Abstract
Background/Aims:
Deoxyschizandrin as one of the most important component of Schisandra chinensis (Turcz.) Baill plays an immunomodulatory role in a variety of diseases, yet its role in ulcerative colitis remains to be elucidated. We aimed to investigate the role of deoxyschizandrin in DSS-induced ulcerative colitis in mice.
Patients and Methods:
In the present study, an inflammation model of cells was constructed to confirm the anti-inflammatory effect of deoxyschizandrin. Then a mouse model with Dextran sulfate sodium sulfate (DSS)-induced ulcerative colitis was constructed, and the effects of deoxyschizandrin on mouse colon inflammation, apoptosis, and CD4 T lymphocyte infiltration in ulcerative colitis were examined.
Result:
Deoxyschizandrin could improve the symptoms of ulcerative colitis, determined by hematoxylin-eosin (HE) staining and histopathological scores. Moreover, deoxyschizandrin reduced the levels of inflammatory cytokines, suppressed CD4 T cell infiltration, and effectively inhibited apoptosis in the colon of DSS-induced ulcerative colitis mice.
Conclusion:
In summary, deoxyschizandrin can effectively rescue the symptoms of DSS-induced ulcerative colitis in mice by inhibiting inflammation. T cell infiltration and apoptosis in the colon, suggesting that deoxyschizandrin could be a potential drug in treating ulcerative colitis.
Keywords: Apoptosis, deoxyschizandrin, inflammation, inflammatory bowel disease, ulcerative colitis
Ulcerative colitis (UC) is a chronic nonspecific inflammatory bowel disease (IBD) with unknown causes. The lesions are mainly concentrated in the mucous layer and submucosa, with ulceration and crypt abscesses as the main features. UC shows wide range of lesions and a long illness, leading to difficulty in treatment and recovery. Moreover, it is prone to recurrence after treatment and can be associated with the risk of concurrent colon cancer, seriously affecting the quality of life of the patients and their families. Thus, the World Health Organization delimits UC as one of the most difficult and complicated modern diseases in terms of treatment.[1,2] In recent years, the incidence of UC in China is in a trend of slow increase,[3,4] yet the pathogenesis of UC remains unclear. With extensive research, it is generally believed that there are multiple causes for UC, including genetic, environmental, infectious, microbiological, and immunological factors.[5,6] In addition, excessive activation and disregulation of the adaptive immune response may be the most direct and important factor.[7] Therefore, understanding the immune-related mechanism underlying the pathogenesis of UC is of great significance for improving cure rate, shortening disease duration, and reducing complications.
Schisandra chinensis (Turcz.) Baill. has been widely used as traditional Chinese medicine for thousands of years owing to its diverse pharmacological effects.[8,9] Deoxyschizandrin, as one of the most important lignans, is a major active compound of S. chinensis.[10,11] Recent studies have reported that deoxyschizandrin possesses beneficial pharmacological effects including anti-inflammatory,[11,12] anti-oxidation,[13] anti-tumor,[14] and provides hepatoprotection activities.[15] However, whether deoxyschizandrin could improve the symptoms of UC underlying these biological activities has not been elucidated. Investigation of these mechanisms could contribute to the development of novel and effective approaches to treating UC.
In this study, a cell model of inflammation and a mouse model of UC were established. Deoxyschizandrin was significantly inhibiting inflammation in cells and improved the symptoms of DSS-induced UC by inhibiting inflammation, T cell infiltration, and apoptosis of the colon tissue. These results provide theoretical evidence for the development of novel UC treatment in the clinical practice.
MATERIALS AND METHODS
Cell culture
Mouse monocyte macrophage leukemia cells RAM264.7 was purchased from the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China), cells were cultured in Dulbecco's Modified Eagle Medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS). The cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Experiments were divided into four groups as DMSO group, lipopolysaccharide (LPS) (1.1 × 10−2 mmol/L) group, dexamethasone (Dex) (1 × 10−3 mmol/L) group, and DSD (5 × 10−6 mmol/L) group.[16]
Animal model construction
Six–eight-week-old female C57BL/6 mice were purchased from the Shanghai experimental animal center, Chinese Academy of Sciences. Prior to the experiment, the mice were housed in a pathogen-free grade animal room and rested for 1 week with access to food and water ad libitum. The experiments were conducted with the approval of Experimental Animal Ethics Committee of China Medical University.
The mice were randomly divided into four groups, namely, Control, DSS, deoxyschizandrin (DSD), and DSS + DSD, with 8 mice in each group. Acute DSS colitis was induced in mice by administering 3% (w/v) dextran sulfate sodium sulfate (DSS) (molecular mass, 36–50kDa; Bitebo, Beijin, China) in drinking water ad libitum for 9 days. Following, DSD 6 μmol/kg/day were administered for 4 weeks. Then the mice were weighted and euthanized, and the colon tissues were dissected. Some tissues were frozen in liquid nitrogen and stored at −70°C for future applications, and others were fixed in 4% paraformaldehyde and embedded in paraffin for subsequent experiments.
Western blot
Cells were lysed by NP-40 Lysate (Beyotime Institute of Biotechnology, Haimen, China), and the total protein from the colon tissues of each group was extracted using radio-immunoprecipitation assay (RIPA) (Beyotime) lysis buffer, and protein concentrations were determined using the bicinchoninic acid (BCA) (Beyotime) method. Forty micrograms of total protein from each sample was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis electrophoresis, followed by transfer to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membrane was incubated with primary antibodies anti-IL-1β, anti-NF-κB, anti-cleaved caspase-3, anti-Bax, and anti-Bcl-2 (1:1000 dilution, bioss, Beijing, China) overnight at 4°C. Subsequently, the membrane was incubated with goat anti-rabbit IgG-HRP (1:5000, Beyotime) at room temperature for 1 h, followed by chromogenic detection using the enhanced chemiluminescence method. The film was scanned and analyzed by Gel-Pro-Analyzer software to determine the optical density value of the target bands using β-actin as the internal control.
Histopathological analysis
The paraffin-embedded mouse colon tissue was sliced into 5-μm thick sections, followed by conventional hematoxylin-eosin (HE) staining. The tissue sections were examined under an optical microscope, and histopathological scores were evaluated according to the methods reported by Xiong et al.[17] The scoring consisted of 5 grades with the following criteria: 0: No inflammation; 1: Low leukocyte infiltration and no structural changes; 2: Moderate leukocyte infiltration, extension, and disfiguration of gland ducts in the mucosa layer with no ulcer; 3: A high degree of leukocyte infiltration, increased vascular density, and bowel wall thickening; and 4: Massive leukocyte infiltration, high vascular density, gland duct extension and disfiguration, obvious goblet cell loss, bowel wall thickening, and associated ulcers.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) kits (USCN, Wuhan, China) were used to detect TNF-α, NO, INF-γ, and IL-6 levels in mouse colon tissues and cells. Procedures were conducted in strict accordance with the kit instructions.
Immunofluorescence staining
Paraffin sections of mouse colon tissues with a thickness of 5 μm were generated. The sections were incubated with 1:100 diluted CD4+ antibody (Sigma-Aldrich, St. Louis, MO, USA) overnight at 4°C, and then with 1:200 diluted Cy3-labeled goat anti-rabbit secondary antibody (Beyotime). After secondary incubation at room temperature for 1 h, the nuclei were counter-stained with 4',6-diamidino-2-phenylindole. The sections were mounted with anti-fading reagent, observed under a fluorescence microscope (BX53, Olympus, Tokyo, Japan), and imaged under 400× magnification lens.
TUNEL
Apoptotic cells were detected using In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Paraffin sections of mouse colon tissues were inactivated by H2O2. Fifty microliter TUNEL reaction mixture was added drop-wise to the sections, followed by incubation in a dark and humidified chamber for 60 min at 37°C. Thereafter, tissue sections were incubated with 50 μl Converter-POD for 30 min. Subsequently, 50 μl DAB chromogenic substrate was added, the reaction was terminated with H2O, and the cells were counter-stained with hematoxylin, followed by conventional dehydration, clarification, and mounting. The staining effect was observed under a microscope and photographed at 400× magnification.
Statistical analysis
Data are expressed as mean ± standard deviation. Comparisons between groups were performed using one-way analysis of variance, and Bonferroni post-hoc test was used for multiple comparisons. Graphpad Prism 5.0 software (GraphPad Software, Inc., CA, USA) was used to process data and images. P < 0.05 indicates statistically significant difference.
RESULTS
Deoxyschizandrin downregulated inflammatory cytokines TNF-α, IL-6, IL-1β and NO, NF-κB in cells RAM264.7
To investigate the role of deoxyschizandrin in cells RAM264.7, we constructed a monocyte macrophage inflammatory model, and a group of cells were received deoxyschizandrin treated. ELISA and Western blot results showed that the expression of inflammatory cytokines TNF-α, IL-6, IL-1β, and nitric oxide synthase (NOS), NF-κB in cells RAM264.7 of monocyte macrophage inflammatory model (LPS) group were all increased compared with the dimethyl sulfoxide (DMSO) group, TNF-α was increased by 169% [Figure 1a; P < 0.001], IL-6 was increased by 132% (Figure 1b; P < 0.001], NOS was increased by 144% [Figure 1c; P < 0.001], and NF-κB and IL-1β were increased by 1.74 folds and 1.44 folds, respectively, [Figure 1d and e; P < 0.001 and P < 0.01]. Deoxyschizandrin significantly reduced the levels of these factors [Figure 1a-e; P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001]. The effects were similar to the positive Dex group. The results indicated that deoxyschizandrin effectively inhibited inflammation in cells.
Figure 1.
Deoxyschizandrin downregulated inflammatory cytokines TNF-α, IL-6, IL-1β and NO, NF-κB in cells RAM264.7. ELISA detection of the levels of TNF-α (a), IL-6 (b), and NO (c) in cells. (c-e) Western blot detection of the levels of cleaved NF-κB and NO in cells. The figure shows the representative result from repeated experiments (n = 6). Data are expressed as mean ± standard deviation. Compared with the DMSO group, **P < 0.01, ***P < 0.001; compared with the LPS group, ###P < 0.001
Deoxyschizandrin attenuated Dextran sulfate sodium-induced ulcerative colitis in mice
HE staining results and histological scores showed that DSS-induced weight loss in mice was relieved by deoxyschizandrin [Figure 2a]. Meanwhile, deoxyschizandrin effectively protected colon tissue and attenuated DSS-induced tissue morphological changes, such as bowel wall thickening, numerous inflammatory cell infiltration, and reduction of goblet cells [Figure 2b and c]. Furthermore, the results were better in the DSS + DSD group. In summary, deoxyschizandrin in the colon tissue attenuated DSS-induced UC symptoms.
Figure 2.
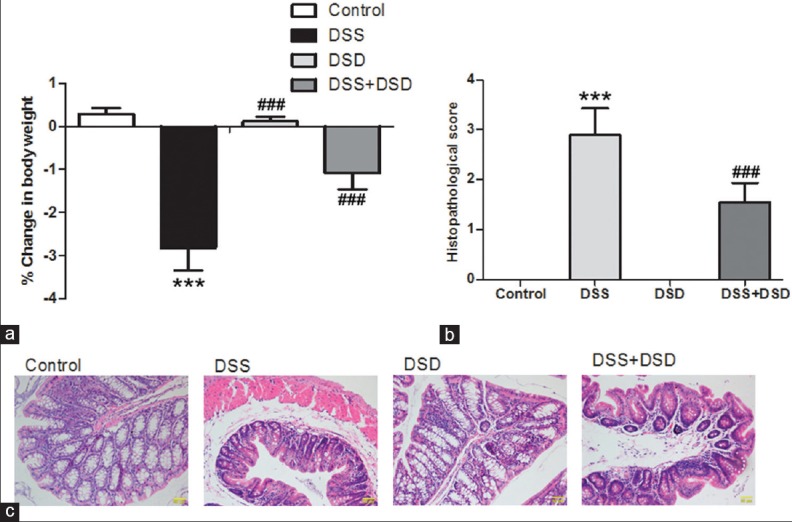
Deoxyschizandrin attenuated DSS-induced UC in mice. (a) Changes in the body weight of various groups of mice before and after the experiment. Histopathological scores (b) and HE staining (c) were used to evaluate the morphological changes in mouse colon tissues after DSS induction with and without deoxyschizandrin. The figure shows the representative results from repeated experiments (n = 6). Data are expressed as mean ± standard deviation. Compared with the Control group, ***P < 0.001; compared with the DSS group, ###P < 0.001
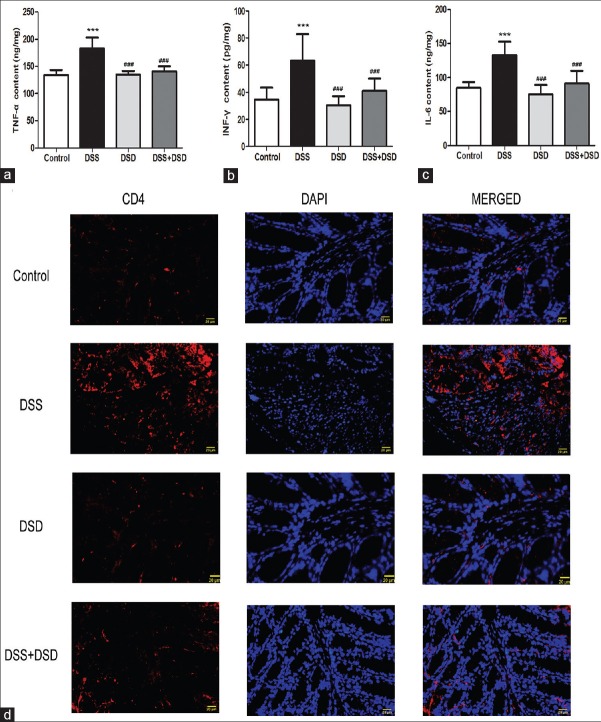
Deoxyschizandrin downregulated inflammatory cytokines and inhibited T cell infiltration in the colonic tissue of Dextran sulfate sodium-induced ulcerative colitis mice
The levels of TNF-α, INF-γ, and IL-6 in mouse colon tissues were detected by ELISA to investigate the effects of deoxyschizandrin on the inflammatory response in DSS-induced UC. Our results showed that DSS induction led to 136% [Figure 3a; P < 0.001], 183% [Figure 3b; P < 0.001], and 158% [Figure 3c; P < 0.001] increases in the TNF-α, INF-γ, and IL-6 levels, respectively, in the mouse colon tissues. Deoxyschizandrin in the colon significantly reduced the levels of these inflammatory factors [Figure 3a-c; P < 0.001, P < 0.001, P < 0.001]. Furthermore, Colonic CD4+ cells, that were mostly T lymphocytes, were examined by immunofluorescence staining, and the results revealed that colonic CD4+ cells increased dramatically in DSS-induced UC, which was attenuated by deoxyschizandrin [Figure 3d]. These results suggest that deoxyschizandrin effectively inhibited inflammation in the colon tissue of DSS-induced UC mice and the results were better in the DSS + DSD group.
Figure 3.
Deoxyschizandrin downregulated inflammatory cytokines and inhibited T cell infiltration in the colonic tissue of DSS-induced UC mice. ELISA detection of the levels of TNF-α (a), INF-γ (b), and IL-6 (c) in the colon tissue of various groups of mice. (d) CD4 + cells were detected by immunofluorescence: CD4 expression appeared red under a fluorescence microscope, while the nucleus was stained blue. The figure shows the representative results from repeated experiments (n = 6). Data are expressed as mean ± standard deviation. Compared with the Control group, ***P < 0.001; compared with the DSS group, ###P < 0.001
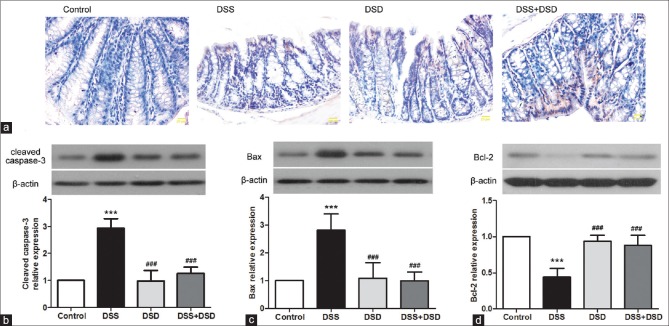
Deoxyschizandrin inhibited apoptosis in the colon tissue of Dextran sulfate sodium-induced ulcerative colitis mice
To investigate the effect of deoxyschizandrin on apoptosis in mouse colon tissues in UC, TUNEL assay was performed to stain the mouse colon tissue for apoptotic cells. DSS induction led to significant apoptosis in mouse colon tissues, concurrently with loose and irregular arrangement of cells, which was attenuated by deoxyschizandrin to a significant extent [Figure 4a]. Furthermore, expression levels of apoptotic genes such as cleaved caspase-3, Bax, and Bcl-2 in mouse colon tissues were examined by Western blot analysis. The results showed that the expression levels of cleaved caspase-3 and Bax were increased by 2.98 folds [Figure 4b; P < 0.001] and 2.81 folds [Figure 4c; P < 0.01], respectively, in DSS-induced colon tissues; whereas Bcl-2 was reduced by 0.44 folds [Figure 4d; P < 0.001]. Compared with the DSS group, deoxyschizandrin led to a significant reversal of the expression profile of these apoptosis indicators [Figure 4b-d; P < 0.001, P < 0.001, P < 0.001]. In summary, deoxyschizandrin could inhibit DSS-induced apoptosis in mouse colon tissues and the results were better in the DSS + DSD group.
Figure 4.
Deoxyschizandrin inhibited apoptosis in the colon tissue of DSS-induced UC mice. (a) TUNEL assay detection of apoptosis in the colon tissue of mice, with cells stained blue. (b-d) Western blot detection of the protein levels of cleaved caspase-3, Bax, and Bcl-2 in the mouse colon tissues. Grayscale analysis was conducted using β-actin as an internal reference. The figure shows the representative results from repeated experiments (n = 6). Data are expressed as mean ± standard deviation. Compared with the Control group, ***P < 0.001; compared with the DSS group, ###P < 0.001
DISCUSSION
The diverse biological functions of traditional Chinese medicine have gained much attention in modern disease research. Deoxyschizandrin has been shown to be associated with a variety of diseases such as chronic cough,[18] hepatoprotective,[19] and cardiovascular disease.[20] It has also been reported that deoxyschizandrin have diverse effects, such as anti-proliferative effects on tumor cells and pretreatment on physical exercise-induced muscle damage. This is in addition to their well-studied influence on UC.[21,22,23,24,25] In this study, we established an inflammation model of cells and a mouse model of DSS-induced UC, and investigated the regulatory roles of deoxyschizandrin in UC, as well as the therapeutic potential of deoxyschizandrin in treating UC.
Pro-inflammatory cytokines such as IL-6, TNF-α IL-1β, and IFN-γ play important roles in the occurrence and development of UC.[26,27] IL-6, IL-1β, and TNF-α act synergistically in regulating inflammatory and immune processes in the body by promoting T cell proliferation and enhancing the activity of cytotoxic T cells.[28] IL-6, IL-1β, and TNF-α also work in concert with IFN-γ to promote inflammation.[29] Studies have shown that IL-6, IL-1β, TNF-α, and IFN-γ levels are upregulated in the intestinal mucosa of UC patients and that these pro-inflammatory cytokines can further induce inflammation and aggravate UC mucosal injury.[17] Deoxyschizandrin showed the strongest anti-inflammarory activity and was able to reduce inflammation and inhibit the expression of inflammatory cytokines.[30,31] which led to the hypothesis that deoxyschizandrin may regulate UC by modulating the expression of inflammatory cytokines. Here, we show that deoxyschizandrin can reduce the levels of inflammatory cytokines IL-6, IL-1β, TNF-α, and IFN-γ [Figures 1a-e and 3a-c]. NF-κB could be activated by pro-inflammatory cytokines and plays a critical role in UC.[32,33,34] NOS, which is secreted by iNOS, could combine with inflammatory mediators and can be detrimental to the integrity of the colon, contributing to the development of the intestinal damage that characterizes the inflammatory reaction.[35] In the present study, we showed that deoxyschizandrin can reduce the levels of NF-κB and NOS through inhibition of inflammation [Figure 1c-e], thereby suppressing colon inflammation and improving UC symptoms. These results suggest that deoxyschizandrin may be critical to maintaining colon homeostasis and inhibiting UC by suppressing the levels of inflammatory cytokines.
T lymphocytes are the core of immune responses. The number of mucosal activated CD4 T cells is closely related to the structural integrity of intestinal mucosa because activated T cells can interact with the intestinal epithelial cells and damage the structure of intestinal mucosa.[36] High levels of CD4 T cell aggregation and T cell infiltration have been observed in the intestinal tract of UC mice.[37] Deoxyschizandrin significantly reduced the level of DSS-induced mucosal T cell infiltration in the colon [Figure 3d], suggesting an inhibitory role of deoxyschizandrin in T lymphocyte aggregation and mucosal infiltration in UC. These results indicate that deoxyschizandrin plays a critical role in T cell infiltration and mucosal homeostasis.
The apoptosis suppressor Bcl-2 is an important player in apoptosis together with the pro-apoptotic protein Bax. The ratio of Bax/Bcl-2 determines whether apoptosis can occur.[38] As one of the caspase family members, caspase-3 plays a pivotal role in the apoptosis pathway.[39] Studies have shown that UC patients present significant apoptosis of intestinal mucosal cells, with significantly increased Bax and caspase-3 levels and reduced Bcl-2 level in the mucosa cells.[40] Moreover, deoxyschizandrin has been demonstrated to inhibit apoptosis by exerting cell-protective effects.[41] Here, we show that deoxyschizandrin inhibited apoptosis in the colon tissue of DSS-induced UC mice [Figure 4a] by increasing the expression level of anti-apoptotic Bcl-2 and reducing the levels of the pro-apoptotic Bax and cleaved caspase-3 [Figure 4b-d]. The results indicate that deoxyschizandrin plays an anti-apoptotic and protective role in the colon by modulating the levels of the apoptosis regulatory proteins.
CONCLUSION
In summary, deoxyschizandrin could inhibit inflammation in cells and improve the pathological symptoms of DSS-induced UC in mice by inhibiting inflammation, T cell infiltration, and apoptosis of the colon tissue. The results demonstrate a positive role of deoxyschizandrin in the pathogenesis of UC, and suggest that deoxyschizandrin might be a promising potential drug for UC treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Science and technology development project of Jilin Province (2014311001YY).
REFERENCES
- 1.Adams SM, Bornemann PH. Ulcerative colitis. Am Fam Physician. 2013;87:699–705. [PubMed] [Google Scholar]
- 2.Lok KH, Hung HG, Ng CH, Kwong KC, Yip WM, Lau SF, et al. Epidemiology and clinical characteristics of ulcerative colitis in Chinese population: Experience from a single center in Hong Kong. J Gastroenterol Hepatol. 2008;23:406–10. doi: 10.1111/j.1440-1746.2007.05079.x. [DOI] [PubMed] [Google Scholar]
- 3.Drugs for inflammatory bowel disease. Treat Guidel Med Lett. 2012;10:19–28. [PubMed] [Google Scholar]
- 4.Ouyang Q, Tandon R, Goh KL, Ooi CJ, Ogata H, Fiocchi C. The emergence of inflammatory bowel disease in the Asian Pacific region. Curr Opin Gastroenterol. 2005;21:408–13. [PubMed] [Google Scholar]
- 5.Biologic targeting in the treatment of inflammatory bowel diseases [Retraction] Biologics. 2014;8:39. doi: 10.2147/BTT.S60301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho JH, Weaver CT. The genetics of inflammatory bowel disease. Gastroenterology. 2007;133:1327–39. doi: 10.1053/j.gastro.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Becker C, Watson AJ, Neurath MF. Complex roles of caspases in the pathogenesis of inflammatory bowel disease. Gastroenterology. 2013;144:283–93. doi: 10.1053/j.gastro.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Wei H, Sun L, Tai Z, Gao S, Xu W, Chen W. A simple and sensitive HPLC method for the simultaneous determination of eight bioactive components and fingerprint analysis of Schisandra sphenanthera. Anal Chim Acta. 2010;662:97–104. doi: 10.1016/j.aca.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118:183–212. doi: 10.1016/j.jep.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Nakajima K, Taguchi H, Ikeya Y, Endo T, Yosioka I. Constituents of Schizandra chinensis Baill. XIII. Quantitative analysis of lignans in the fruits of Schizandra chinensis Baill. by high performance liquid chromatography. Yakugaku Zasshi. 1983;103:743–9. doi: 10.1248/yakushi1947.103.7_743. [DOI] [PubMed] [Google Scholar]
- 11.Opletal L, Sovova H, Bartlova M. Dibenzo[a, c] cyclooctadiene lignans of the genus Schisandra: Importance, isolation and determination. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:357–71. doi: 10.1016/j.jchromb.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 12.Burgos RA, Hidalgo MA, Matthei SM, Hermosilla R, Folch H, Hancke J. L. Determination of specific receptor sites for platelet activating factor in bovine neutrophils. Am J Vet Res. 2004;65:628–36. doi: 10.2460/ajvr.2004.65.628. [DOI] [PubMed] [Google Scholar]
- 13.Lu H, Liu GT. Anti-oxidant activity of dibenzocyclooctene lignans isolated from Schisandraceae. Planta Med. 1992;58:311–3. doi: 10.1055/s-2006-961473. [DOI] [PubMed] [Google Scholar]
- 14.Fong WF, Wan CK, Zhu GY, Chattopadhyay A, Dey S, Zhao Z, et al. Schisandrol A from Schisandra chinensis reverses P-glycoprotein-mediated multidrug resistance by affecting Pgp-substrate complexes. Planta Med. 2007;73:212–20. doi: 10.1055/s-2007-967120. [DOI] [PubMed] [Google Scholar]
- 15.Maeda S, Sudo K, Miyamoto Y, Takeda S, Shinbo M, Aburada M, et al. Pharmacological studies on schizandra fruits. II. Effects of constituents of shizandra fruits on drugs induced hepatic damage in rats. Yakugaku Zasshi. 1982;102:579–88. doi: 10.1248/yakushi1947.102.6_579. [DOI] [PubMed] [Google Scholar]
- 16.Wang XY, Chen XL, Wang L, Chen HW. High-dose glucocorticoids increases the expression of mineralocorticoid receptor in vascular endothelial cells. Eur Rev Med Pharmacol Sci. 2015;19:4314–23. [PubMed] [Google Scholar]
- 17.Abdin AA. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol. 2013;718:145–53. doi: 10.1016/j.ejphar.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Ahn YT, Kim YS, Cho SI, An WG. Antiasthmatic effects of schizandrae fructus extract in mice with asthma. Pharmacogn Mag. 2014;10:S80–5. doi: 10.4103/0973-1296.127348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Fan X, Wang Y, Tan H, Chen P, Zeng H, et al. Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem Biol Interact. 2015;231:83–9. doi: 10.1016/j.cbi.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Chun JN, Cho M, So I, Jeon JH. The protective effects of Schisandra chinensis fruit extract and its lignans against cardiovascular disease: A review of the molecular mechanisms. Fitoterapia. 2014;97:224–33. doi: 10.1016/j.fitote.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Teschke R, Wolff A, Frenzel C, Eickhoff A, Schulze J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J Gastroenterol. 2015;21:4466–90. doi: 10.3748/wjg.v21.i15.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, Jiang M, Lu A. Considerations of traditional Chinese medicine as adjunct therapy in the management of ulcerative colitis. Clin Rev Allergy Immunol. 2013;44:274–83. doi: 10.1007/s12016-012-8328-9. [DOI] [PubMed] [Google Scholar]
- 23.Sałaga M, Zatorski H, Sobczak M, Chen C, Fichna J. Chinese herbal medicines in the treatment of IBD and colorectal cancer: A review. Curr Treat Options Oncol. 2014;15:405–20. doi: 10.1007/s11864-014-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ, Wu JC. Systematic review: The efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:854–63. doi: 10.1111/apt.12464. [DOI] [PubMed] [Google Scholar]
- 25.Ke F, Yadav PK, Ju LZ. Herbal medicine in the treatment of ulcerative colitis. Saudi J Gastroenterol. 2012;18:3–10. doi: 10.4103/1319-3767.91726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv YN, Wei XH, Xiao P. Study on action mechanism of Danhong injection based on computational system biology approach. Zhongguo Zhong Yao Za Zhi. 2015;40:538–42. [PubMed] [Google Scholar]
- 27.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–96. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 28.de Caralt S, Uriz MJ, Wijffels RH. Cell culture from sponges: Pluripotency and immortality. Trends Biotechnol. 2007;25:467–71. doi: 10.1016/j.tibtech.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Padia FN, Yaseen M, Gore B, Rogers S, Bell G, Lu JR. Influence of molecular structure on the size, shape, and nanostructure of nonionic C (n) E (m) surfactant micelles. J Phys Chem B. 2014;118:179–88. doi: 10.1021/jp409808c. [DOI] [PubMed] [Google Scholar]
- 30.Qiu HT, Zhao XP, Li Z, Wang LL, Wang Y. Study on main pharmacodynamic effects for Schisandra lignans based upon network pharmacology. Zhongguo Zhong Yao Za Zhi. 2015;40:522–7. [PubMed] [Google Scholar]
- 31.Zhong S, Nie YC, Gan ZY, Liu XD, Fang ZF, Zhong BN, et al. Effects of Schisandra chinensis extracts on cough and pulmonary inflammation in a cough hypersensitivity guinea pig model induced by cigarette smoke exposure. J Ethnopharmacol. 2015;165:73–82. doi: 10.1016/j.jep.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–69. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Ann Review Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–84. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura H, Hokari R, Miura S, Shigematsu T, Hirokawa M, Akiba Y, et al. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180–7. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu XF, Xu R, Ouyang ZJ, Qian C, Shen Y, Wu XD, et al. Beauvericin ameliorates experimental colitis by inhibiting activated T cells via downregulation of the PI3K/Akt signaling pathway. PloS One. 2013;8:e83013. doi: 10.1371/journal.pone.0083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardizzone S, Cassinotti A, de Franchis R. Immunosuppressive and biologic therapy for ulcerative colitis. Expert Opin Emerg Drugs. 2012;17:449–67. doi: 10.1517/14728214.2012.744820. [DOI] [PubMed] [Google Scholar]
- 38.Bu P, Keshavarzian A, Stone DD, Liu J, Le PT, Fisher S, et al. Apoptosis: One of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J Immunol. 2001;166:6399–403. doi: 10.4049/jimmunol.166.10.6399. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg I, Mc CH, Dotter CT. Angiocardiographic findings in pulmonary tuberculosis. Dis Chest. 1951;19:510–20. doi: 10.1378/chest.19.5.510. [DOI] [PubMed] [Google Scholar]
- 40.Zhao QJ, Yu YB, Zuo XL, Dong YY, Li YQ. Milk fat globule-epidermal growth factor 8 is decreased in intestinal epithelium of ulcerative colitis patients and thereby causes increased apoptosis and impaired wound healing. Mol Med. 2012;18:497–506. doi: 10.2119/molmed.2011.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu BH, Minh NV, Lee SH, Lim SW, Lee YM, Lee KS, et al. Deoxyschisandrin inhibits H2O2-induced apoptotic cell death in intestinal epithelial cells through nuclear factor-kappaB. Int J Mol Med. 2010;26:401–6. [PubMed] [Google Scholar]