Abstract
Background/Aims:
Hepatic fibrosis results in defective liver regeneration following partial hepatectomy. Angiotensin converting enzyme (ACE) inhibitors can enhance liver regeneration and are also involved in the reduction of hepatic fibrosis. The present study has been conducted to evaluate the potential effect of an ACE inhibitor, lisinopril, on the morphological and biochemical aspects of fibrotic liver regeneration.
Materials and Methods:
Eight-week old female Sprague Dawley rats were made fibrotic by intragastric carbon tetrachloride treatment. Rats were given saline or lisinopril (1 mg/kg) orally for 1 week and were subjected to sham surgery or two-third partial hepatectomy. Liver regenerative and functional capacities were determined 48 hours post surgery.
Results:
Lisinopril administration did not affect the regeneration rate, proliferation cell nuclear antigen count, and hepatocellular area of fibrotic livers following partial hepatectomy. No statistically significant difference between treated and control rats regarding mitotic count, hepatocyte nuclear area, and binuclear hepatocyte frequency was observed. Serum biochemical analysis showed that lisinopril non-significantly decreased the partial hepatectomy induced elevated levels of alanine aminotransferase, aspartate transaminase, and alkaline phosphatase whereas lactate dehydrogenase and total bilirubin levels were significantly reduced. No marked reduction in hepatic collagen content and alpha smooth actin positive cells was observed by lisinopril treatment.
Conclusion:
ACE inhibitor lisinopril did not produce major histomorphological alterations in regenerating fibrotic liver following partial hepatectomy, however, it may improve its functional capability.
Keywords: ACE-inihibitors, hepatic fibrosis, partial hepatectomy
The liver has a remarkable capacity to regenerate following loss of hepatic tissue with marvelous sequential changes in gene expression, growth factors, and morphologic structure.[1] Liver regeneration following partial hepatectomy (PHx) involves the proliferation of all mature cell populations comprising the organ, especially the hepatocytes, the main cellular components of liver. They are highly differentiated cells that do not divide under normal physiological conditions; however, hepatic resection leads to their hypertrophy and hyperplasia allowing the liver to regenerate to its original size.[2,3] In contrast to normal liver, fibrotic and cirrhotic liver has impaired and slow regeneration.[4,5] Defective regeneration and dysfunction of cirrhotic liver result in postoperative deaths after surgery.[6] Reduced hepatocellular proliferation and increased extracellular matrix are main reasons for the retarded regeneration of fibrotic and cirrhotic livers.[4,7,8]
Several substances including angiotensin converting enzyme (ACE) inhibitors have been identified that potentiate liver regeneration following PHx.[9,10,11] These drugs enhance liver regeneration by increased production of hepatocyte growth factor (HGF); a potent growth factor for hepatocytes.[9,10] Renin-angiotensin axis blockade by angiotensin receptor antagonists also results in improved liver regeneration following PHx.[10] In addition to facilitating liver regeneration, ACE inhibitors have been reported to affect hepatic fibrogenesis.[12,13] Several studies concerning potential benefits of ACE inhibitors against hepatic fibrosis have been conducted.[14,15,16] Angiotensin II, the product of renin-angiotensin system, enhances hepatic fibrosis by hepatic stellate cells activation and augmented transforming growth factor-β1 (TGF-β1) production; the major profibrotic cytokine.[17,18] ACE inhibitors by inhibiting the production of Angiotensin II inhibits the progression of hepatic fibrosis due to decreased extracellular matrix production.[15] In view of the role of ACE inhibitors in normal liver regeneration and hepatic fibrosis, the present study has been designed to evaluate the efficacy of an ACE inhibitor, lisinopril, on fibrotic liver regeneration.
MATERIAL AND METHODS
Animals
Eight-weeks old female Sprague-Dawley rats weighing approximately 200-225 g and bred in animal house facilities of Quaid-i-Azam university Islamabad were used in the study. Animals were kept under controlled environmental conditions at 25°C with a 12 h light/dark cycle.
Induction of fibrosis and experimental procedure
The already established carbon tetrachloride (CCl4) induced fibrotic rat model[19] was used for scheduling the CCl4 dose regimen. In brief, rats were orally treated with CCl4 at an initial dose of 412 mg/kg for 9 weeks. After establishment of fibrosis, animals were divided into 3 groups:
First group received 1 ml saline for 1 week followed by sham surgery (n = 5)
The second group served as control. Animals in this group also received saline for 1 week followed by 70% partial hepatectomy (n = 6)
The animals in the third group received oral dose of 1 mg/kg lisinopril for 1 week followed by 70% partial hepatectomy (n = 6).
Partial hepatectomy
The animals were subjected to sham surgeries or 70% partial hepatectomy according to the already reported instructions.[20] In brief, animals were anesthetized with diethyl ether and a midline incision was made; the median and left lateral lobes were ligated by silk suture and resected. The peritoneum was then reapproximated with catgut followed by closure of the skin with silk sutures. Animals were dissected 48 h after the surgery. The liver remnants were removed and weighed for liver regeneration rate (LRR) determination. Blood was collected by cardiac puncture; serum was collected and stored at − 20°C for biochemical analysis. For histopathological examination, parts of the excised livers were processed for histology.
Liver regenerative capacity following lisinopril pretreatment was determined by estimating LRR through the following formula[21]
LRR (%) =100 × {(C − (A − B)}/A
where A is the estimated liver weight at surgery; B is the excised liver weight at surgery; and C is the remnant liver weight at dissection. Estimated liver weight was calculated by the equation A = B/0.70
Histopathological examination
Liver specimens were fixed in 4% paraformaldehyde followed by dehydration in ascending grades of alcohol, clearing in xylene, and embedding in paraffin. Thin sections were stained with hematoxylin and eosin (H and E) for histomorphological examination. Mitotic figures and binuclear hepatocyte cell frequency were manually enumerated at 400× magnification and were expressed as percentage. Using ImageJ2X (Rawak Software), hepatocyte nuclear and cellular area was calculated in the same sections. For collagenous connective tissue assessment in liver tissue, Gomori's trichrome staining was done. In brief, sections were deparaffinized in xylene and hydrated with descending alcohol grades to distilled water and stained with Weigert's hematoxylin for 10 mins. Slides were washed in running water for 10 mins. Sections were stained for 15 to 20 mins in Gomori's trichrome stain. Differentiation was done in 0.5% acetic acid by placing slides in it for 2 mins followed by dehydration with alcohol, clearing in xylene, and mounting with distyrene plasticizer xylene (DPX).
Immunohistochemistry
Proliferating cell nuclear antigen staining
Cell proliferation was also evaluated with immunohistochemical staining for proliferating cell nuclear antigen (PCNA) using Invitrogen PCNA staining kit, UK. After deparaffinization in xylene and rehydration with alcohol, sections were treated with 3% hydrogen peroxide in methanol for 10 min to block the endogenous peroxidase activity. Heat induced epitope retrieval (HIER) was used to enhance the specific antigen staining by placing the slides in antigen retrieval solution at 89°C for 10 min. The solution was slowly cooled to room temperature and the specimens were incubated with ready to use blocking solution at room temperature for 10 mins. Tissues were then incubated with biotinylated monoclonal mouse anti-PCNA primary antibody in a moist chamber for an hour followed by rinsing with phosphate buffer saline (PBS). The specimens were incubated with streptavidin-peroxidase at room temperature for 10 min and with diaminobenzidine (DAB) chromogen for 5 min. Hematoxylin was used for counterstaining. Hepatocytes with PCNA-positive nuclei and the total number of hepatocytes were counted in five random microscopic fields at 400× magnification to compute the cell proliferation index.
Alpha-smooth muscle actin staining
Activated hepatic stellate cells were identified by alpha smooth muscle actin (α-SMA) immunostaining. In brief, following deparaffinization and rehydration, the sections were treated with 3% hydrogen peroxidase in methanol for 15 min to block endogenous peroxidase activity and were then washed with PBS. Antigen retrieval was performed by heating the slides for 10 min in a microwave oven in 0.1 mol/L citrate buffer (pH 6.0). Tissue sections were treated with normal horse serum for 10 min to avoid non-specific immunoreactivity and were again washed twice with PBS. Sections were then incubated with primary mouse actin smooth muscle (BioGenex, USA clone 1A4) antibody for 30 min at room temperature. After washing with PBS, tissues were incubated at room temperature with biotinylated goat anti-mouse antibody for 10 min followed by incubation with streptavidin horseradish peroxidase conjugate at room temperature for 10 min. Slides were again washed with PBS and the bound peroxidase was detected using DAB chromogen. Hematoxylin was used for counterstaining.
Biochemical analysis
Hepatic functional capability
Liver functional capability following PHx under lisinopril pretreatment was evaluated by serum biochemical analysis. Serum concentrations of alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and total bilirubin were spectrophotometrically determined by using commercially available AMP Diagnostics kits, Austria. All the reactions were performed at 37°C.
Hepatic hydroxyproline content
Hepatic hydroxyproline levels were determined through alkaline hydrolysis of dry liver samples, as was previously described.[22] Hydroxyproline levels in tissue hydrolyzates were analyzed using Chloramine-T and Ehrlich's reagent. The absorbencies of chromophore produced by standards and samples were read at 550 nm and the results are reported as mg hydroxyproline/g of dry tissue.
Statistical analysis
Data were presented as mean ± SD and was analyzed by using student's t-test and one way analysis of variance (ANOVA) with Tukey post hoc test. Statistical significance was taken at P < 0.05.
RESULTS
Liver regeneration assessment
Effect of lisinopril pretreatment on fibrotic liver regeneration at 48 h following 70% PHx was assessed by estimating somatometric and histomorphological parameters. Results showed that lisinopril did not affect LRR and PCNA count, however, a decreasing effect on mitotic activity was seen [Table 1]. PHx increased the nuclear and hepatocyte area whereas binucler cells were decreased. No statistically significant effect of lisinopril on hepatocyte cellular area, nuclear area, and binuclear count was observed [Table 2 and Figure 1].
Table 1.
Effect of ACE-inhibitor, lisinopril, on liver regeneration rate, proliferating cell nuclear antigen (PCNA) index, and mitotic activity at 48 h following partial hepatectomy
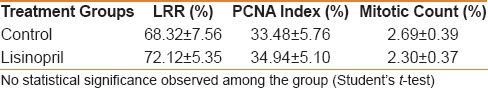
Table 2.
Effect of ACE-inhibitor, lisinopril, on hepatocyte cellular area, hepatocyte nuclear area, and binuclear cell count at 48 h following partial hepatectomy
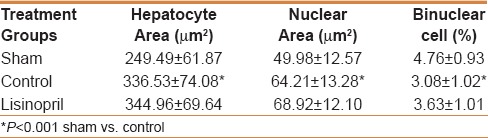
Figure 1.
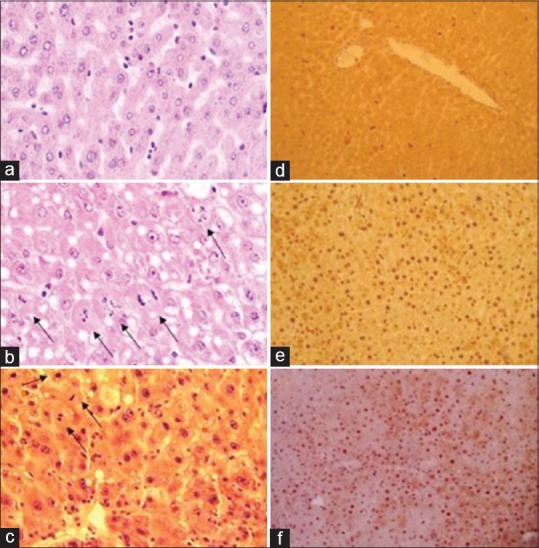
Effect of lisinopril treatment on hepatic histomorphology following partial hepatectomy (PHx). Compared to the sham (a and d), cells at different stages of cell cycle could be seen in regenerating livers (b and c, e and f). Lisinopril did not affect PCNA count, however, a decreasing effect on hepatic mitotic activity had been seen (f and c). PHx significantly increased the hepatocyte nuclear and cellular area whereas binuclear hepatocyte frequency was decreased (b). No significant morphological alterations regarding these parameters under lisinopril treatment was observed (c). (H and E stain; ×400)
Liver functional capability
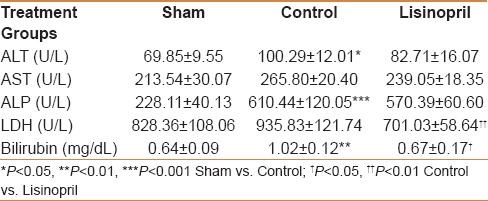
In order to evaluate liver functional capability, serum biochemical analysis of different liver-specific enzymes and metabolite was done. PHx resulted in elevation of all the studied parameters. Levels of ALT, ALP, and total bilirubin were significantly increased whereas a non-significant increase in AST and LDH levels were seen. A considerable decrease in LDH and total bilirubin levels in lisinopril-treated rats 48 h following PHx was observed. ALT, AST, and ALP levels were also low in that group, however, the reduction was not statistically significant [Table 3].
Table 3.
Effect of ACE-inhibitor, lisinopril on liver specific enzymes and total bilirubin at 48 h following partial hepatectomy
Extracellular matrix content
Hepatic hydroxyproline content
Lisinopril administration could not reduce hepatic hydroxyproline levels 48 h following PHx [Figure 2].
Figure 2.
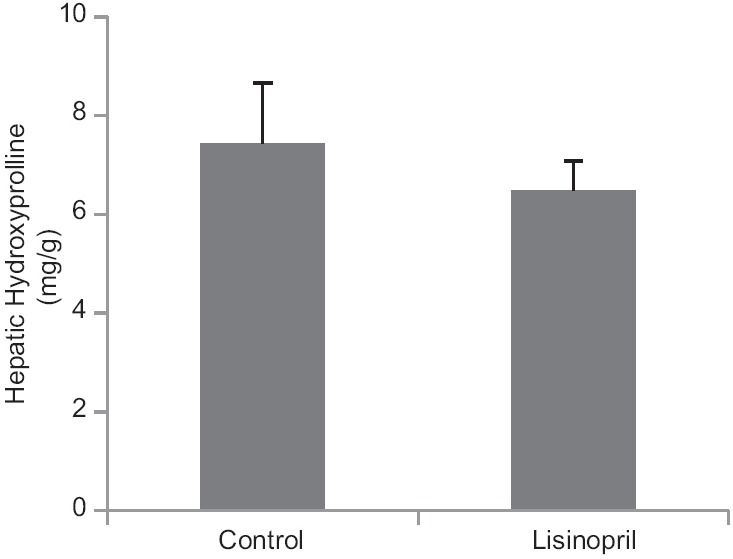
Effect of ACE-inhibitor, lisinopril on hepatic hydroxyproline content (mg/g dry mass) 48 hours following partial hepatectomy
Histopathological collagen estimation
All partially hepatectomized rats showed lipid droplets and increased extracellular matrix 48 h post PHx during histopathological liver evaluation. Activated hepatic stellate cells, as detected by α-SMA immunostaining, were more numerous in partially hepatectomized rats compared to sham. These cells could be detected at vascular and sinusoidal spaces. Gomori triple staining also showed the same result with increased collagen fibers in hepatectomized rats. Lisinopril treatment did not produce marked reduction in hepatic collagen content following PHx because, in both control and treated groups, prominent collagen fibers along with α-SMA positive cells were visible [Figure 3].
Figure 3.
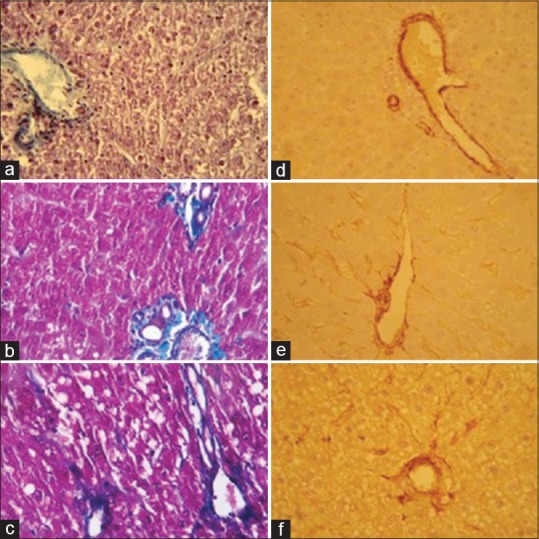
Changes in hepatic extracellular matrix under lisinopril treatment following partial hepatectomy (PHx). Gomori triple stain indicated increased extracellular matrix in regenerating livers compared to sham during histopathological liver evaluation (a-c). Activated hepatic stellate cells as detected by α-SMA immunostaining were also more numerous in partially hepatectomized rats than sham. α-SMA positive cells could be seen at vascular and sinusoidal spaces (d-f). No marked reduction in hepatic collagen content following PHx under lisinopril treatment was seen (c and f). (×400)
DISCUSSION
In this study, the efficacy of an ACE inhibitor, lisinopril, in improving the fibrotic liver regeneration has been evaluated. Liver regeneration following PHx is a combined effect of hypertrophy and hyperplasia.[3] Liver regenerative ability of fibrotic rats 48 h post PHx under lisinopril treatment had been assessed by estimating different hyperplasic and hypertrophic parameters. ACE inhibitor, lisinopril, has been reported to enhance normal liver regeneration after 70% hepatectomy.[9,10] Hepatic PCNA expression has been shown to significantly increase by ACE inhibitors and other related drugs during normal liver regeneration.[10] The present study showed that lisinopril did not enhance the regeneration rate of fibrotic livers because LRR was almost unchanged in the treated group compared to control. PCNA count also remained unchanged with lisinopril treatment, however, a decreasing trend in hepatocyte mitotic activity had been seen after hepatectomy, showing that lisinopril administration did not affect hepatocytes in the S phase of cell cycle, however, it inhibited the cells to enter in the M phase [Figure 1]. Studies reported on the effect of ACE inhibitors and angiotensin receptor antagonists on liver regeneration used PCNA, bromo-deoxyuridine (BrdU), and Ki-67 as proliferative markers,[9,10,11] but no study showed the effect of these drugs on mitotic count. The reasons of less mitotic count are unknown because ACE inhibitors enhance the hepatic regeneration after PHx by bradykinin B2 receptor activation and angiotensin II production inhibition, both of which may result in increased HGF production, thus resulting in enhancement of liver regeneration as HGF is the most important growth factor of hepatocytes.[23] One reason could be the involvement of HSCs because lisinopril treatment could not decrease their number. An ACE inhibitor captopril was found to enhance the liver regeneration at earlier stages by increasing HSCs rather than hepatocytes. In fact, it decreased the hepatocyte proliferation, as assessed by Ki-67 antibody.[11] HSCs generate such factors at later stages of liver regeneration such as TGF-β1, which inhibit hepatocyte proliferation.[24]
Moreover, angiotensin receptor antagonists have been reported to produce inhibition of hepatic proliferative activity after PHx when PCNA was used as a proliferative marker[25] whereas these receptors have been found to enhance the hepatic regeneration ability like lisinopril when liver regenerative response was measured by BrdU incorporation.[10] It can be said that different regenerative indexes may show different results under a certain treatment.
Partial hepatectomy results in the hypertrophy of hepatocytes, as indicated by increased hepatocyte area;[3] the same hypertrophic effect could be seen during fibrotic liver regeneration because the size of hepatocytes increased significantly after PHx [Figure 1]. Along with hepatocellular area, increase in hepatocyte nuclear was also observed. Increased hepatocyte ploidy during liver regeneration following PHx has already been reported.[26] ACE inhibitor captopril was found to decrease the hepatocyte cellular size at 48 h after PHx during normal liver regeneration[11] whereas in this study we observed that lisinopril treatment did not affect hepatocyte nuclear or cellular area, leading to conclude that it had no effect on hepatocyte hypertrophy during fibrotic liver regeneration.
Binucleation is an interesting feature of hapatocytes; whose number decreases after 70% PHx.[27,28] During fibrotic liver regeneration, the binuclear cell count decreased significantly, and in lisinopril-treated rats a non-significant increase of binuclear cells was seen. More binucleate cells and less mitotic activity during hepatic regeneration after lisinopril treatment are related events because there exists a close parallel relation between mitotic division and the fall in binucleation after 70% PHx.[29]
Although lisinopril failed to enhance the hyperplasic or hypertrophic events during fibrotic liver regeneration, it did not negatively affect the studied parameters, with the exception of hepatocyte mitosis, because most of the parameters remained unchanged rather than reduced by the lisinopril treatment.
Serum biochemical analysis showed that liver function enzymes, i.e., ALT, AST, ALP, LDH and total bilirubin levels, were elevated after PHx due to increased cellular damage by surgery.[30] Lisinopril was successful in significantly or non-significantly decreasing all the studied liver functional parameters of regenerating fibrotic liver at 48 h following PHx. Lisinopril pretreatment has already been reported to significantly decrease the hepatic ischemia reperfusion induced increase in hepatic enzymes and total bilirubin levels.[31] Captopril was also found to improve the liver function during early stages of normal liver regeneration;[11] we previously reported that lisinopril administration significantly decrease the hepatic functional markers 24 h post PHx during fibrotic liver regeneration.[32] Moreover, ACE-inhibitors and Angiotensin II blockers have been shown to reduce the above mentioned liver function markers in several studies.[33,34]
ACE inhibitors are known to reduce oxidative stress in different pathophysiological conditions.[35,36] They can reduce plasma malondialdehyde and increase erythrocyte glutathione content in rats.[37] The decreased lipid peroxidative stress by ACE inhibitors may result in the structural integrity of hepatocytes, thus improving the overall liver function.
A characteristic feature of lipid accumulation after PHx[38] and increased collagen content[39] had been seen in all the hepatectomized rats in the study. Anti-fibrogenic effect of ACE inhibitors has been observed by histopathological examination.[14,40] In the present study, no considerable reduction in hepatic collagen content in fibrotic rats under lisinopril treatment after hepatectomy was seen, as is evident by Gomori triple staining. α-SMA immunostaining cleared the picture and showed almost unaffected number of activated hepatic stellate cells in treated rats compared to control. It could be said that lisinopril treatment can reduce fibrosis in normal rats significantly, however, in regenerating fibrotic livers, it cannot produce a significant effect. These drugs are reported to activate stellate cells in regenerating livers,[41] which are the major fibrogenic cells of liver.[42]
We concluded that ACE inhibitor, lisinopril did not produce major histomorphological alterations in regenerating fibrotic livers 48 h following PHx with the exception of non-significant mitosis inhibition and increased hepatocyte binucleation. However, it may improve the hepatic functional capability of fibrotic rats after surgery. More detailed studies with sampling at earlier time points after surgery may better explain the effect of lisinopril on fibrotic liver regeneration.
Financial support and sponsorship
Higher Education Commission of Pakistan.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Fausto N. Liver regeneration. J hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 3.Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166–75. doi: 10.1016/j.cub.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Andiran F, Ayhan A, Tanyel FC, Abbasoğlu O, Sayek I. Regenerative capacities of normal and cirrhotic livers following 70% hepatectomy in rats and the effect of α-tocopherol on cirrhotic regeneration. J Surg Res. 2000;89:184–8. doi: 10.1006/jsre.2000.5825. [DOI] [PubMed] [Google Scholar]
- 5.Suárez-Cuenca JA1, Chagoya de Sánchez V, Aranda-Fraustro A, Sánchez-Sevilla L, Martínez-Pérez L, Hernández-Muñoz R. Partial hepatectomy induced regeneration accelerates reversion of liver fibrosis involving participation of hepatic stellate cells. Exp Biol Med. 2008;233:827–39. doi: 10.3181/0709-RM-247. [DOI] [PubMed] [Google Scholar]
- 6.Redaelli CA, Wagner M, Krähenbühl L, Gloor B, Schilling MK, Dufour JF, et al. Liver surgery in the era of tissue-preserving resections: Early and late outcome in patients with primary and secondary hepatic tumors. World J Surg. 2002;26:1126–32. doi: 10.1007/s00268-002-6321-9. [DOI] [PubMed] [Google Scholar]
- 7.Kato A, Bamba H, Shinohara M, Yamauchi A, Ota S, Kawamoto C, et al. Relationship between expression of cyclin D1 and impaired liver regeneration observed in fibrotic or cirrhotic rats. J Gastroenterol Hepatol. 2005;20:1198–205. doi: 10.1111/j.1440-1746.2005.03829.x. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Togo S, Kunihiro O, Fujii Y, Kurosawa H, Tanaka K, et al. Clinicohistological features of liver failure after excessive hepatectomy. Hepatogastroenterology. 2001;49:354–8. [PubMed] [Google Scholar]
- 9.Ramalho FS, Ramalho LNZ, Castro-e-Silva Júnior O, Zucoloto S, Corrêa FMA. Angiotensin-converting enzyme inhibition by lisinopril enhances liver regeneration in rats. Braz J Med Biol Res. 2001;34:125–7. doi: 10.1590/s0100-879x2001000100016. [DOI] [PubMed] [Google Scholar]
- 10.Yayama K, Sugiyama K, Miyagi R, Okamoto H. Angiotensin-converting enzyme inhibitor enhances liver regeneration following partial hepatectomy: Involvement of bradykinin B2 and angiotensin AT1 receptors. Biol Pharm Bull. 2007;30:591–4. doi: 10.1248/bpb.30.591. [DOI] [PubMed] [Google Scholar]
- 11.Koh SL, Ager E, Malcontenti-Wilson C, Muralidharan V, Christophi C. Blockade of the renin–angiotensin system improves the early stages of liver regeneration and liver function. J Surg Res. 2013;179:66–71. doi: 10.1016/j.jss.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Warner F, Lubel J, McCaughan G, Angus P. Liver fibrosis: A balance of ACEs. Clin Sci. 2007;113:109–18. doi: 10.1042/CS20070026. [DOI] [PubMed] [Google Scholar]
- 13.Yoshiji H, Noguchi R, Ikenaka Y, Kitade M, Kaji K, Tsujimoto T, et al. Renin-angiotensin system inhibitors as therapeutic alternatives in the treatment of chronic liver diseases. Curr Med Chem. 2007;14:2749–54. doi: 10.2174/092986707782360169. [DOI] [PubMed] [Google Scholar]
- 14.Toblli JE, Muñoz MC, Cao G, Mella J, Pereyra L, Mastai R. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese Zucker rats. Obesity. 2008;16:770–6. doi: 10.1038/oby.2007.114. [DOI] [PubMed] [Google Scholar]
- 15.Türkay C, Yönem O, Arici S, Koyuncu A, Kanbay M. Effect of angiotensin-converting enzyme inhibition on experimental hepatic fibrogenesis. Dig Dis Sci. 2008;53:789–93. doi: 10.1007/s10620-007-9941-y. [DOI] [PubMed] [Google Scholar]
- 16.Huang ML, Li X, Meng Y, Xiao B, Ma Q, Ying SS, et al. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol Physiol. 2010;37:e1–6. doi: 10.1111/j.1440-1681.2009.05302.x. [DOI] [PubMed] [Google Scholar]
- 17.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFβ1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 18.Bataller R, Ginès P, Nicolás JM, Görbig MN, Garcia-Ramallo E, Gasull X, et al. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–56. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 19.Proctor E, Chatamra K. Controlled induction of cirrhosis in the rat. Br J Exp Pathol. 1983;64:320–30. [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins GM, Anderson RM. Experimental pathology of the liver-Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 21.Fishback FC. A morphologic study of regeneration of the liver after partial removal. Arch Pathol. 1929;7:955–77. [Google Scholar]
- 22.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–3. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 24.Karkampouna S, Ten Dijke P, Dooley S, Julio MK. TGF-beta signaling in liver regeneration. Curr Pharm Des. 2012;18:4103–13. doi: 10.2174/138161212802430521. [DOI] [PubMed] [Google Scholar]
- 25.Ramalho FS, Ramalho LN, Castro-e-Silva Júnior O, Zucoloto S, Corrêa FM. Effect of angiotensin-converting enzyme inhibitors on liver regeneration in rats. Hepatogastroenterology. 2002;49:1347–51. [PubMed] [Google Scholar]
- 26.Gandillet A, Alexandre E, Royer C, Cinqualbre J, Jaeck D, Richert L. Hepatocyte ploidy in regenerating livers after partial hepatectomy, drug-induced necrosis, and cirrhosis. Eur Surg Res. 2003;35:148–60. doi: 10.1159/000070044. [DOI] [PubMed] [Google Scholar]
- 27.Beams HW, King RL. The origin of binucleate and large mono nucleate cells in the liver of the rat. Anat Rec. 1942;83:281–97. [Google Scholar]
- 28.Sulkin NM. A study of the nucleus in the normal and hyperplastic liver of the rat. Am J Anat. 1943;73:107–25. [Google Scholar]
- 29.Wheatley DN. Binucleation in mammalian liver: Studies on the control of cytokinesis in vivo. Exp Cell Res. 1972;74:455–65. doi: 10.1016/0014-4827(72)90401-6. [DOI] [PubMed] [Google Scholar]
- 30.Aly HF, Mohamad HE, Soliman AM, Shams SGE. Role of melatonin in management of partially hepatectomized and/or propagated-cirrhotic livers of rats. Int J Pharm Sci Rev Res. 2014;26:11–31. [Google Scholar]
- 31.Morsy MA. Protective effect of lisinopril on hepatic ischemia/reperfusion injury in rats. Indian J Pharmacol. 2011;43:652–5. doi: 10.4103/0253-7613.89820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambreen A, Samina J, Sarwat J, Naushaba M, Riffat G. Effect of lisinopril, an angiotensin-converting enzyme inhibitor, on fibrotic liver regeneration. Am J Biol Life Sci. 2015;3:228–31. [Google Scholar]
- 33.Wei HS, Lu HM, Li DG, Zhan YT, Wang ZR, Huang X, et al. The regulatory role of AT 1 receptor on activated HSCs in hepatic fibrogenesis: Effects of RAS inhibitors on hepatic fibrosis induced by CCl4. World J Gastroenterol. 2000;6:824–8. doi: 10.3748/wjg.v6.i6.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abd-Allah OM, Sharaf EI-Din AAI. Evaluation of antifibrotic and antioxidant effects of olmesartan medoximil, a new angiotensin II blocker, on experimentally-induced liver fibrosis in rats. J Egypt Soc Pharmacol Exp Ther. 2008;29:553–77. [Google Scholar]
- 35.Khan BV, Sola S, Lauten WB, Natarajan R, Hooper WC, Menon RG, et al. Quinapril, an ACE inhibitor, reduces markers of oxidative stress in the metabolic syndrome. Diabetes care. 2004;27:1712–5. doi: 10.2337/diacare.27.7.1712. [DOI] [PubMed] [Google Scholar]
- 36.Mashhoody T, Rastegar K, Zal F. Perindopril may improve the hippocampal reduced glutathione content in rats. Adv Pharm Bull. 2014;4:155–9. doi: 10.5681/apb.2014.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huda S, Akhter N. Modulation of oxidative stress by enalapril and valsartan in adrenaline treated rats: A comparative study. Bangladesh Med Res Counc Bull. 2014;40:25–30. doi: 10.3329/bmrcb.v40i1.20333. [DOI] [PubMed] [Google Scholar]
- 38.Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, et al. Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models. Hepatology. 2008;48:1097–105. doi: 10.1002/hep.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojkind M, Rojkind MH, Cordero-Hernandez J. In vivo collagen synthesis and deposition in fibrotic and regenerating rat livers. Coll Relat Res. 1983;3:335–47. doi: 10.1016/s0174-173x(83)80015-6. [DOI] [PubMed] [Google Scholar]
- 40.Tuncer I, Ozbek H, Ugras H, Ugras S, Bayram I. Anti-fibrogenic effects of captopril and candesartan cilexetil on the hepatic fibrosis development in rat: The effect of AT1-R blocker on the hepatic fibrosis. Exp Toxicol Pathol. 2003;55:159–66. doi: 10.1078/0940-2993-00309. [DOI] [PubMed] [Google Scholar]
- 41.Ramalho LN, Zucoloto S, Ramalho FS, Castro-e-Silva Júnior O, Corrêa FMA. Effect of antihypertensive agents on stellate cells during liver regeneration in rats. Arq Gastroenterol. 2003;40:40–4. doi: 10.1590/s0004-28032003000100009. [DOI] [PubMed] [Google Scholar]
- 42.Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–40. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]


