Abstract
Context:
Celiac plexus block (CPB) (is an effective way to reduce cancer-associated pain in upper abdominal malignancies.
Aims:
To evaluate the efficacy and safety of different volumes of 70% alcohol in CPB.
Settings and Design:
Prospective, randomized, controlled clinical study.
Subjects and Methods:
Thirty patients of carcinoma gall bladder were randomly divided into three groups (n = 10) to receive 20, 30, and 40 ml of 70% alcohol in CPB.
Statistical Analysis Used:
All the continuous data were assessed analysis of variance followed by post-hoc tests (Tukey's Honestly Significant Difference test). Ordinal data were compared using Kruskal–Wallis H-test followed by Mann–Whitney U-test. Categorical comparisons were performed using Chi-square test.
Results:
A significant difference in visual analog scale (VAS) score of Group I, Group I and Group III was observed from week 6 onward until the end of the study. At all these time intervals, VAS scores in Group I was higher than both Groups II and III during this time interval. VAS scores in Group III were significantly lower as compared to Group II from week 10 onward until the end of the study. As compared to baseline, at all the follow-up intervals, mean morphine requirement was significantly lower in Group II and Group III. A quality of life (QOL) score of Group III were higher as compared to Group I. Between Group II and Group III, significant difference was observed at week 16 only when Group III had a higher score as compared to Group II.
Conclusions:
VAS score, QOL, and reduction in morphine consumption were increased on increasing the volume of alcohol in CPB, 40 ml being most effective.
Keywords: Alcohol, celiac plexus, lignocaine, malignancy, neurolysis, pain, transdiscal
Introduction
Abdominal pain is a common debilitating problem in patients with abdominal malignancy and often dramatically affects the quality of life (QOL) and survival.[1,2,3] Management of cancer-related abdominal pain is a complex and challenging issue.[4,5,6] An effective means of alleviating the intractable pain associated with abdominal malignancy is imaging-guided celiac plexus block (CPB) and neurolysis.[7] A cocktail of absolute ethanol (95–100%), bupivacaine, and contrast material, with a ratio of 6:3:1 is the most frequently used neurolytic blocking mixture.[8,9] This prospective, randomized, controlled clinical study was conducted to compare the degree of pain relief in patients of upper abdominal malignancies using 20, 30, and 40 ml of 70% alcohol in CPB.
Subjects and Methods
After getting approval from Institutional Ethics Committee, this prospective, randomized study was conducted on 30 patients of upper abdominal malignancies, of either sex having age between 25 and 70 years and in who pain was not relieved by nonsteroidal anti-inflammatory drug (NSAID) or strong opioids like morphine (according to WHO ladder III). Patient on anticoagulantion therapy, with coagulopathy, local or intra-abdominal infection, sepsis and huge ascites, patients with bowel obstruction, with physical opioid dependence and drug seeking behavior, and patient not willing to participate in the study were excluded from the study. An informed consent was taken from all the patients. The CPB was performed in patient and subjective evaluation of degree of pain relief was done. Patients were randomly divided into three groups of 10 patients each using a computer generated the table.
Sample size estimation
We are comparing the degree of pain relief in patients of upper abdominal malignancies using different volume 20, 30, and 40 ml of 70% alcohol. We are targeting a mean difference in visual analog scale (VAS) score to the tune of 2 with a pooled variability of 1 to be detrimental in the selection of the regimen. For this purpose, the sample size was calculated using the formula: n = (16 × σ2)/d2 + 1.
For the purpose of the present study, d = 2 and σ =1. Now putting these values in the above equation we get: n = ([16 × 12]/22) +1 = 16/4 + 1 = 5.
Thus, the calculated sample size was 5 for each group, However, we targeted a sample size of 10 in each group
A detailed patient check-up was done, and the procedure was explained to the patient. Patients were kept nil orally as per American Society of Anesthesiologists preoperatively fasting guidelines. An IV access was obtained by 18 gauge intravenous (IV) cannula and sensitivity to lignocaine were done, using 0.1 ml intradermal injection. After that IV ondansetron (0.1 mg/kg), IV midazolam (0.02 mg/kg), and IV ceftriaxone antibiotic (25–50 mg/kg) was given 30 min before performing procedure. After shifting the patient to operation theater, the patient was preloaded with intravenous fluid (Ringer's lactate) 10 ml/kg body weight prior to the procedure. Meanwhile, monitors such as pulseoximeter, noninvasive blood pressure (BP), and electrocardiogram were attached. Baseline HR, BP, and SPO2 were recorded. C- arm guided transdiscal technique was chosen to block the celiac plexus. The insertion site was the lateral margin of the superior articular process of T12 and it was marked 5 cm from the midline line. The skin and subcutaneous tissues were anesthetized using local anesthetic 2% lidocaine preservative free. The 15 cm long 25 gauge spinal needle was inserted on the left side through a skin wheal. After feeling of contact of the disc, the fluoroscope was rotated to a lateral position. The needle was inserted through the disc while checking the tip position with AP and lateral fluoroscopic images. After penetrating the disc, frequent fluoroscopic images for both AP and lateral views were used to guide the needle when advancing it in the correct plane. While advancing the needle, there was a feeling of ‘loss of resistance’. It can then be concluded that the needle is outside the T12/L1 disc. When needle was in position, observation for leakage of blood, urine, or cerebrospinal fluid was made before careful aspiration. 2ml contrast (urograffin) was injected in order to check the position of needle in the disc. The needle position was confirmed in lateral images by seeing the hugging of dye anterior to the intervertebral disc and in antero-posterior images dye was midline in a position anterior to the intervertebral disc. After this depth is ascertained, the right sided needle was inserted in a similar fashion to a depth of 1.0–1.5 cm farther. After checking the position of the needle, a 3 ml of local anesthetic was given before injection of study solution to prevent the irritation of alcohol. 10, 15, and 20 ml of study solution were used in each needle, in 10 patients of each group, respectively. Before removal of the needle, 2 ml normal saline was injected into each needle to prevent alcohol from tracking back along the needle path.
The effect on pain relief, requirement of oral analgesics and QOL were compared. The patients were divided into one of the following groups using a computer generated random number list:
Group I: Patient on oral morphine (60–90 mg/day) and to block celiac plexus 20 ml of study solution was used
Group II: Patient on oral morphine (60–90 mg/day) and to block celiac plexus 30 ml of study solution was used
Group III: Patient on oral morphine (60–90 mg/day) and to block celiac plexus 40 ml of study solution was used.
The following parameters were recorded:
Hemodynamic parameters - heart rate (HR), systolic, diastolic and mean arterial BP and SpO2. hypotension was defined as a decrease in systolic arterial pressure ≥20% from baseline and was treated with fluid boluses and intermittent IV mephentermine (0.1 mg/kg). Bradycardia was defined as decrease in HR <60 beats/min and was treated with IV atropine (0.01 mg/kg)
Degree of pain relief - was assessed by using VAS score (0–10) at weekly interval up to 16 weeks. Based on 10 cm line, the left extremity represented no pain at all (score 0) and right extremity represented unbearable pain (score 10) the procedure was considered successful if there was satisfactory pain relief VAS score ≤3 with or without morphine or reduction in the dose of morphine
Requirement of oral analgesics - were also assessed at baseline and weekly interval up to 16 weeks. All the patients before CPB were consuming 60–90 mg morphine per day and they were advised to consume same amount of morphine for 1 week after CPB and after that their dosing were changed according to VAS score
QOL of the patients - was assessed using 100 point scale. The patients were asked to define deterioration in QOL assuming QOL before the disease as 100 on the scale
Incidence of side effects/complications i.e., pain at injection site, back pain, hypotension, bradycardia, diarrhea, respiratory depression, arrhythmias, paraplegia, visceral puncture, pneumothorax, retroperitoneal fibrosis, impotence were recorded, and treated accordingly.
Data were analyzed using Statistical Package for Social Sciences, version 15.0 (IBM). As sample size was small, hence normality check was performed. As the distributions were normal, hence all the continuous data were assessed using analysis of variance followed by post-hoc tests (Tukey's Honestly Significant Difference test). Ordinal data were compared using Kruskal–Wallis H-test followed by Mann–Whitney U-test for between group comparisons. Categorical comparisons were performed using Chi-square test. The confidence level of this study was kept at 95%, hence a P < 0.05 indicated a statistically significant difference.
Results
Demographic variables of patients are shown in Table 1. As systolic BP, diastolic BP, mean arterial pressure, and HR were continuous variables, but the sample size was small (<30 for each group); hence, normality of the distributions was checked to determine the plan of analysis. Normality of distribution was assessed only at baselines. All the distributions were normal; hence, a parametric evaluation plan was adopted for evaluation of these parameters in different groups. No clinically significant changes were noted in hemodynamic parameters.
Table 1.
Demographic evaluation and diagnostic characteristics

VAS scores are shown in Table 2 and Figure 1. Statistically, significant intergroup differences were observed from week 6 to the end of study (P < 0.001). A significant difference in VAS score of Group I, Group II and Group III was observed from week 6 onward to the end of study. At all these time intervals, VAS scores in Group I was higher than both Groups II and III during this time interval. VAS scores in Group III were significantly lower as compared to Group II from week 10 onward to the end of study.
Table 2.
Intergroup comparison of VAS scores at different time intervals
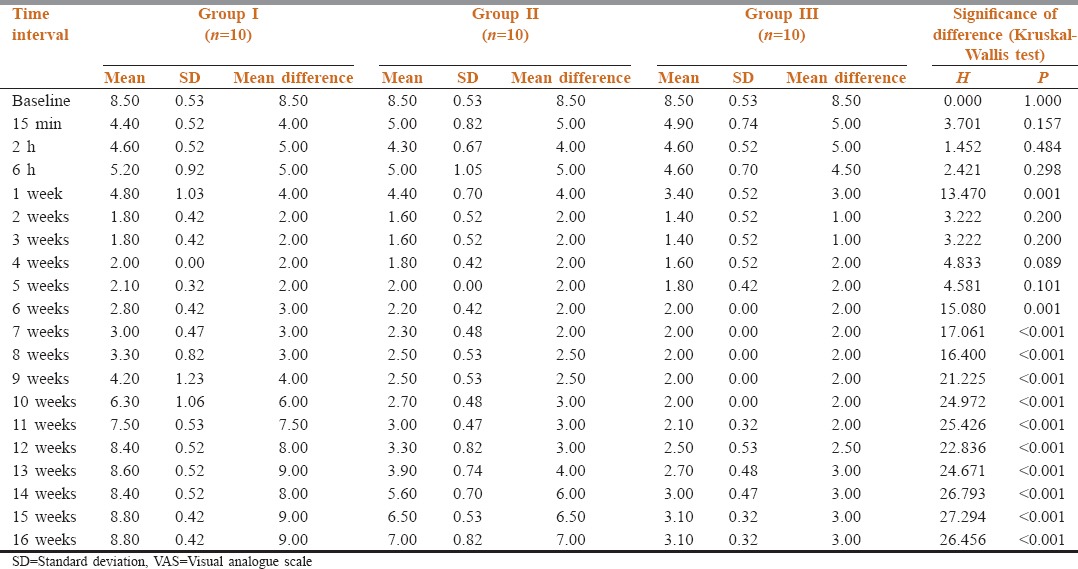
Figure 1.
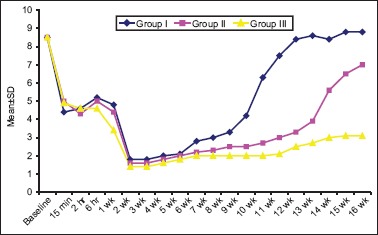
Intergroup comparison of visual analogue scale scores at different time intervals
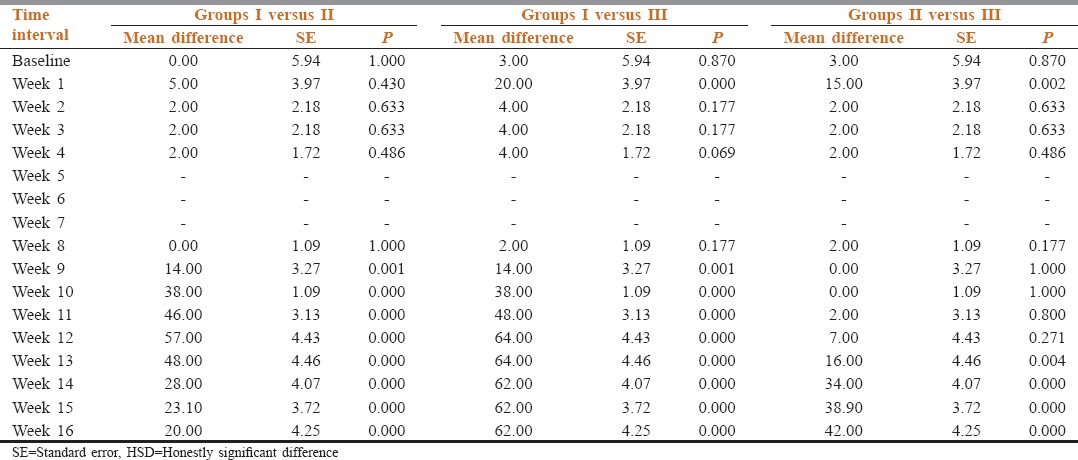
At baseline, all the patients had same requirement of Morphine. After CPB the morphine requirement was reduced as shown in Tables 3 and 4.
Table 3.
Comparison of morphine requirement in mg/day in different groups at baseline and at different follow-up intervals
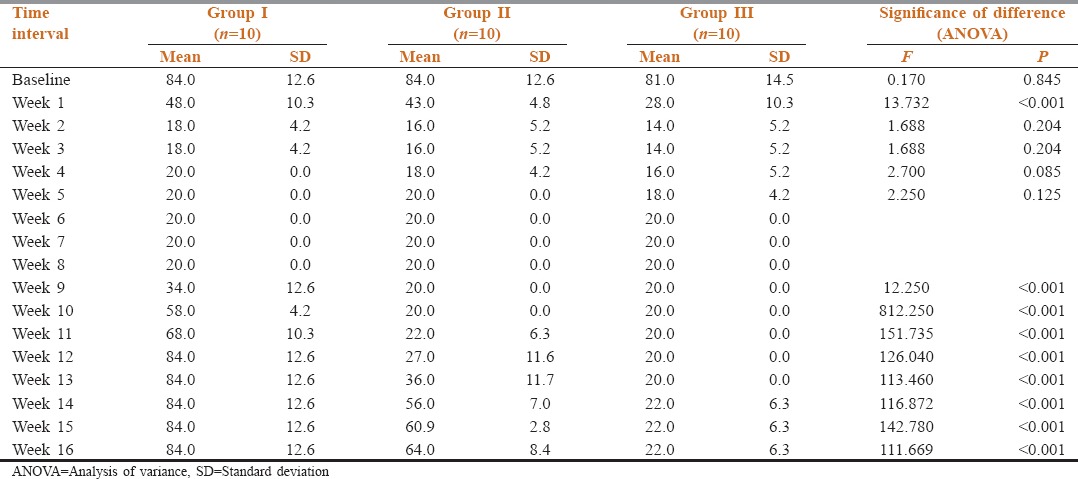
Table 4.
Between group comparison of morphine requirement (mg/day) at different time intervals (Tukey's HSD test)
At baseline, QOL scores did not show a significant intergroup difference (P = 0.165). An intergroup difference in QOL scores was observed at week 3 onward until the end of study as shown in Table 5 and Figure 2.
Table 5.
Intergroup comparison of QOL scores at different time intervals
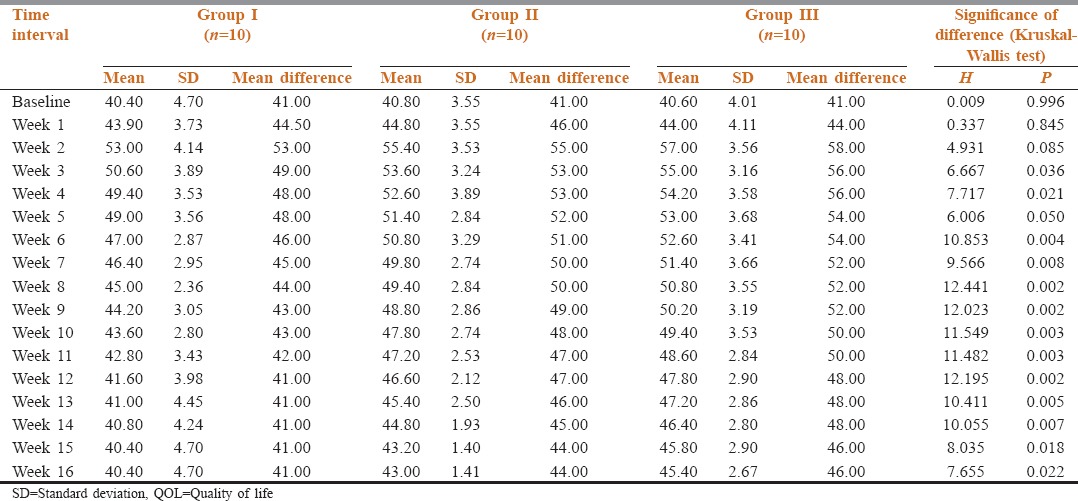
Figure 2.
Intergroup comparison of quality of life scores at different time intervals
Except for pain at injection site, mild back pain or diarrhea in few patients, no significant side effects were observed in any of the groups. Statistically too, the differences in these features among different groups were not significant (P > 0.05) [Table 6 and Figure 3].
Table 6.
Side effect profile
Figure 3.
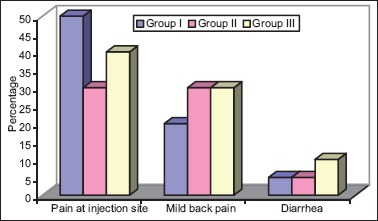
Side effect profile
Discussion
Upper Abdominal cancer patients may experience severe pain that is, resistant to oral opioids. In addition, excessive sedation or other side effects may limit the acceptability and usefulness of oral opioids therapy. Neurolysis of celiac plexus (NCPB) appeared boon to cancer pain. It appears to be a safe, cost-effective approach to treating visceral pain associated with cancer. The benefits include improved analgesia, reduced opioid consumption, favorable economic implications, and superior clinical effects due to the avoidance of deleterious properties of high-dose chronic opioid therapy.
Degree of pain relief
There was significant pain relieved in patients of all the three groups after CPB. This was demonstrated by decrease in VAS score. In the present study, VAS score ≤3 with or without opioid medication was taken as a successful CPB. Mercadante[10] found that CPB made pain control possible with a reduction in opioid consumption for a mean survival period of about 51 days. Furthermore, Eisenberg et al.[4] suggested that NCPB has long-lasting benefit for 70–90% of patients with pancreatic and other intra-abdominal cancers, regardless of the technique used. Bridenbaugh et al.[11] concluded that this simple procedure proved effective in controlling the pain without any serious complications. Kawamata et al.[12] found that VAS scores significantly improved for 4 weeks after CPB and concluded that the VAS scores were lower in CPB group than MOR group. Amr and Makharita[13] found that the analgesia induced by the celiac block after medically controlling pain was better and more sustained when compared with the outcome on performing a celiac block at a high VAS score >7.
In the present study, a fall in VAS scores was observed 15 min after intervention in all the three groups, which was due to the effect of local anesthetic agent lidocaine. The lowest VAS score in all the three groups was observed at the end of 2nd week. This finding of our study is supported by Eisenberg et al.[4] In their study, good to excellent pain relief was reported in 89% of patients during the first 2 weeks after NCPB. Furthermore, Soweid and Azar[14] found that 78% of patients reported a drop in pain score 2 weeks after the procedure. In 2013, Seicean[15] also found that average pain and worst pain had decreased significantly by 2 weeks after the procedure, but complete relief using pain killers was not significantly different, although some patients gave up their morphine-based medication.
In the present study, VAS scores in Group I were lower as compared to baseline at all follow-up intervals except from 12 weeks onward to the end of study where the difference from baseline was not significant statistically. In Group I, the VAS score remained to ≤3 up to 8th week i.e., duration of blockade was 8 weeks. In Groups II and III, at all the follow up intervals, VAS scores were significantly lower as compared to baseline. However in Group II after 12th week the VAS score became more than 3 i.e. duration of blockade was 12 weeks. In Group III until the end of study i.e. up to 16 week the VAS score remained ≤3 i.e., duration of blockade was 16 weeks. This finding of the study is supported by Rykowski and Hilgier,[9] they found that recurrence of significant and severe pain (VAS score 4 or more) occurred gradually, mostly from the 4th month from the primary neurolysis (mean, 3.4 months or 119 days of effective pain relief without strong opioids) and concluded that after NCPB, 74% had effective pain relief during the first 3 months. Similarly, Eisenberg et al.[4] concluded that partial to complete pain relief continued in approximately 90% of patients alive at 3 months post-NCPB and in 70–90% until death even if beyond 3 months post-NCPB. Hence, we can conclude that patients of Group III had a longer duration of pain relief after CPB as compared to other groups.
Dosing of morphine
At baseline, all the patients had the same requirement of morphine. In the present study as compared to baseline, after CPB from week 1 itself the morphine requirement was reduced at all the follow-up intervals in the Group II and Group III. In Group I the morphine requirement was reduced to up to 11 weeks, from 12th week onward morphine dosing returned to baseline. In the present study, morphine was not completely stopped in any patients of three groups. This result of the present study is supported by other studies.[9,12] Opioids were withdrawn totally in 47% of NCPB and reduced in 53% in these studies.
In the present study, Group I had significantly higher morphine requirement as compared to Group II from week 9 onward to the end of the study. Group II had significantly higher morphine requirement as compared to Group III from week 12 to the end of the study. Therefore, we can state that patients of Group III required less doses of morphine after CPB as compared to other groups.
Assessment of quality of life
At baseline, QOL scores did not show a significant intergroup difference (P = 0.165). In all the three groups, differences in QOL scores at different follow-up intervals as compared to base line was significant statistically (P < 0.05) except in Group I from week 12 onward and Group II at week 16 intervals. At all these intervals, the values were significantly higher (improved QOL) than that at baseline. An intergroup difference in QOL scores was observed at week 3 onward until the end of study (P < 0.05). A significant difference in QOL scores between Groups I and Group II was observed from week 6 until week 14, QOL scores of Group II were higher (improved QOL) as compared to Group I. Between Group I and Group III, significant differences were observed from week 2 onwarduntil the end of study, QOL score of Group III were higher (improved QOL) as compared to Group I. Between Group II and Group III, significant difference was observed at week 16 only when Group III had higher score (improved QOL) as compared to Group II.
This result of our study is supported by Amr and Makharita.[13] They found that QLQ-C30 assessment revealed a significant improvement in daily life activity and QOL after injection in both groups but with more significant improvement in the group in which the celiac block was performed after medical therapy. Similarly, Rykowski and Hilgier[9] concluded that patients who had good pain relief after neurolysis had improved alertness and QOL. Matamala et al.[16] concluded that decreased opioid consumption may improve the QOL by decreasing sedative effect of opioids and enhance the immune system as it was shown that opioids had a negative effect on immunity at cellular level. However, Kawamata et al.[12] found that sufficient pain management with the least side effects does not remarkably improve QOL in patients with pancreatic cancer pain, but it can prevent deterioration in QOL. To improve the QOL significantly, socio-environmental supports including home care are necessary, as well as pain management and palliative care, and concluded that CPB does not directly improve QOL in patients with pancreatic cancer pain, but it may prevent deterioration in QOL by the long-lasting analgesic effect, limitation of side effects, and the reduction of morphine consumption, compared to treatment only with NSAID-morphine. Similarly, Wong et al.[2] concluded that NCPB improves pain relief as compared to systemic analgesic intervention alone but it does not influences QOL or survival.
From the observations of our study, we cannot comment on the improvement survival of the patients received CPB because first; we had excluded those patients, who did not complete the 16 weeks follow-up period for any reason, second, due to the short follow-up period, and finally, it was not included in the objective of our study.
Complications
In the present study except for pain at injection site, mild back pain, and diarrhea were noticed in few patients, and they were transient in nature, no other side effects were observed in any of the groups. Eisenberg et al.[4] concluded that common adverse effects were transient, including local pain (96%), diarrhea (44%), and hypotension (38%). In the present study, none of the patient experienced bradycardia or hypotension. The may be due to preloading with Ringer's lactate by increasing amount of 70% alcohol up to 40 ml the incidence of side effects were not increased in the present study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Staats PS, Hekmat H, Sauter P, Lillemoe K. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: A double-blind, randomized, placebo-controlled study. Pain Med. 2001;2:28–34. doi: 10.1046/j.1526-4637.2001.002001028.x. [DOI] [PubMed] [Google Scholar]
- 2.Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: A randomized controlled trial. JAMA. 2004;291:1092–9. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 3.De Oliveira R, dos Reis MP, Prado WA. The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain. 2004;110:400–8. doi: 10.1016/j.pain.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: A meta-analysis. Anesth Analg. 1995;80:290–5. doi: 10.1097/00000539-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Markman JD, Philip A. Interventional approaches to pain management. Med Clin North Am. 2007;91:271–86. doi: 10.1016/j.mcna.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, et al. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–34. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- 7.De Cicco M, Matovic M, Balestreri L, Fracasso A, Morassut S, Testa V. Single-needle celiac plexus block: Is needle tip position critical in patients with no regional anatomic distortions? Anesthesiology. 1997;87:1301–8. doi: 10.1097/00000542-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZJ, Webb EM, Westphalen AC, Coakley FV, Yeh BM. Multi-detector row computed tomographic appearance of celiac ganglia. J Comput Assist Tomogr. 2010;34:343–7. doi: 10.1097/RCT.0b013e3181d26ddd. [DOI] [PubMed] [Google Scholar]
- 9.Rykowski JJ, Hilgier M. Efficacy of neurolytic celiac plexus block in varying locations of pancreatic cancer: Influence on pain relief. Anesthesiology. 2000;92:347–54. doi: 10.1097/00000542-200002000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain. 1993;52:187–92. doi: 10.1016/0304-3959(93)90130-H. [DOI] [PubMed] [Google Scholar]
- 11.Bridenbaugh LD, Moore DC, Campbell DD. Management of upper abdominal cancer pain: Treatment with celiac plexus block with alcohol. JAMA. 1964;190:877–80. [PubMed] [Google Scholar]
- 12.Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64:597–602. doi: 10.1016/0304-3959(95)00189-1. [DOI] [PubMed] [Google Scholar]
- 13.Amr YM, Makharita MY. Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: One-year follow-up. Clin J Pain. 2013;29:807–13. doi: 10.1097/AJP.0b013e3182757673. [DOI] [PubMed] [Google Scholar]
- 14.Soweid AM, Azar C. Endoscopic ultrasound-guided celiac plexus neurolysis. World J Gastrointest Endosc. 2010;2:228–31. doi: 10.4253/wjge.v2.i6.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seicean A. Celiac plexus neurolysis in pancreatic cancer: The endoscopic ultrasound approach. World J Gastroenterol. 2014;20:110–7. doi: 10.3748/wjg.v20.i1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montero Matamala A, Vidal Lopez F, Aguilar Sanchez JL, Donoso Bach L. Percutaneous anterior approach to the coeliac plexus using ultrasound. Br J Anaesth. 1989;62:637–40. doi: 10.1093/bja/62.6.637. [DOI] [PubMed] [Google Scholar]


