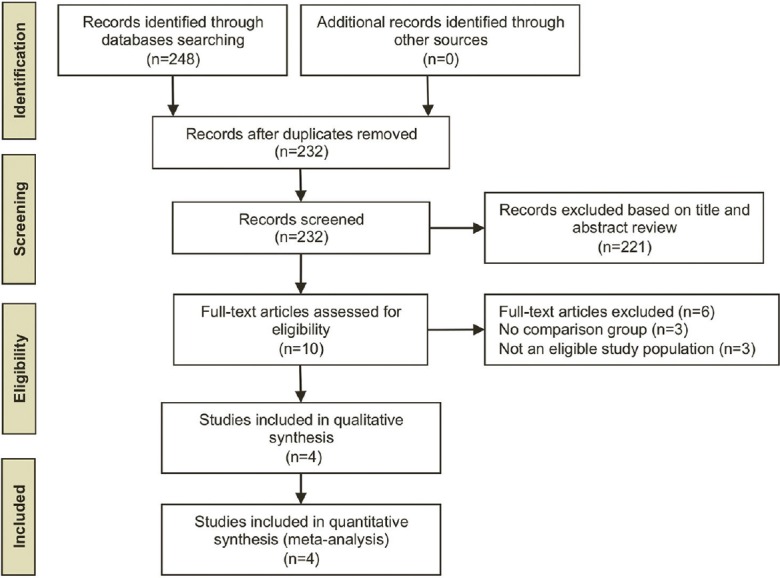
Search methodology and selection process
Table 1.
Characteristics of included studies
Quality assessment of included studies
Quality assessment scores of the RCTs and non-randomized trials are summarized in Item S2 of the Supplementary Material. All included RCTs had 3-point scores, indicating that they were of high quality. We noted that the double-blind design is impractical because of the nature of an interventional study. All nonrandomized studies had a total score of 9, indicating that they were of high quality.
Meta-analysis results
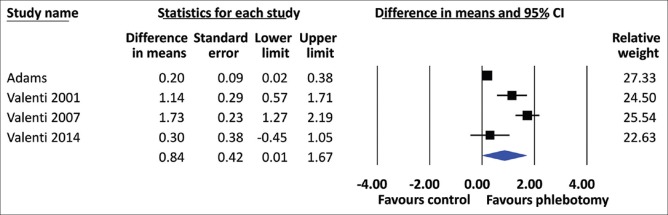
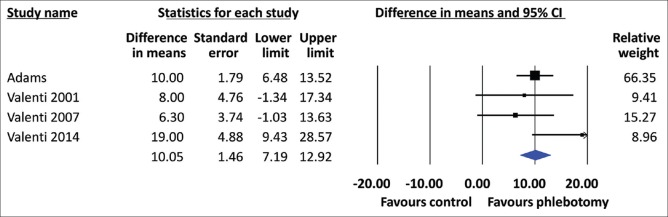
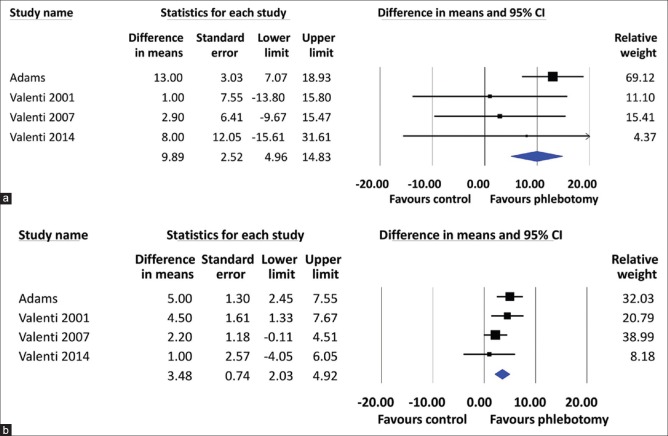
All included studies showed that the HOMA-IR was significantly decreased in the phlebotomy groups compared to the control groups. The heterogeneity among studies (I2) was 72%, Pheterogeneity= 0.01 [Figure 2]. Using a random-effects model, the pooled MD of HOMA-IR was found to be significantly lower in the phlebotomy group than in the control group (MD = 0.84, 95% CI 0.01–1.67). For the secondary outcomes, phlebotomy significantly reduced the ALT level (MD = 10.05, 95% CI 7.19–12.92) [Figure 3] and the TG level (MD = 9.89, 95% CI 4.96–14.83) and increased the HDL-C level (MD = 3.48, 95% CI 2.03–4.92) [Figure 4]. The between-study heterogeneity was not statistically significant, with low to moderate heterogeneity (I2 = 34%, Pheterogeneity= 0.21; I2 = 22%, Pheterogeneity= 0.28; and I2 = 18%, Pheterogeneity= 0.30, respectively) [Figure 2].
Figure 2.
Forest plot of insulin resistance values, according to the homeostasis model assessment of insulin resistance, for patients with nonalcoholic fatty liver disease who underwent phlebotomy and NAFLD patients without phlebotomy (controls). CI: Confidence interval
Figure 3.
Forest plot of serum alanine aminotransferase levels (IU/L) for the NAFLD patients with phlebotomy and those without phlebotomy (controls). CI: Confidence interval
Figure 4.
Forest plot of triglyceride levels (mg/dL) (a) and high-density lipoprotein levels (mg/dL) (b) for NAFLD patients with phlebotomy and those without phlebotomy (controls). CI: Confidence interval
Exploration of heterogeneity
Because pre-intervention ferritin might cause heterogeneity, a regression analysis was used to assess the effect of this factor on the outcomes and whether differences in this variable between the studies accounted for observed heterogeneity. According to the regression meta-analysis, changes in ferritin had a significant predictive effect on the difference in HDL-C after phlebotomy, with a coefficient of 0.002 (95% CI 0.0002–0.0038, P = 0.03). None of the other post-intervention outcomes were moderated by the change in ferritin: HOMA-IR (β = −0.0008, 95% CI −0.006 to +0.005, P = 0.78); ALT (β = −0.013, 95% CI −0.046 to 0.021, P = 0.46); TG (β =0.03, 95% CI −0.005 to +0.006, P = 0.09).
DISCUSSION
This meta-analysis investigated the effect of phlebotomy on insulin resistance, the lipid profile, and liver enzymes in NAFLD patients. Our main findings suggest that phlebotomy can significantly decrease HOMA-IR compared to that in NAFLD patients who did not undergo phlebotomy. In addition, larger decreases in ALT and TG and increases in HDL-C were observed in the phlebotomy groups than in the controls. Changes in ferritin after phlebotomy could explain the increased HDL-C in NAFLD patients.
A high serum ferritin level is a component of insulin-resistance syndrome. A recent meta-analysis showed that patients with high levels of ferritin were at 1.67 times higher risk of developing DM than patients with low ferritin levels−a finding that was still significant after adjusting for inflammation.[26] Another meta-analysis demonstrated that increased ferritin levels resulted in 1.73 times higher odds of a patient having metabolic syndrome than in those with low ferritin levels.[27] A recent prospective study showed that the baseline serum ferritin level was independently and prospectively associated with insulin resistance in muscle, liver, and adipocytes over a 7-year follow-up period.[28] This finding suggested that iron metabolism contributes to the development of insulin resistance and DM. Serum ferritin was also associated with central adiposity and other obesity markers.[29] A potential reason for this association is that iron might impair beta cell function by inducing oxidative stress. It could also impair glucose uptake in muscle and adipose tissues.[30] High iron intake in animal models was shown to worsen insulin resistance and result in increased TG.[31] Vice versa, iron-depletion therapy improved metabolic markers including blood glucose, the low-density/high density lipoprotein ratio, hemoglobin A1C, and blood pressure in patients with metabolic syndrome.[32]
Numerous recent studies have investigated the role of phlebotomy in improving metabolic markers−e.g., insulin resistance, the lipid profile, liver enzymes−in NAFLD patients. They, however, reported contradictory results. Our meta-analysis confirmed that phlebotomy significantly improved insulin resistance and ALT, TG, and HDL-C levels. One of the included studies assessed histological improvement after phlebotomy. It showed that the prevalence of NAFLD activity score improvement without worsening of fibrosis at 2 years was significantly higher in patients who underwent phlebotomy.[19] Beaton et al.[33] performed paired liver biopsies before and after phlebotomy in 31 subjects and showed that iron depletion resulted in significant improvement of the NAFLD activity score (by two points) but did not improve any histological features (including fibrosis and HOMA-IR) at the 6-month follow-up. The two-point improvement in NAFLD activity score without worsening fibrosis, however, provided a clinically meaningful endpoint because this improvement was rarely seen in recent placebo-controlled trials.[34,35]
The exact mechanism underlying these associations remains poorly understood. However, there are several plausible mechanisms for the association between iron-depleting therapy and the improved metabolic profile and liver function in NAFLD patients. First, the liver is the major reservoir of iron in the body. Excess iron would interfere with glucose metabolism, causing hyperinsulinemia by both decreasing the insulin extraction and creating impaired insulin signaling.[36] Second, iron, a transitional metal, might induce insulin resistance by catalyzing oxidative stress.[37] Under this condition, the overproduction of oxidants exceeds antioxidant activity, which is mechanistically associated with insulin resistance by mitochondrial overproduction of the superoxide ion and enhanced activity of cellular NADPH oxidase.[38] Third, iron might affect insulin resistance by the altered regulation of adipose tissue and adipokines. Based on a recent meta-analysis, hypoadiponectinemia might play an important role in the progression from NAFLD to, specifically, NASH, and serum ferritin is significantly correlated with serum adiponectin.[39,40]
Although phlebotomy appears to be an effective method for decreasing iron storage in the liver of NAFLD patients, there is no significant correlation between the serum ferritin level and the erythrocyte sedimentation rate, C-reactive protein level, or grade of liver inflammation on biopsy.[9] In addition, serum ferrit in levels do not predict the severity of NAFLD, as seen in a biopsy specimen.[41] Thus, iron concentration in the liver might be lower than expected for a given serum ferritin level.[42] Adams et al.[17] reported that the mean ferritin level for a treatment group was 225 μg/L, which is within the reference range, and the hepatic iron concentration of this group was 23 μmol/g, which is in the mid-normal range as measured by magnetic resonance imaging. Hoki et al.[43] studied 40 subjects with a mean serum ferritin level of 182 μg/L. Only 5% had + 3 iron staining, and none had + 4 staining. Beaton et al.[33] recruited 31 subjects with a mean serum ferritin level of 383 μg/L. Only 45% had detectable liver iron on biopsy, which was mild (+1) in all cases. Thus, patients may not display obvious benefit because, in fact, they had no hepatic iron overload.[42]
Current mainstays for treating fatty liver are lifestyle interventions, including a healthy diet and physical activity, which are not easy to maintain. Some patients progress to end-stage liver disease, ultimately requiring liver transplantation. Phlebotomy, which is a safe, well-tolerated procedure, has become an attractive alternative. Based on our results, we believe that this iron-depleting procedure can improve insulin resistance and ALT and HDL-C levels. Data regarding liver histological improvement and phlebotomy, however, were limited in a small study.[19] Larger RCTs are required to investigate the effect of phlebotomy on liver histological activity.
There are some limitations of this study. First, only four studies met our inclusion criteria, most of which were conducted in Italy, possibly limiting the external validity of this meta-analysis. Although all four studies were of high quality, further studies should be done in other geographical areas. Second, the average age was 49-55 years old, so our findings might not be applicable to individuals who are above or below this age range. Third, the included studies with biopsy-proven NAFLD, which is more specific, were smaller than those adopting imaging definition (ultrasonography, computed tomography), leaving a possibility of study bias. Moreover, because of the relative insensitivity of imaging studies to detect NAFLD, it is possible to misclassify NAFLD patients as healthy controls, which would lead to underestimating the strength of the association. Fourth, there is heterogeneity in the study that is likely due to differences in study designs and background characteristics of the subjects. We investigated the source of heterogeneity by regression meta-analysis and found that the change in ferritin after phlebotomy explained the change in HDL-C levels.
Our meta-analysis of clinical trials demonstrated that phlebotomy improved insulin resistance and the ALT and HDL-C levels in NAFLD patients. Physicians may consider phlebotomy as an alternative option in NAFLD patients in addition to lifestyle interventions. We suggest that further large, prospective, controlled trials are required to investigate the long-term outcomes and histological improvement in patients with NAFLD after phlebotomy.
SUPPLEMENTARY MATERIAL
S1: Search strategy
S2: Quality assessment of the included studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank Matthew Roslund for validating the search. V.J. and S.U. conceived the study, searched the literature, assessed the quality of the studies, and drafted the manuscript. T.R. participated in the statistical analysis and drafted the manuscript. A.S. assessed the quality of the studies and performed the statistical analysis. This article does not contain any studies with human participants or animals performed by any of the authors.
REFERENCES
- 1.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 2.Rinella ME. Nonalcoholic fatty liver disease: A systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 3.Vernon G, Baranova A, Younossi ZM. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: Diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol. 2015;13:2062–70. doi: 10.1016/j.cgh.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Kim MJ, Mitchell DG, Ito K, Hann HW, Park YN, Kim PN. Hepatic iron deposition on MR imaging in patients with chronic liver disease: Correlation with serial serum ferritin concentration. Abdom Imaging. 2001;26:149–56. doi: 10.1007/s002610000121. [DOI] [PubMed] [Google Scholar]
- 7.Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, et al. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:2448–55. doi: 10.1111/j.1572-0241.2001.04052.x. [DOI] [PubMed] [Google Scholar]
- 8.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700–7. doi: 10.1016/j.jhep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Beaton MD, Chakrabarti S, Adams PC. Inflammation is not the cause of an elevated serum ferritin in non-alcoholic fatty liver disease. Ann Hepatol. 2014;13:353–6. [PubMed] [Google Scholar]
- 10.Valenti L, Dongiovanni P, Fracanzani AL, Santorelli G, Fatta E, Bertelli C, et al. Increased susceptibility to nonalcoholic fatty liver disease in heterozygotes for the mutation responsible for hereditary hemochromatosis. Dig Liver Dis. 2003;35:172–8. doi: 10.1016/s1590-8658(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 11.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology. 2008;48:792–8. doi: 10.1002/hep.22429. doi: 10.1002/hep. 22429. [DOI] [PubMed] [Google Scholar]
- 12.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dongiovanni P, Fracanzani AL, Fargion S, Valenti L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J Hepatol. 2011;55:920–32. doi: 10.1016/j.jhep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, et al. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155–63. doi: 10.1016/s0016-5085(99)70401-4. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H, Takikawa T, Nishimura N, Yano M, Isomura T, Sakamoto N. Improvement of serum aminotransferase levels after phlebotomy in patients with chronic active hepatitis C and excess hepatic iron. Am J Gastroenterol. 1994;89:986–8. [PubMed] [Google Scholar]
- 16.Girelli CM, Mirata C, Casiraghi A. Effect of blood letting on serum aminotransferase levels of patients with chronic hepatitis C and iron overload. Recenti Prog Med. 1998;89:241–4. [PubMed] [Google Scholar]
- 17.Adams LA, Crawford DH, Stuart K, House MJ, St Pierre TG, Webb M, et al. The impact of phlebotomy in nonalcoholic fatty liver disease: A prospective, randomized, controlled trial. Hepatology. 2015;61:1555–64. doi: 10.1002/hep.27662. [DOI] [PubMed] [Google Scholar]
- 18.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: Evidence from a case–control study. Am J Gastroenterol. 2007;102:1251–8. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 19.Valenti L, Fracanzani AL, Dongiovanni P, Rovida S, Rametta R, Fatta E, et al. A randomized trial of iron depletion in patients with nonalcoholic fatty liver disease and hyperferritinemia. World J Gastroenterol. 2014;20:3002–10. doi: 10.3748/wjg.v20.i11.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenti L, Moscatiello S, Vanni E, Fracanzani AL, Bugianesi E, Fargion S, et al. Venesection for non-alcoholic fatty liver disease unresponsive to lifestyle counselling—a propensity score-adjusted observational study. QJM. 2011;104:141–9. doi: 10.1093/qjmed/hcq170. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 26.Orban E, Schwab S, Thorand B, Huth C. Association of iron indices and type 2 diabetes: A meta-analysis of observational studies. Diabetes Metab Res Rev. 2014;30:372–94. doi: 10.1002/dmrr.2506. [DOI] [PubMed] [Google Scholar]
- 27.Abril-Ulloa V, Flores-Mateo G, Solà-Alberich R, Manuel-y-Keenoy B, Arija V. Ferritin levels and risk of metabolic syndrome: Meta-analysis of observational studies. BMC Public Health. 2014;14:483. doi: 10.1186/1471-2458-14-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wlazlo N, van Greevenbroek MM, Ferreira I, Jansen EH, Feskens EJ, van der Kallen CJ, et al. Iron metabolism is prospectively associated with insulin resistance and glucose intolerance over a 7-year follow-up period: The CODAM study. Acta Diabetol. 2015;52:337–48. doi: 10.1007/s00592-014-0646-3. [DOI] [PubMed] [Google Scholar]
- 29.Gillum RF. Association of serum ferritin and indices of body fat distribution and obesity in Mexican American men—the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord. 2001;25:639–45. doi: 10.1038/sj.ijo.0801561. [DOI] [PubMed] [Google Scholar]
- 30.Swaminathan S, Fonseca VA, Alam MG, Shah SV. The role of iron in diabetes and its complications. Diabetes Care. 2007;30:1926–33. doi: 10.2337/dc06-2625. [DOI] [PubMed] [Google Scholar]
- 31.Dongiovanni P, Ruscica M, Rametta R, Recalcati S, Steffani L, Gatti S, et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am J Pathol. 2013;182:2254–63. doi: 10.1016/j.ajpath.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Houschyar KS, Lüdtke R, Dobos GJ, Kalus U, Broecker-Preuss M, Rampp T, et al. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: Results from a randomized clinical trial. BMC Med. 2012;10:54. doi: 10.1186/1741-7015-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaton MD, Chakrabarti S, Levstik M, Speechley M, Marotta P, Adams P. Phase II clinical trial of phlebotomy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:720–9. doi: 10.1111/apt.12255. [DOI] [PubMed] [Google Scholar]
- 34.Garg R, Goodman Z, Younossi Z. Commentary: Phlebotomy in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:1112. doi: 10.1111/apt.12322. doi: 10.1111/apt. 12322. [DOI] [PubMed] [Google Scholar]
- 35.Loomba R, Wesley R, Pucino F, Liang TJ, Kleiner DE, Lavine JE. Placebo in nonalcoholic steatohepatitis: Insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–8. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: Impaired hepatic insulin degradation? Diabetologia. 1984;26:441–4. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 37.Wolff SP. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull. 1993;49:642–52. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- 38.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–9. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ku BJ, Kim SY, Lee TY, Park KS. Serum ferritin is inversely correlated with serum adiponectin level: Population-based cross-sectional study. Dis Markers. 2009;27:303–10. doi: 10.3233/DMA-2009-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. 2011;60:313–26. doi: 10.1016/j.metabol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Chandok N, Minuk G, Wengiel M, Uhanova J. Serum ferritin levels do not predict the stage of underlying non-alcoholic fatty liver disease. J Gastrointestin Liver Dis. 2012;21:53–8. [PubMed] [Google Scholar]
- 42.Adams PC. The (II) logic of iron reduction therapy for steatohepatitis. Hepatology. 2015;62:668–70. doi: 10.1002/hep.27866. [DOI] [PubMed] [Google Scholar]
- 43.Hoki T, Miyanishi K, Tanaka S, Takada K, Kawano Y, Sakurada A, et al. Increased duodenal iron absorption through up-regulation of divalent metal transporter 1 from enhancement of iron regulatory protein 1 activity in patients with nonalcoholic steatohepatitis. Hepatology. 2015;62:751–61. doi: 10.1002/hep.27774. [DOI] [PubMed] [Google Scholar]