Abstract
Background/Aims:
Hepatic injury caused by ischemia/reperfusion (I/R) is a clinical problem associated with major liver surgery. Among other flavonoids, apigenin has shown a promising effect on I/R cases. In this study, we have investigated the effects of apigenin after liver I/R injury in rats.
Materials and Methods:
Forty eight rats were randomized into the following eight groups: (1) Control-sham group: rats subjected to the surgical procedure, except for liver I/R; (2) DMSO group: rats subjected to surgery, except for liver I/R given the apigenin solvent dimethyl-sulfoxide intraperitoneally; (3) C60 group; (4) C120 group; (5) C240 group: rats underwent liver ischemia for 45 min followed by reperfusion for 60 min, 120 min, and 240 min; (6) AP60 group; (7) AP120 group; (8) AP240 group: rats underwent liver ischemia for 45 min, and then given apigenin (5 mg) intraperitoneally followed by reperfusion for 60 min, 120 min, and 240 min. Reverse transcription polymerase chain reaction was performed on liver tissues to measure BCL-2/BAX expression, enzyme-linked immunosorbent assay to measure M30/M65 and ICAM-1. Immunohistochemistry was used to identify M30 biomarker in liver tissues.
Statistical Analysis:
Quantitative variables were tested by Kolmogorov–Smirnov test, repeated measures analysis of variance/Friedman test. Gene levels were assessed by Student's t-test/Mann–Whitney U-test.
Results:
BCL-2 levels were significantly higher in I/R apigenin groups than in I/R control groups. BAX levels were lower in the AP240 group than in C240 group. Prolongation of reperfusion resulted in increased activation of M30. ICAM-1 levels were lower in the AP240 group than in C240 group.
Conclusions:
Apigenin seems to inhibit the process of apoptosis and ameliorate the hepatic I/R injury.
Keywords: Apigenin, BCL-2, ICAM-1, liver ischaemia/reperfusion injury, M30, M65
Hepatic ischemia/reperfusion (I/R) injury is common in major liver surgery including liver transplantation and hepatectomies. Despite improvement in surgical techniques and perioperative management, liver failure remains one of the major complications.[1] Liver ischemia and reperfusion involve a variety of mechanisms each of which contributes to the overall concomitant injury. More specifically, I/R injury can be divided into two distinct phases, the initial phase, 2 hours after reperfusion, which is characterized by Kupffer cell activation, reactive oxygen species (ROS) production, and the release of proinflammatory chemokines and cytokines, including tumor necrosis factor alpha (TNF-a).[2] The cytokines upregulate adhesion molecules, such as ICAM-1, and cause polymorphonuclear neutrophils (PMN) to adhere to endothelial cells followed by sequestration of PMNs and further production of ROS.[3] The second phase of injury, 6 hours after reperfusion, is characterized by the recruitment and extravasation of polymorphonuclear leukocytes (neutrophils) into the parenchyma, significant ROS production, and the release of proteases and additional inflammatory mediators, leading to further liver tissue injury. Incomplete restoration of the blood flow, including heterogeneous distribution in the hepatic microcirculation, would also appear to form a part of the underlying mechanism of I/R-induced liver injury. This major cell injury can lead to either hepatic cell necrosis or apoptosis. These two types of cell death coexist in liver pathology as a result of common pathways and signals.[4] The apoptotic changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation, and global mRNA decay and death. There are two pathways through which apoptosis can be initiated. Via the intrinsic pathway, the cell kills itself because of cell stress, whereas via the extrinsic pathway, the cell kills itself because of signals from other cells. In both the pathways, cell death is induced by the activation of caspases.[5]
A number of experimental studies have been conducted to test the effect of several pharmacological agents in liver injury inhibition, including the use of flavonoids.[6] Flavonoids are a group of plant polyphenols that are abundant in fruits and vegetables, and represent substantial constituents of the nonenergetic part of human diet. In addition to their physiological role in plants, they exhibit an antioxidant activity, and this is why they are known to possess a therapeutic potential in some diseases, including cancer, ischemic heart disease, atherosclerosis, and liver diseases.[7,8,9,10,11,12] Apigenin is a natural flavonoid reported to have several biologic effects such as cancer chemopreventive properties and an antioxidant effect including protection of the endothelium and liver cells against oxidative stress.[13,14,15,16,17,18] It is not clear yet which are the receptors that apigenin binds to liver cells. The mechanism through which apigenin protects living cells against oxidative stress has not been fully investigated yet, however, it has been shown that apigenin upregulates the gene expression of antioxidant enzymes such as superoxide dismutase (SOD) 1, SOD2, and glutathione peroxidase (GPx) 1, suggesting that it attenuates oxidative-induced cell damage in living cells.[19] Apigenin is also a Pregnane X receptor (PXR) activator, the expression of which is related to the clearance of toxic substances from the body, but it does not directly bind to PXR suggesting that an alternative mechanism may be responsible for flavonoid-mediated PXR activation in HepG2 liver carcinoma cells.[20] In addition, we know that apigenin can inhibit the procedure of apoptosis by leading to lower Tumor Necrosis Factor-Related Apoptosis–Inducing Ligand Receptor-1 (TRAIL-R1) expression level on macrophages.[21]
The aim of the present study was to evaluate the possible protective effect of apigenin against I/R induced hepatic injury by measuring the expression of apoptosis controlling genes BCL-2 (anti-apoptotic) and BAX (pro-apoptotic) using the reverse transcription polymerase chain reaction (RT-PCR) method.[22] In addition, the M30/M65 ratio was measured in order to be used in defining the relative proportion of apoptosis versus total cell death. M30 measures caspase-cleaved CK18 produced during apoptosis and M65 measures the levels of both caspase-cleaved and intact CK18, the latter being released from cells undergoing necrosis.[23,24] Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum levels and the serum levels of the adhesion molecule ICAM-1 were measured to use as indicators of the level of hepatic I/R injury.[3]
MATERIALS AND METHODS
Animals and experimental protocol
All experiments described here were performed according to the European union guidelines for the ethical treatment of experimental animals. Forty-eight Wistar type rats, aged 14–16 weeks and weighing 220–350 g were used. The animals were living under controlled temperature and lighting conditions. They were housed individually in cages, and were allowed free access to standard rat food and water before and after the experiments. The animals were fasted overnight before the experiments, but were given free access to water. Rats were anesthetized by using diethyl ether and sevoflurane. After the central incision, the ligament attachments connecting the liver, diaphragm, abdominal wall, and neighboring organs were divided. After the organ was carefully isolated, the liver hilus was exposed to find the common hepatic artery and portal vein. These vessels were obstructed using an atraumatic vascular microclip for 45 min to interrupt the blood supply to the liver. Apigenin was administrated intraperitoneally dissolved in dimethyl sulfoxide solvent (DMSO).[25,26] The exact consistency of the solution was: 5 mg apigenin + 0.2 ml DMSO + 0.3 ml NaCl 0.9%. Reperfusion was followed for 60, 120, and 240 minutes (AP60, AP120, and AP240 groups). The same number of rats was used as control group and were subjected only to ischemia and reperfusion for the respective times (C60, C120, and C240 groups). Other rats were subjected to a sham operation, which was identical to the surgical procedure used for the I/R group rats without clamping (group C) and the rest were sham operated and given the DMSO solvent intraperitoneally (DMSO group). The rats were kept under anesthesia for the same period of time. The animals were euthanized and liver tissues were obtained and stored individually in liquid nitrogen at −80°C until the end of the experiment in paraben for histologic examinations by light microscopy and immunocytochemistry, and additionally blood was obtained by cardiac puncture, centrifuged, and the serum was kept at -80°C pending analysis.
Measuring the expression of BCL-2/BAX genes by reverse transcription polymerase chain reaction method
Real-time RT-PCR was performed in order to determine the relative amounts of RNA transcripts for BCL-2 and BAX genes in total RNA isolated from liver tissue in the experimental model of liver ischemia reperfusion.[27] Total RNA was extracted from rat liver tissue samples using Trizol reagent, according to the manufacturer's instructions. Reverse transcription was performed using the SuperScript Preamplification System (Invitrogen, Paisley, UK) and random hexamers in a total volume of 20 ml, following DNase treatment (Invitrogen). Using the Light Cycler MX3005P (Stratagene, La Jolla, CA), reactions were carried out using the SYBER Green MM QPCR Brilliant mix (Stratagene), 0.2 µM of each primer and 0.5 µl of cDNA in a final volume of 25 µl. Cycling parameters were as follows: a pre-amplification cycle (denaturation for 10 min at 95°C), 40 cycles of amplification (denaturation for 30 s at 94°C, annealing for 30 s at 60°C, and extension for 30 s at 72°C), and a final extension cycle (denaturation for 60 s at 94°C, annealing for 30 s at 60°C, and extension for 30 s at 95°C followed by dissociation). Results were calculated using MaxPro QPCR Software Version 3 (Sratagene California USA), according to the comparative threshold cycle method. Analysis of relative gene expression data was performed according to the 2-DDCT method[28] using β-actin as an endogenous reference (internal control) gene and cDNA from total rat brain RNA extract as a control reference sample, the discrete cosine transform (DCT) value, of which was deducted from all sample values (also used as an intra-assay standard).
Histology and immunohistochemistry
Liver tissues were fixed in 10% neutral-buffered formalin, processed routinely, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H and E). Liver sections were also stained immunohistochemically with M30 CytoDeath Monoclonal Antibody, 1:50 dilution, (Alexis Biochemical).
ELISA M30/M65, ICAM-1
The values of ELISA M30 represent the apoptotic cell death because monoclonal antibody M30 is used to trace the apoptotic epithelial cells. The monoclonal antibody M30 is specific for the epitope in caspase-3 broken CK-18 (CK18Asp396; NE M30 neo-epitope), which appears during apoptosis whereas it is not identifiable in live epithelial cells. The quantitative designation of rat ICAM-1 levels was done through the immunoabsorption technique ELISA (rat ICAM-1, ORB 50029 Biorbyt, UK), in blood serum according to the manufacturer's instructions. Blood samples were collected immediately after the end of the reperfusion time according to the protocol.
Statistical analysis
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM, NY, USA). The normality of quantitative variables was tested by Kolmogorov–Smirnov test. Levels of M65, M30, BCL-2, BAX, and AST/ALT were expressed as mean ± standard deviation (SD), whereas ICAM-1 levels were expressed as median value and range. Within group differences of normally distributed quantitative variables (M65, M30, BCL-2, and BAX) were examined by one-way repeated measures ANOVA (rmANOVA) and Friedman test (ICAM-1). Because of the small size of the samples, no correction of the significance level in multiple comparisons was performed. Between group differences and the differences of levels of the genes (BCL-2 and BAX) were assessed by Student's t-test and Mann–Whitney U-test. All tests were two tailed and statistical significance was considered for P values less than 0.05.
RESULTS
Histology
There was an evident Kupffer cells activation in groups subjected to liver ischemia and reperfusion. In all groups receiving apigenin, significant inhibition of Kupffer cells activation was noted, with a more intense impact on the apigenin treated 240 min I/R group compared to 240 min I/R group. In all I/R groups, there was vascular congestion, and hepatocytes frequently showed cytoplasmic edema. Nuclear pyknosis was also frequent in both Kupffer and endothelial cells. Increased number of neutrophils was seen in the dilated sinusoids. The typical histopathological findings of I/R-induced liver injury described above were more rarely found in the groups that were pretreated with apigenin [Figures 1 and 2].
Figure 1.
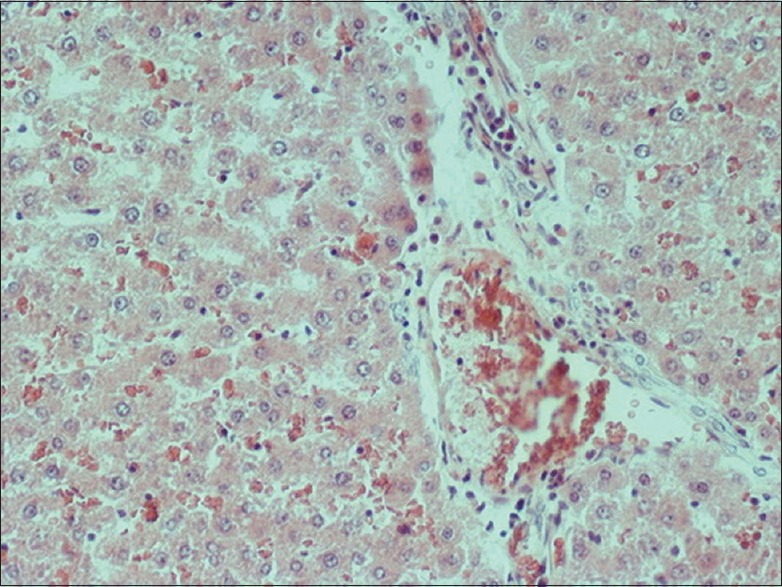
Liver tissue after 240 min I/R 240 × 200
Figure 2.
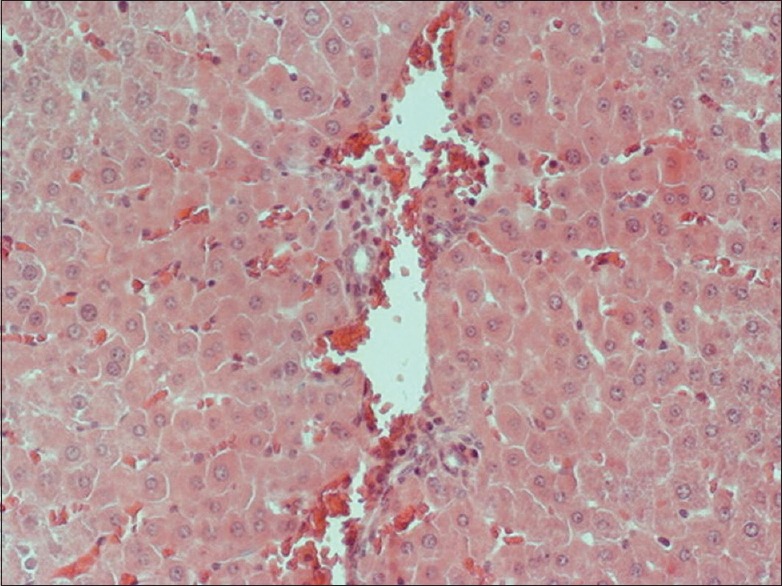
Apigenin treated liver after 240 min I/R 240 × 200
Immunohistochemistry of M30
The stain for M30 demonstrated a significant activation of the gene in I/R groups compared to the sham operation group. Prolongation of reperfusion resulted in an increased activation of M30. I/R groups pretreated with apigenin and subjected to different periods of reperfusion exhibited reduced expression of M30 compared to the respective nontreated animals' groups [Figures 3-8]. This suggests an inhibitory effect of apigenin on liver cell apoptosis.
Figure 3.
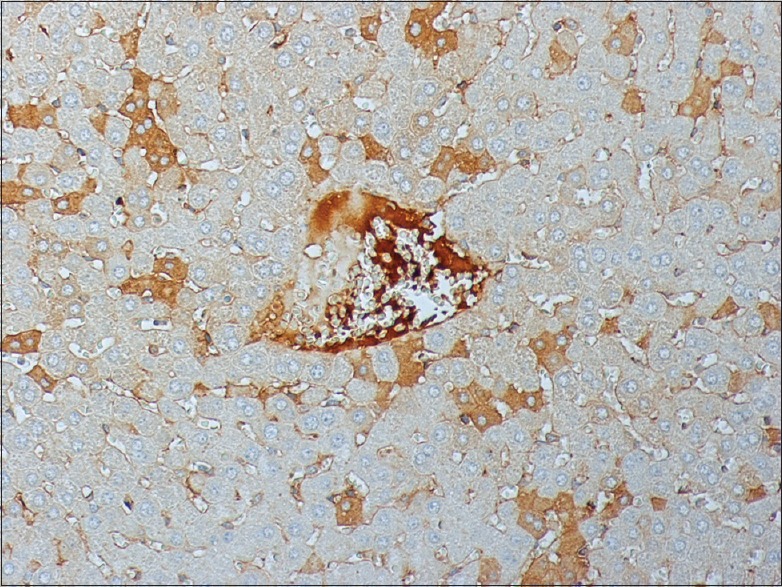
Liver tissue after 60 min I/R stained for M30 240 × 200
Figure 8.
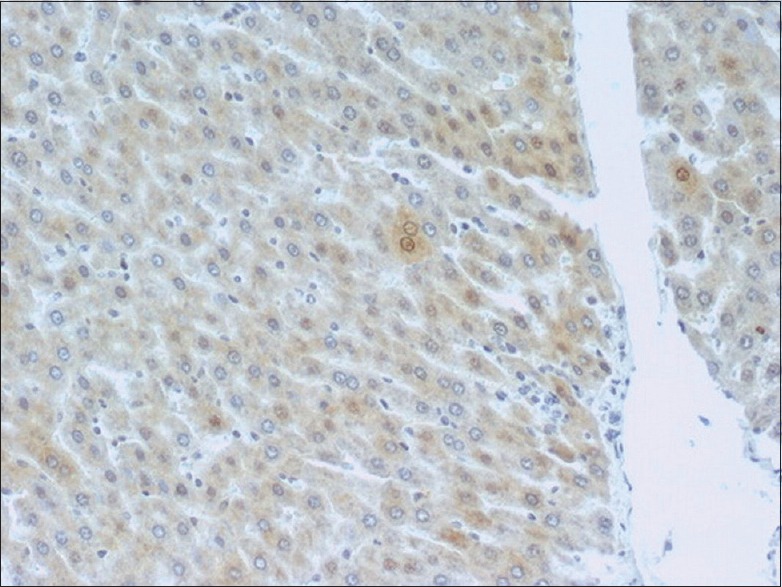
Apigenin treated liver after 240 min I/R stained for M30 240 × 200
Figure 4.
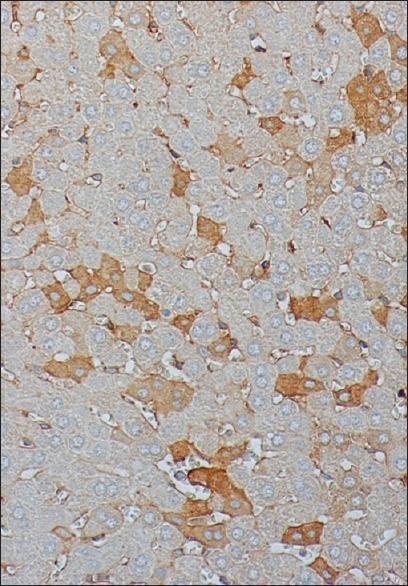
Liver tissue after 120 min I/R stained for M30 240 × 200
Figure 5.
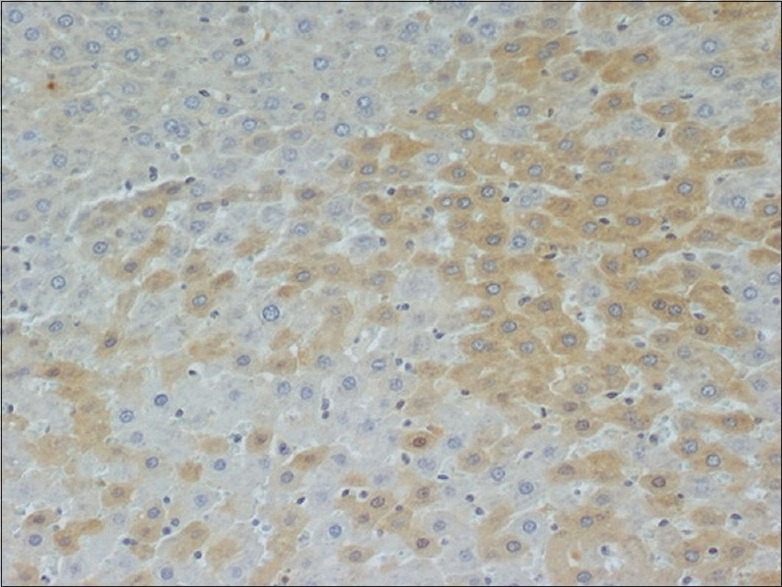
Liver tissue after 240 min I/R stained for M30 240 × 200
Figure 6.
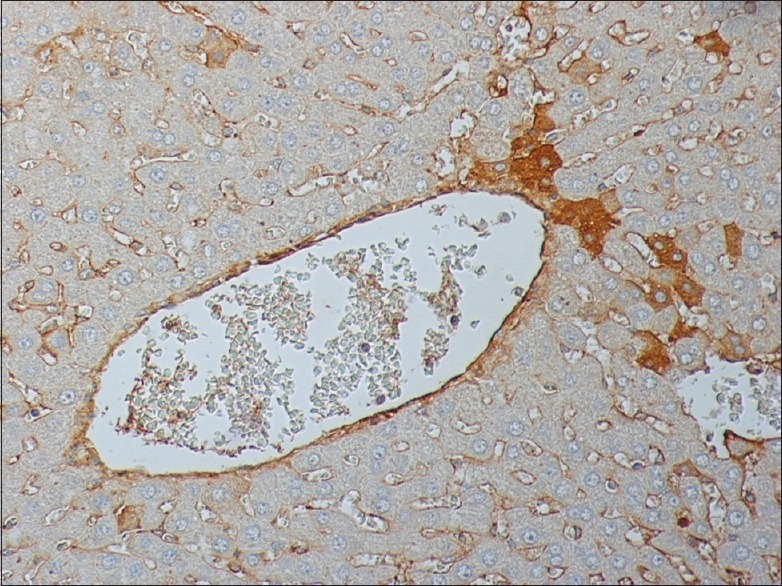
Apigenin treated liver after 60 min I/R stained for M30 240 × 200
Figure 7.
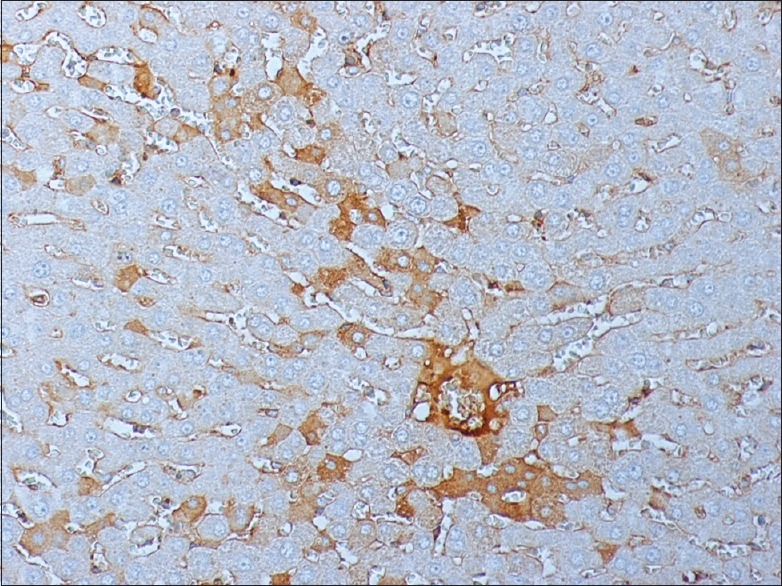
Apigenin treated liver after 120 min I/R stained for M30 240 × 200
Real time polymerase chain reaction
Regulation of expression of BCL-2 and BAX by Apigenin
Liver ischaemia and reperfusion for 60 min resulted in increased expression of BCL-2 compared to the downregulated BCL-2 expression after 120 and 240 min of reperfusion. This can be explained by the enhanced apoptotic activity after 120 min of reperfusion. Administration of apigenin resulted in upregulation of the expression of BCL-2 in groups subjected to 60 min, 120 min, and 240 min of reperfusion compared to the nontreated groups subjected to 60 min, 120 min, and 240 min of reperfusion, respectively [Figure 9]. The increase in the levels of BCL-2 expression was even higher between treated and nontreated groups of 240 min of reperfusion. Sham and DMSO groups showed no difference [Figure 10].
Figure 9.
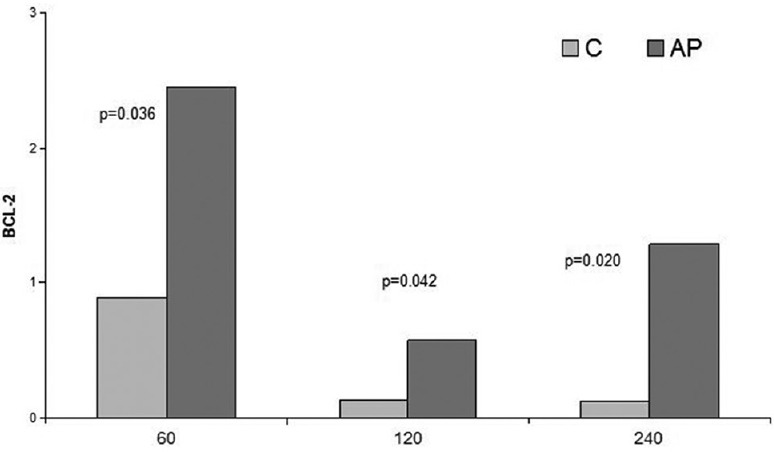
Comparison of I/R groups to respective apigenin treated I/R groups in terms of BCL-2 levels
Figure 10.
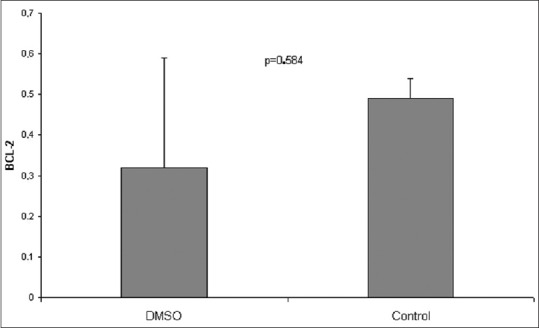
Comparison of BCL-2 levels between dimethyl sulfoxide and sham operation groups
Expression of BAX in liver I/R, sham, and DMSO groups showed no statistically significant differences between all groups. Comparison of the apigenin treated groups with the nontreated groups showed no differences in 60 min and 120 min of reperfusion. After 240 min of reperfusion, the apigenin treated group demonstrated a statistically significant decrease in the expression of proapoptotic BAX compared to the 240 min nontreated group [Figure 11].
Figure 11.
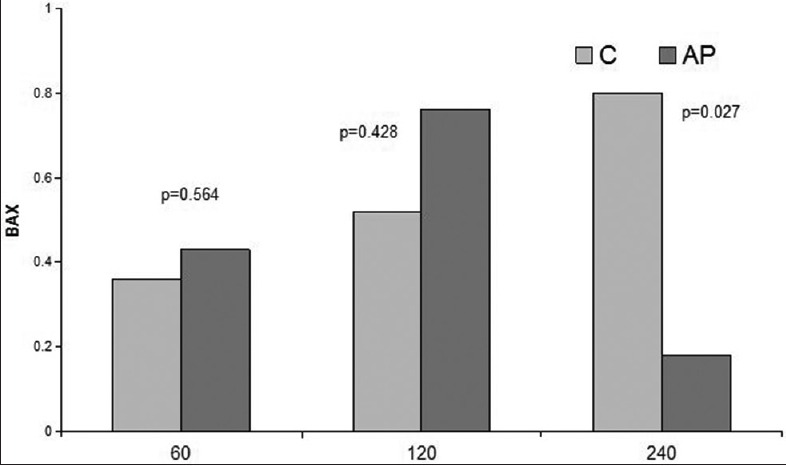
Comparison of I/R groups to respective apigenin treated I/R groups in terms of BAX levels
Enzyme linked immunosorbent assay
M30/M65
As far as M30 was concerned, there was a slight decrease in its concentration in the apigenin treated 60 min and 240 min groups. However, there was a surprising but not significant rise in its concentration in the 120 min group [Figure 12].
Figure 12.
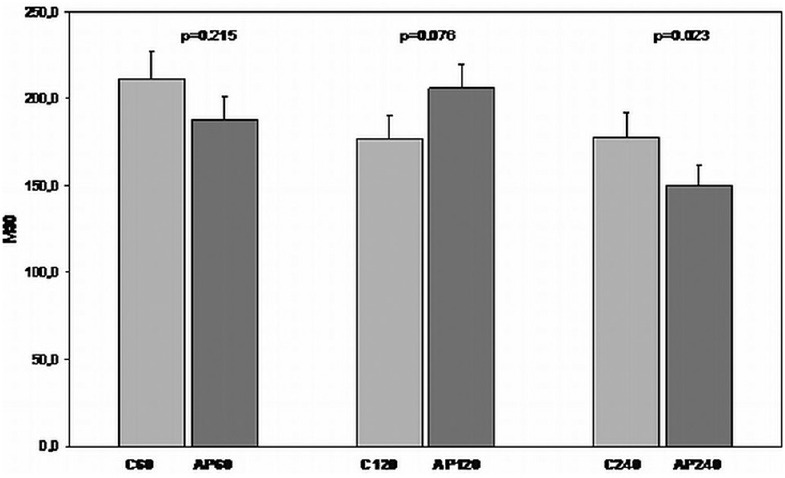
Comparison of I/R groups to respective apigenin treated I/R groups in terms of M30 levels
In the case of M65, apigenin treated groups subjected to 60 min, 120 min, and 240 min of reperfusion showed decreased expression compared with the non-treated animals' groups subjected to 60 min, 120 min, and 240 min of reperfusion, respectively [Figure 13].
Figure 13.
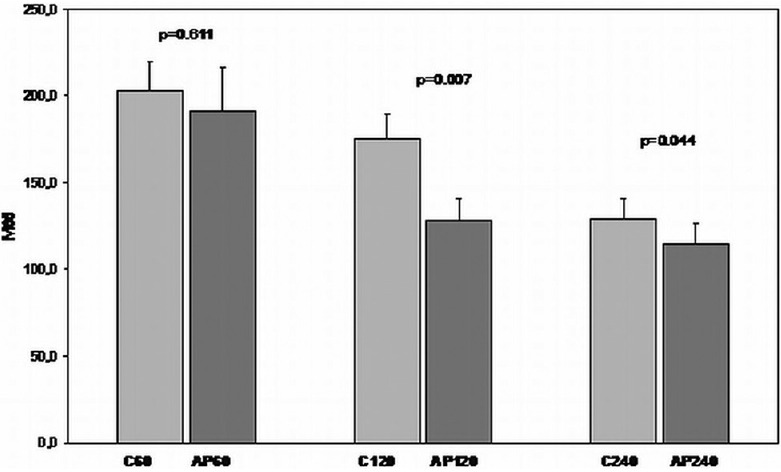
Comparison of I/R groups to respective apigenin treated I/R groups in terms of M65 levels
Regarding the M30/65 ratio, that illustrates how apoptosis is correlated to necrosis, there were no statistically significant differences between the treated and nontreated groups. The ratio did not seem to be affected by the reperfusion time either.
ICAM-1
The expression of ICAM-1 increased after 60 min of reperfusion, decreased after 120 min, and increased again after 240 min of reperfusion. Differences did not reach statistically significant levels. Animals treated with apigenin showed decreased mean levels of ICAM-1 expression in all the different reperfusion periods, however, this reduction was statistically significant only for the apigenin treated 240 min group in comparison with the 240 min nontreated group [Figure 14].
Figure 14.
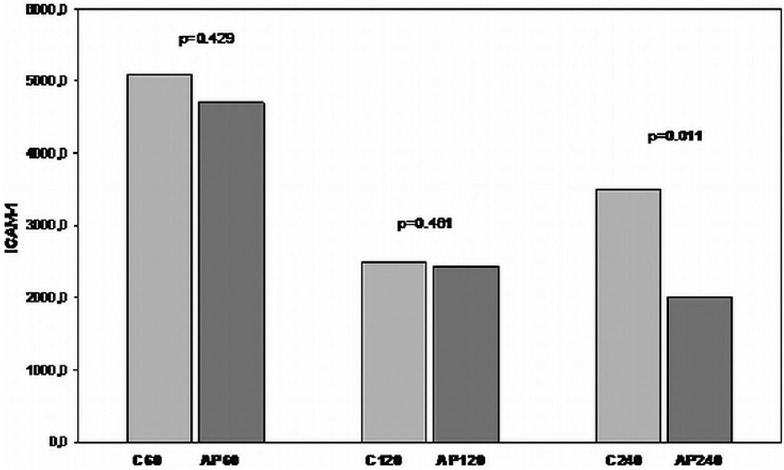
Comparison of I/R groups to respective apigenin treated I/R groups in terms of ICAM-1 levels
Aspartate aminotransferase/alanine aminotransferase
The liver enzymes' serum levels were elevated for all the I/R groups. The mean values for the 60 min I/R group were AST = 914 ± 585.58 U/L and ALT = 122.33 ± 52.78 U/L, compared to the levels for the apigenin treated 60 min I/R group with means of AST 224.4 ± 39.54 U/L and ALT 24.6 ± 3.82 U/L (P = 0.01 and P = 0.0007, respectively). For the 120 min I/R group, AST was 701.6 ± 286.69 U/L and ALT 170.7 ± 138.12 U/L; values were significantly higher compared to those of the 120 min apigenin group; AST 205.25 ± 31.52 U/L and ALT 31 ± 14.61 U/L (P = 0.0005 and P = 0.014, respectively). The results were similar for the 240 min groups with a mean AST of 997.33 ± 394.5 U/L and ALT of 326.83 ± 80.62 U/L for the nontreated group and a mean AST of 84.5 ± 15.2 U/L and ALT of 14.16 ± 2.6 U/L for the apigenin treated group (P = 0.0023 and P = 0.00033, respectively).
DISCUSSION
Liver I/R is a clinical phenomenon presenting in several conditions such as in liver surgery and transplantation as well as in hypovolemic shock leading to hepatic cell apoptosis or necrosis.[1]
In our study, we have illustrated that the intraperitoneal administration of apigenin resulted in an upregulation of the expression of BCL-2 antiapoptotic proteins in groups subjected to 60 min, 120 min, and 240 min of reperfusion compared to the nontreated groups subjected to 60 min, 120 min, and 240 min of reperfusion. The increase in the levels of BCL-2 expression was remarkably higher between treated and nontreated groups of 240 min of reperfusion. The comparison of the apigenin treated groups with the nontreated groups showed no significant differences in BAX levels after 60 min and 120 min of reperfusion. However, after 240 min of reperfusion, the apigenin treated group demonstrated a statistically significant decrease in the expression of BAX compared to the nontreated group. The protective and antiapoptotic action of apigenin through the mitochondrial pathway has been demonstrated in previous studies.[29] Liu et al. in 2014 have shown that apigenin has neuroprotective effects through the inhibition of oxidative damage and the suppression of apoptosis by demonstrating an increase in BCL-2 levels in the apigenin treated groups.[30] Even more specifically in hepatic ischemia-reperfusion injury, Tsalkidou et al., in 2014, have demonstrated that apigenin seems to affect the Fas/FasL mediated pathway of apoptosis, resulting in apoptosis inhibition.[31]
In this study, we have also shown that animals treated with apigenin showed remarkably decreased mean levels of ICAM-1 expression in the apigenin treated 240 min group compared to the 240 min nontreated group, suggesting a limitation of the inflammatory process occurring during reperfusion. A similar study in rats by Savvanis et al. in 2014, also showed a decrease in the ICAM-1 levels as evidence of the attenuation of the I/R injury by sildenafil.[3]
Regarding the M30/65 ratio, that illustrates how apoptosis is correlated to necrosis,[32] there were no statistically significant differences between the treated and nontreated groups.
Finally, according to our histology results, in animals pretreated with apigenin before the onset of hepatic ischemia, attenuation of hepatocellular apoptosis and inflammatory infiltration was noted in contrast to the animals of the I/R group, which exhibited a greater degree of necrosis and inflammation under microscopic evaluation. In all groups receiving apigenin, significant inhibition of Kupffer cells activation was noted, with a more intense impact on the apigenin treated 240 min I/R group compared to 240 min I/R group. As previously shown by Lee et al. in 2016, the Kupffer cell activation contributes to the inflammatory response, and inhibition of this activation can lead to less hepatocellular damage during I/R injury.[33]
Immunohistochemichal stain revealed that I/R groups pretreated with apigenin and subjected to different periods of reperfusion exhibited reduced expression of M30 compared with the nontreated animals with respective reperfusion time, suggesting a decrease in apoptosis for the pretreated groups.[31]
Finally, the serum liver enzymes values of AST and ALT were statistically significantly lower in all the apigenin treated groups compared to the respective nontreated groups, suggesting that apigenin-induced protection against liver injury in the treated groups. As it has been illustrated before by Yang et al. in 2013, apigenin could protect against acute liver damage caused by hepatotoxic agents.[34]
These findings suggest that apigenin has potent actions against hepatic I/R injury through suppression of inflammation, oxidative stress, and apoptosis. Further studies are definitely needed to investigate the exact mechanisms through which apigenin acts at a cellular level and to support the potential benefits of pretreatment with this flavonoid.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Andersson R, Fan J, Wang X. Liver ischaemia following vascular occlusion: A century's experience. Scand J Gastroenterol. 2008;43:1413–5. doi: 10.1080/00365520802008157. [DOI] [PubMed] [Google Scholar]
- 2.Lanteri R, Acquaviva R, Di Giacomo C, Sorrenti V, Li Destri G, Santangelo M, et al. Rutin in rat liver ischemia/reperfusion injury: Effect on DDAH/NOS pathway. Microsurgery. 2007;27:245–51. doi: 10.1002/micr.20345. [DOI] [PubMed] [Google Scholar]
- 3.Savvanis S, Nastos C, Tasoulis MK, Papoutsidakis N, Demonakou M, Karmaniolou I, et al. Sildenafil attenuates hepatocellular injury after liver ischemia reperfusion in rats: A preliminary study. Oxid Med Cell Longev 2014. 2014:161942. doi: 10.1155/2014/161942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr JF, Winterford CM, Harmon BV. Apoptosis: Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saris NE, Eriksson KO. Mitochondrial dysfunction in ischaemia-reperfusion. Acta Anaesthesiol Scand Suppl. 1995;107:171–6. doi: 10.1111/j.1399-6576.1995.tb04353.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F, Mao Y, Qiao H, Jiang H, Zhao H, Chen X. Protective effects of taurine against endotoxin-induced acute liver injury after hepatic ischemia reperfusion. Amino Acids. 2010;38:237–45. doi: 10.1007/s00726-009-0233-z. [DOI] [PubMed] [Google Scholar]
- 8.Mabuchi A, Wake K, Marlini M, Watanabe H, Wheatley A. Protection by Glycyrrhizin against Warm Ischemia-Reperfusion-Induced Cellular Injury and Derangement of the Microcirculatory Blood Flow in the Rat Liver. Microcirculation. 2009;16:164–76. doi: 10.1080/10739680902796917. [DOI] [PubMed] [Google Scholar]
- 9.Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Phenolic compounds protect HepG2 cells from oxidative damage: Relevance of glutathione levels. Life Sci. 2006;79:2056–68. doi: 10.1016/j.lfs.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Acquaviva R, Lanteri R, Li Destri G, Caltabiano R, Vanella L, Lanzafame S, et al. Beneficial effects of rutin and L-arginine coadministration in a rat model of liver ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G664–70. doi: 10.1152/ajpgi.90609.2008. [DOI] [PubMed] [Google Scholar]
- 11.Canbek M, Uyanoglu M, Bayramoglu G, Senturk H, Erkasap N, Koken T, et al. Effects of carvacrol on defects of ischemia-reperfusion in the rat liver. Phytomedicine. 2008;15:447–52. doi: 10.1016/j.phymed.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Tsaroucha A, Papalois A, Vernadakis S, Adamopoulos S, Papadopoulos K, Lambropoulou M, et al. The effect of U-74389G on liver recovery after acute liver ischemia-reperfusion injury in a swine model. J Surg Res. 2009;151:10–4. doi: 10.1016/j.jss.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Sui H, Xu H, Yin SA. Effect of high dose apigenin on antioxidase activity and DNA damage in rats. Wei Sheng Yan Jiu. 2009;38:36–8. [PubMed] [Google Scholar]
- 14.Crespo I, García-Mediavilla MV, Almar M, González P, Tuñón MJ, Sánchez-Campos S, et al. Differential effects of dietary flavonoids on reactive oxygen and nitrogen species generation and changes in antioxidant enzyme expression induced by proinflammatory cytokines in Chang Liver cells. Food Chem Toxicol. 2008;46:1555–69. doi: 10.1016/j.fct.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Jin BH, Qian LB, Chen S, Li J, Wang HP, Bruce IC, et al. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. Eur J Pharmacol. 2009;616:200–5. doi: 10.1016/j.ejphar.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Zhou K, Zhao F, Liu Z, Zhuang Y, Chen L, Qiu F. Triterpenoids and flavonoids from celery (Apium graveolens) J Nat Prod. 2009;72:1563–7. doi: 10.1021/np900117v. [DOI] [PubMed] [Google Scholar]
- 17.Navarro-Núñez L, Rivera J, Guerrero JA, Martínez C, Vicente V, Lozano ML. Differential effects of quercetin, apigenin and genistein on signalling pathways of protease-activated receptors PAR (1) and PAR (4) in platelets. Br J Pharmacol. 2009;158:1548–56. doi: 10.1111/j.1476-5381.2009.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang SW, Kulkarni KH, Tang L, Wang J, Yin T, Daidoji T, et al. Disposition of Flavonoids via Enteric Recycling: UGT1As Deficiency In Gunn Rats Is Compensated by Increases in UGT2Bs Activities. J Pharmacol Exp Ther. 2009;329:1023–31. doi: 10.1124/jpet.108.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung WW. Protective effect of apigenin against oxidative stress-induced damage in osteoblastic cells. Int J Mol Med. 2014;33:1327–34. doi: 10.3892/ijmm.2014.1666. [DOI] [PubMed] [Google Scholar]
- 20.Dong H, Lin W, Wu J, Chen T. Flavonoids activate pregnanexreceptor-mediated CYP3A4 gene expression by inhibiting cyclin-dependent kinases in HepG2 liver carcinoma cells. BMC Biochem. 2010;11:23. doi: 10.1186/1471-2091-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warat M, Szliszka E, Korzonek-Szlacheta I, Król W, Czuba ZP. Chrysin, Apigenin and Acacetin Inhibit Tumor Necrosis Factor-Related Apoptosis—Inducing Ligand Receptor-1 (TRAIL-R1) on Activated RAW264.7 Macrophages. Int J Mol Sci. 2014;15(7):11510–22. doi: 10.3390/ijms150711510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawatzky DA, Willoughby DA, Colville-Nash PR, Rossi AG. The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo. Am J Pathol. 2006;168:33–41. doi: 10.2353/ajpath.2006.050058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simopoulos C, Tsaroucha AK, Asimakopoulos B, Giatromanolaki A, Gavriilidis P, Polychronidis A, et al. Total and caspase-cleaved cytokeratin 18 in chronic cholecystitis: A prospective study. BMC Gastroenterol. 2008;8:14. doi: 10.1186/1471-230X-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig DG, Lee P, Pryde EA, Masterton GS, Hayes PC, Simpson KJ. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 2011;31:1127–36. doi: 10.1111/j.1478-3231.2011.02528.x. [DOI] [PubMed] [Google Scholar]
- 25. [Last accessed on 2016 Jul 18]. http://www.dmso.org/articles/information/herschler.htm .
- 26.Tsai TI, Bui BV, Vingrys AJ. Dimethyl sulphoxide dose-response on rat retinal function. Doc Ophthalmol. 2009;119:199–207. doi: 10.1007/s10633-009-9191-8. [DOI] [PubMed] [Google Scholar]
- 27.Kubista M, Andrade JM, Bengtsson M, Forootan A, Jonák J, Lind K, et al. The real-time polymerase chain reaction. Mol Aspects Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 22DDCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Chan LP, Chou TH, Ding HY, Chen PR, Chiang FY, Kuo PL, et al. Apigenin induces apoptosis via tumor necrosis factor receptor- and Bcl-2-mediated pathway and enhances susceptibility of head and neck squamous cell carcinoma to 5-fluorouracil and cisplatin. Biochim Biophys Acta. 2012;1820:1081–91. doi: 10.1016/j.bbagen.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Kong S, Xie Q, Su J, Li W, Guo H, et al. Protective effects of apigenin against 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Int J Mol Med. 2015;35:739–46. doi: 10.3892/ijmm.2014.2056. [DOI] [PubMed] [Google Scholar]
- 31.Tsalkidou EG, Tsaroucha AK1 Chatzaki E, Lambropoulou M, Papachristou F, Trypsianis G, et al. The effects of apigenin on the expression of Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion injury in rats. Biomed Res Int 2014. 2014:157216. doi: 10.1155/2014/157216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlachos S, Tsaroucha AK, Konstantoudakis G, Papachristou F, Trypsianis G, Schizas D, et al. Serum profiles of M30, M65 and interleukin-17 compared with C-reactive protein in patients with mild and severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2014;21:911–8. doi: 10.1002/jhbp.162. [DOI] [PubMed] [Google Scholar]
- 33.Lee LY, Harberg C, Matkowskyj KA, Cook S, Roenneburg D, Werner S, et al. Cell-Specific Over-activation of Nrf2-mediated Gene Expression in Myeloid Cells Decreases Hepatic Ischemia Reperfusion Injury. Liver Transpl. 2016 doi: 10.1002/lt.24473. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Wang XY, Xue J, Gu ZL, Xie ML. Protective effect of apigenin on mouse acute liver injury induced by acetaminophen is associated with increment of hepatic glutathione reductase activity. Food Funct. 2013;4:939–43. doi: 10.1039/c3fo60071h. [DOI] [PubMed] [Google Scholar]


