Dear Editor,
Breast cancer is the most common malignancy affecting young women, with 10% of cases diagnosed before the age of 40. Tamoxifen is the mainstay of adjuvant hormonal treatment in premenopausal breast cancer patients. Due to its high incidence and increasing disease-free survival time, oncologists are increasingly confronted with patients on tamoxifen and desirous to become pregnant or who unexpectedly become pregnant during tamoxifen treatment. In the initial phase of tamoxifen treatment, it may stimulate ovulation and thus making the women more fertile. With continued use, in some women (approximately half) periods become less regular, lighter or stop altogether. In general, periods will start again once the tamoxifen is stopped; however, it may take 4–5 months for the cycle to become regular. Animal studies have shown that tamoxifen can cause genitourinary developmental defects.[1] One case-report showing that tamoxifen can cause genital defects in humans,[2] while on the other hand few case studies report delivery of healthy babies by women using tamoxifen.[3,4] The metabolism and mechanism of action of tamoxifen are complex. The most common side effects are similar to menopausal symptoms, including hot flushes, night sweats and sleep disturbance, vaginal irritation (such as dryness, itching or discharge), loss of sex drive (libido) and mood changes. In pregnant women, tamoxifen and its metabolites interact with embryonic and fetal tissues, which may lead to teratogenicity. There should be proper discussion and counseling regarding the possible teratogenic and fetal adverse effects of tamoxifen. However, there is insufficient data for the possible consequences of tamoxifen exposure during pregnancy. Here, we are reporting a case with inadvertent usage of tamoxifen during pregnancy.
A 22-year-old lady presented with a left breast lump in 2002; incisional biopsy revealed infiltrative ductal carcinoma grade III (IDC) [Figure 1a and b]. She underwent modified-radical-mastectomy in January 2002. Histology showed a pT1N1, infiltrating ductal carcinoma III. Immunohistochemical analysis showed this tumor to be estrogen receptor (ER)-positive (100% [+]), progesterone receptor 100% (+), and HER2-negative. She was recently married, nulliparous women and very much concerned about her subsequent reproductive outcome. She was explained about the side effects of adjuvant-chemotherapy and hormone therapy in great detail. She was also counseled about the contraception and possible teratogenicity of chemotherapy and subsequent hormone therapy. She received six cycles of adjuvant-chemotherapy with cyclophosphamide, doxorubicin and 5-fluorouracil in standard doses. Subsequently, she was started on oral tamoxifen 20 mg once daily. At this juncture also, patient and family has shown their concern about the reproductive outcome. She was again counseled at length about the need to continue tamoxifen and to practice contraception and possible teratogenicity of the tamoxifen. She continued tamoxifen and was on regular follow-up. She tolerated it well and experienced occasional hot flashes. No other side effects was experienced. After 32 months of therapy, she presented with a diagnosis of pregnancy and ultrasound showed a viable 7 weeks fetus. Despite explaining about possible fetal adverse effects due to tamoxifen exposure during pregnancy and possible consequences of early stoppage of tamoxifen, she chose to stop tamoxifen, continued pregnancy and delivered by caesarian section at the week 39 of pregnancy. She delivered a full term healthy normal weight baby who subsequently attained developmental milestones as per age. She did not commence tamoxifen and again conceived after 2 years, but the neonate died in immediate postpartum period due to neonatal asphyxia. Subsequently patient gave birth to another healthy female child by caesarian section in 2009. Both children are doing well, physical examination is normal, developmental milestones are normal until. However, patient developed loco-regional recurrence after 6 years of stoppage of tamoxifen. She received second line taxane-based chemotherapy. She was counseled in great detail regarding the benefits and risks of surgical removal of ovaries. As she completed her family, she agreed for the procedure. Post-chemotherapy she had a good local response and subsequently underwent wide local excision of the tumor on the chest wall with bilateral salpingoopherectomy. Histopathology revealed IDC grade III with hormone receptor positivity. She was started on adjuvant therapy with aromatase inhibitors after attaining post-menopausal status. She was doing well at last follow-up. Later on, she requested for follow-up at native place.
Figure 1.
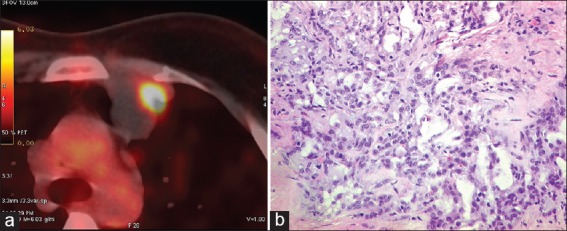
(a) with positron emission tomography - computed tomography scan fluorodeoxyglucose (FDG) avid an anterior mediastinal node in pre-vascular region measuring (2.8 cm × 2.7 cm) with central necrosis and a focal area of increased FDG uptake. (b) Microphotograph showing infiltrating duct carcinoma, grade III, amidst sclerotic stroma (H and E, original magnification, ×200)
Young breast cancer survivors and their spouses may face a difficult dilemma regarding their wish to have children while on tamoxifen. Animal studies with tamoxifen have revealed evidence of feto-toxicity, low birth weight, spontaneous abortions, birth defects, and still birth.[5,6] Tamoxifen may act as an estrogen in the fetus and high fetal estrogenic activity, may increase subsequent aggressive breast cancer risk. Tamoxifen metabolites like endoxifen and 4-OH-tamoxifen have high binding affinities to ERs, antiproliferative activity, and inhibitory effects on the expression of typical estrogen-related genes.[7] Pregnancy is characterized by growth and development, both of which are tightly linked to transcription and translation in strict time frames. Knowing the potent actions of tamoxifen and its metabolites, it is evident that there is a strong concern about the use of tamoxifen during pregnancy. Tamoxifen is also known to cause chromosomal aberrations by formation of DNA adducts without phenotypic abnormality.[8] Perinatal exposure may induce precocious puberty, reproductive toxicities uterine anomalies, uterine neoplasia, and uterine atrophy.[9,10]
Studies on human beings revealed, tamoxifen exposure during pregnancy resulted in 16 live births with congenital malformations and a total of 122 live births without malformations. AstraZeneca Safety Database reported 11 babies with congenital malformations, of 44 live births; additionally, 18 fetal losses and 3 stillbirths.[11] Cullins,[12] published a single case of Goldenhar's syndrome that is, oculo-auriculo-vertebral dysplasia and Berger and Clericuzio described a Pierre Robin sequence associated with first trimester fetal tamoxifen exposure.[13] In 1993, Clark published a report of 85 women who became pregnant while receiving prophylactic tamoxifen as part of a prevention trial. No fetal abnormalities were observed which is in sharp contrast with above observations.[14] This case is one of those rare cases as the lady delivered full term normal baby despite in utero exposure to tamoxifen; further did not resume tamoxifen and delivered two more times. One succumb to neonatal asphyxia while another was again full term normal baby. One must remember that these are anecdotal reports and, tamoxifen exposure during pregnancy is potentially teratogenic.
Food Drug Administration has classified the drug as a pregnancy category D medication and guidelines contraindicates tamoxifen exposure during pregnancy and mandates the use of barrier contraception during and 2 months after stopping tamoxifen treatment due to the extended half-life of its metabolite, N-desmethyl tamoxifen.[15,16] Tamoxifen suppresses prolactin release, hence contraindicated during lactation as well.[17] Women with breast cancer, who elected to discontinue tamoxifen prematurely, carry higher chances of recurrent disease and may also be less likely to conceive due to their prior treatment effects. Accidental conception on tamoxifen and its consequences on maternal and fetal health are not well-explored. Tamoxifen was not used during lactation in the present case.
To summarize, the current evidence based on animal studies and few human case-reports demonstrating teratogenic effects of tamoxifen and the lack of long-term data on outcome, mandates thorough counseling of the women with reproductive potential taking tamoxifen about the need for contraceptives and hazards of getting pregnant during tamoxifen. It is prudent to stop tamoxifen before planning to conceive. In case of an inadvertent pregnancy, possible teratogenicity and risk to mother for future recurrences due to early stoppage of tamoxifen should be discussed in detail before opting for or against continuing the pregnancy and further management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Iguchi T, Hirokawa M, Takasugi N. Occurrence of genital tract abnormalities and bladder hernia in female mice exposed neonatally to tamoxifen. Toxicology. 1986;42:1–11. doi: 10.1016/0300-483x(86)90087-9. [DOI] [PubMed] [Google Scholar]
- 2.Tewari K, Bonebrake RG, Asrat T, Shanberg AM. Ambiguous genitalia in infant exposed to tamoxifen in utero. Lancet. 1997;350:183. doi: 10.1016/S0140-6736(97)24029-8. [DOI] [PubMed] [Google Scholar]
- 3.Oksüzoglu B, Güler N. An infertile patient with breast cancer who delivered a healthy child under adjuvant tamoxifen therapy. Eur J Obstet Gynecol Reprod Biol. 2002;104:79. doi: 10.1016/s0301-2115(01)00552-8. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs RJ, Hunter W, Clark K. Tamoxifen as systemic treatment of advanced breast cancer during pregnancy – Case report and literature review. Gynecol Oncol. 2001;80:405–8. doi: 10.1006/gyno.2000.6080. [DOI] [PubMed] [Google Scholar]
- 5.Halakivi-Clarke L, Cho E, Onojafe I, Liao DJ, Clarke R. Maternal exposure to tamoxifen during pregnancy increases carcinogen-induced mammary tumorigenesis among female rat offspring. Clin Cancer Res. 2000;6:305–8. [PubMed] [Google Scholar]
- 6.Pugh DM, Sumano HS. The anti-implantation action of tamoxifen in mice. Arch Toxicol Suppl. 1982;5:209–13. doi: 10.1007/978-3-642-68511-8_38. [DOI] [PubMed] [Google Scholar]
- 7.Lim YC, Li L, Desta Z, Zhao Q, Rae JM, Flockhart DA, et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006;318:503–12. doi: 10.1124/jpet.105.100511. [DOI] [PubMed] [Google Scholar]
- 8.Kedia-Mokashi N, Makawy AE, Saxena M, Balasinor NH. Chromosomal aberration in the post-implantation embryos sired by tamoxifen treated male rats. Mutat Res. 2010;703:169–73. doi: 10.1016/j.mrgentox.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Clark RM, Chua T. Breast cancer and pregnancy: The ultimate challenge. Clin Oncol (R Coll Radiol) 1989;1:11–8. doi: 10.1016/s0936-6555(89)80004-4. [DOI] [PubMed] [Google Scholar]
- 10.Poulet FM, Roessler ML, Vancutsem PM. Initial uterine alterations caused by developmental exposure to tamoxifen. Reprod Toxicol. 1997;11:815–22. doi: 10.1016/s0890-6238(97)00065-8. [DOI] [PubMed] [Google Scholar]
- 11.Barthelmes L, Gateley CA. Tamoxifen and pregnancy. Breast. 2004;13:446–51. doi: 10.1016/j.breast.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Cullins SL, Pridjian G, Sutherland CM. Goldenhar's syndrome associated with tamoxifen given to the mother during gestation. JAMA. 1994;271:1905–6. [PubMed] [Google Scholar]
- 13.Berger JC, Clericuzio CL. Pierre Robin sequence associated with first trimester fetal tamoxifen exposure. Am J Med Genet A. 2008;146A:2141–4. doi: 10.1002/ajmg.a.32432. [DOI] [PubMed] [Google Scholar]
- 14.Clark S. Prophylactic tamoxifen. Lancet. 1993;342:168. [Google Scholar]
- 15.Patterson JS, Settatree RS, Adam HK, Kemp JV. Serum concentrations of tamoxifen and major metabolite during longterm nolvadex therapy, correlated with clinical response. Eur J Cancer Suppl. 1980;1:89–92. [PubMed] [Google Scholar]
- 16.MacCallum J, Cummings J, Dixon JM, Miller WR. Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br J Cancer. 2000;82:1629–35. doi: 10.1054/bjoc.2000.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 6th ed. Philadelphia: Williams and Wilkins; 2002. pp. 1307–13. [Google Scholar]


