Abstract
Reactive oxygen species (ROS) can be used to kill bacterial cells, and thus the selective generation of ROS from material surfaces is an emerging direction in antibacterial material discovery. We found the polarization of piezoelectric ceramic causes the two sides of the disk to become positively and negatively charged, which translate into cathode and anode surfaces in an aqueous solution. Because of the microelectrolysis of water, ROS are preferentially formed on the cathode surface. Consequently, the bacteria are selectively killed on the cathode surface. However, the cell experiment suggested that the level of ROS is safe for normal mammalian cells.
Keywords: piezoceramics, reactive oxygen, biocompatibility, antibacterial mechanism, potassium sodium niobate
Graphical abstract
Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen, such as peroxides, superoxide, hydroxyl radicals, and singlet oxygen.1,2 ROS are highly reactive molecules with unpaired electrons and are generated in normal physiological processes.3,4 The regulation of oxidation– reduction (redox) reactions is critical to cells because it influences metabolic and other signal transduction pathways.5,6 When ROS generation exceeds the cellular antioxidant defense, cells are damaged.7,8 It is increasingly clear that the generation of ROS can be exploited therapeutically to develop antimicrobial agents,9,10 to treat cancer,11–13 and for organ transplantation.14 Ideally, antibacterial biomaterials research should include methods for tuning ROS production. There has been no effort to control ROS production on the specific surface of a functional material. To address this gap, we exploited the physical properties of piezoelectric ceramics to control ROS production without changing the material’s composition.
An electric field can trigger the micro electrolysis of water15 and subsequent production of ROS on the cathode surface.16,17 Piezoelectric ceramic surfaces can form a micro electric field after polarization under a strong DC field (ceramic electric domain along the direction of the electric field). Therefore, we hypothesized that polarized piezoceramics would produce ROS on the cathode side to permit ROS release from a particular side of the material in a controllable manner. Potassium sodium niobate (KNN), a lead-free piezoelectric ceramic, has been employed as an implant material for dental and orthopedic applications due to its stable piezoelectricity.18,19 Hence, we used KNN with different piezoelectric constants to achieve surface-selective production of ROS (Figure 1).
Figure 1.
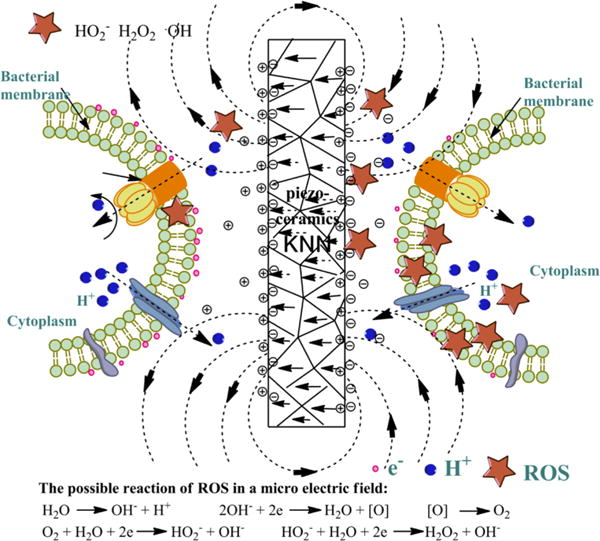
Schematic illustration of the possible selective antibacterial mechanism of polarized KNN. After polarization, the electric domains of the piezoelectric KNN become aligned, which results in the distribution of positive charges on one surface and the distribution of negative charges on the opposite surface. Consequently, an electric field is generated around the KNN substrates that promotes the formation of ROS (e.g., H2O2, HO2− and •OH) at the cathode surface through the reactions listed in the figure. Due to the presence of the electric double layer, the positive surface of KNN is the cathode of the micro electric field. Consequently, when bacteria are cultured on the positive surfaces (termed positively polarized surfaces) of the KNN substrates, they are killed by ROS. Conversely, when bacteria are cultured on the negative surfaces (termed negatively polarized surfaces) of the KNN substrates, they are not killed because of the low levels of ROS.
After polarization of KNN, a piezoelectric constant of 70 pC/N was obtained. The positively and negatively polarized sides were denoted KNN+70 and KNN−70. KNN+70 produced the largest amount of ROS, whereas KNN−70 produced a negligible amount of ROS. To verify ROS generation and selective release on the positively polarized side, we assessed the antibacterial activity of KNN with different piezoelectric constants against S. aureus using a plate colony-counting method.20 We observed that the bacteria were killed selectively on the positively polarized surface because of the selective production of ROS on this surface.
The polarization of KNN led to the formation of positively and negatively charged surfaces on opposite sides,21 which further attracted ions of opposite charges to form a micro electric field in phosphate-buffered saline (PBS) (Figure 1). The electric field disrupted the hydrogen bonding of H2O, producing more charged ions and free-radicals.22 Such micro electrolysis can further produce ROS. As shown in the electrolysis reaction (Figure 1), ROS are produced, including H2O2, HO2−, and •OH.23 The ROS can kill bacteria by increasing the oxidative stress in the cells and the permeability of the cell membranes, resulting in the penetration and disruption of the bacterial cell membranes.24
KNN was polarized under an electric field of 25 kV/cm for 30 min. to get a piezoelectric constants of 70 pC/N. For the polarized KNN substrate, two surfaces were studied, one positively charged and another negatively charged, to determine which side generated more ROS and thus selectively killed bacteria. KNN substrates with four different piezoelectric constants (10, 30, 50, and 70 pC/N) were prepared. The scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) results (Figure S1) indicated that the polarized KNN had the same morphology and chemical composition as nonpolarized KNN. Hydroxyapatite (HA) was used as a control surface. Figure S2 shows the confirmation of the successful preparation of KNN and HA substrates by XRD analysis.
The surface potentials of nonpolarized KNN, KNN+70, and KNN−70 were 136 ± 5, 187 ± 8, and 67 ± 3 mV as determined by scanning Kelvin probe microscopy (SKPM) (Figure 2). The surface potential was measured and calculated by averaging the data from 3 randomly selected areas (500 nm × 500 nm) on the substrates. The surface potential of the three KNN surfaces increased in the order KNN−70 < KNN < KNN+70. Thus, the potential of the KNN surface was increased by positive polarization and reduced by negative polarization.
Figure 2.
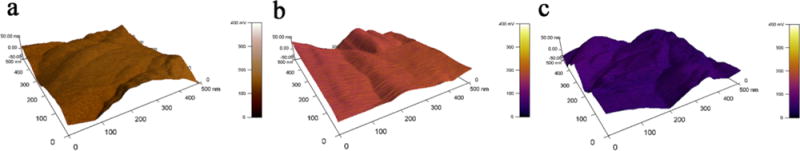
SKPM study of the surface potential of (a) nonpolarized KNN, (b) KNN+70 (positively polarized KNN), and (c) KNN−70 (negatively polarized KNN). The 3D map combination of height and potential profiles illustrates that the observed potential change is not caused by height change. This figure shows that the surface potential of KNN is increased by positive polarization and reduced by negative polarization.
A high ROS level can increase oxidative stress in cells, resulting in damage to both the cell membrane and proteins. Intracellular ROS formation was measured on the polarized KNN using dichlorofluorescein diacetate (DCFH-DA) as a fluorescent staining probe after 24 h of incubation with S. aureus. Quantification of ROS in S. aureus treated with KNN samples (Figure 3) indicated that ROS increased when S. aureus was cultured on KNN+70. However, when S. aureus was cultured on KNN−70, very little ROS were detected. These results further support our hypothesis that piezoelectric ceramics can realize surface-selective controllable ROS production.
Figure 3.
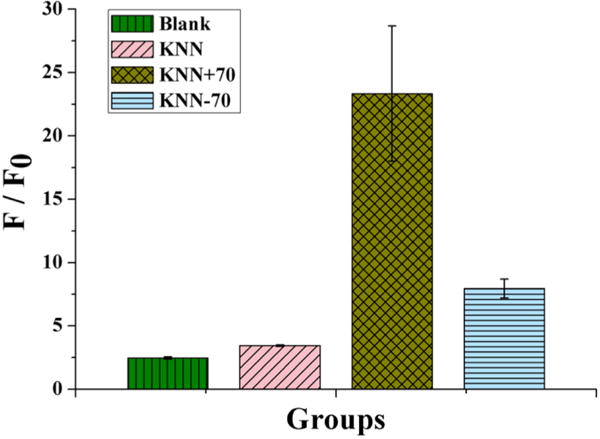
Quantification of the ROS level. The DCFH fluorescence intensity was tested after different substrates were cocultured with S. aureus for 24 h. F and F0 are the fluorescence intensities of the experimental group and the positive control included in the commercial kit, respectively. F/F0 is the relative ROS intensity of the experimental group. The results demonstrate that the ROS level of KNN+70 is higher than those of the other groups (Blank, KNN, and KNN−70).
The prevailing hypothesis is that the production of ROS leads to oxidative damage to cell membrane and proteins, subsequently resulting in cellular death. To verify that selective ROS generation can lead to antibacterial behavior on selective surfaces (positively vs negatively polarized), the antibacterial activity of polarized KNN against S. aureus was evaluated using a plate colony-counting method. In the plate colony-counting test, polarized KNN+70 samples were compared with nonpolarized KNN, KNN−70 and blank 48-well plates. The positively polarized KNN surface exhibited antibacterial properties against S. aureus, whereas both the nonpolarized and negatively polarized KNN surfaces did not (Figure 4). The positively polarized KNN surface was the most effective in killing S. aureus. Its antibacterial ratio reached 100%, meeting the standard of medicine. The antibacterial results (Figure 4) were consistent with the generation of ROS (Figure 3).
Figure 4.
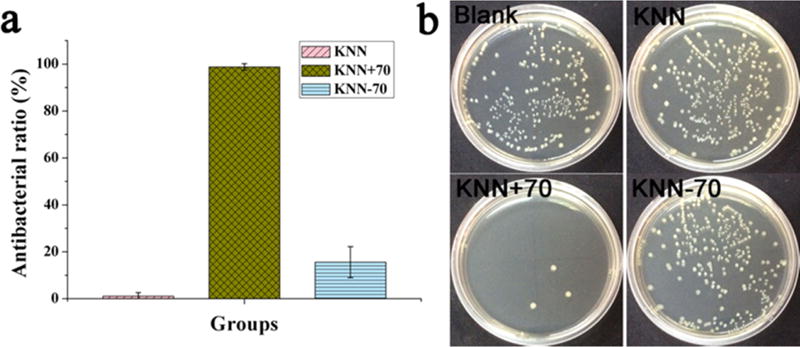
(a) Antibacterial ratio of Staphylococcus aureus at a concentration of 106 CFU/mL after coculture with nonpolarized KNN, KNN+70 and KNN−70. The data are presented as the mean ± standard deviation (n = 4); (b) Bacterial colonies were grown on the different substrates, and counts were obtained using a standard plate counting method. The data revealed that the positively polarized KNN with a piezoelectric constant of +70 had good antibacterial properties against S. aureus, whereas KNN−70 or KNN did not have antibacterial properties.
An ideal antibacterial surface should only kill bacteria and not normal mammalian cells. We therefore performed a CCK-8 assay to detect the proliferation of mouse mesenchymal stem cells (MSCs) on the different KNN surfaces (Figure S3). No significant difference in cell proliferation was observed on the polarized KNN surface compared to HA. In addition, the biocompatibility of the polarized KNN surface was greater than that of nonpolarized KNN. Because HA is a biocompatible implant material, these results indicate that KNN + 70 is both antibacterial and biocompatible and that the level of ROS generated is sufficient to kill bacteria but safe for normal mammalian cells.25,26 Importantly, healthy mammalian cells such as MSCs can survive under higher ROS stress levels.27–30 Our results further confirmed that the ROS levels generated on KNN+70 were sufficiently high to kill bacteria but not MSCs.
As shown in Figure 1, the polarization of KNN will generate microelectric fields on both positively and negatively charged surfaces. Because ROS tend to be preferentially formed on cathodes compared to anodes,17,18 ROS are expected to be produced on the positively polarized surface, which represents a cathode surface terminated with negative charges (Figure 1). Our findings confirmed this expectation, that is, the microelectric field due to the polarization of the piezoelectric ceramics produced ROS and hence killed bacteria selectively on the positively polarized surface rather than on the negatively polarized surface.
In conclusion, we have developed a new method to produce and release ROS on the surface of a material. The polarization of KNN results in the formation of a micro electric field on its surface, which in turn generates ROS and kills bacteria selectively on the positively polarized surface. The KNN+70 surface exhibited the most efficient antibacterial activities against S. aureus as well as biocompatibility comparable to that of HA. The present study provides new insights on controlling the antibacterial properties of a surface using piezoelectric ceramics without changing the material’s composition.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the financial support of National Basic Research Program of China (Grant No. 2012CB619100), the National Natural Science Foundation of China (Grant Nos. 51541201, 51372087), Natural Science Foundation of Guangdong Province (Grant No. 2015A030313493), and the Science and Technology Planning Project of Guangdong Province (Grant no. 2014A010105048). Y. Zhu and C.B.M would like to thank the financial support from National Institutes of Health (EB015190), National Science Foundation (CMMI-1234957), Department of Defense office of the Congressionally Directed Medical Research Programs (W81XWH-15-1-0180), Oklahoma Center for Adult Stem Cell Research (434003) and Oklahoma Center for the Advancement of Science and Technology (HR14-160).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.6b07440.
Experimental section describing the morphologies and compositions of KNN and HA; additional antibacterial effects of nonpolarized KNN, KNN+70, and KNN−70; and cell proliferation on the surface of the three types of KNN and HA (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.Chen XQ, Tian XZ, Shin I, Yoon J. Fluorescent and Luminescent Probes for Detection of Reactive Oxygen and Nitrogen Species. Chem Soc Rev. 2011;40(9):4783–4804. doi: 10.1039/c1cs15037e. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nystrom T, Belousov V, Schumacker PT, Winterbourn CC. Unraveling the Biological Roles of Reactive Oxygen Species. Cell Metab. 2011;13(4):361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Circu ML, Aw TY. Reactive Oxygen Species, Cellular Redox Systems, and Apoptosis. Free Radical Biol Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial Reactive Oxygen Species (Ros) and Ros-Induced Ros Release. Physiol Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sena LA, Chandel NS. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray PD, Huang BW, Tsuji Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell Signalling. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleury C, Mignotte B, Vayssiere JL. Mitochondrial Reactive Oxygen Species in Cell Death Signaling. Biochimie. 2002;84(2–3):131–141. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 8.Dixon SJ, Stockwell BR. The Role of Iron and Reactive Oxygen Species in Cell Death. Nat Chem Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 9.Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, Agerberth B, Gudmundsson GH, Gallo RL. Cutaneous Injury Induces the Release of Cathelicidin Antimicrobial Peptides Active against Group A Streptococcus. J Invest Dermatol. 2001;117(1):91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 10.Hou AQ, Feng GC, Zhuo JY, Sun G. UV Light-Induced Generation of Reactive Oxygen Species and Antimicrobial Properties of Cellulose Fabric Modified by 3,3′, 4,4′-Benzophenone Tetracarboxylic Acid. ACS Appl Mater Interfaces. 2015;7(50):27918–27924. doi: 10.1021/acsami.5b09993. [DOI] [PubMed] [Google Scholar]
- 11.Renschler MF. The Emerging Role of Reactive Oxygen Species in Cancer Therapy. Eur J Cancer. 2004;40(13):1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 12.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Shi JJ, Chen ZY, Wang BH, Wang L, Lu TT, Zhang ZZ. Reactive Oxygen Species-Manipulated Drug Release from a Smart Envelope-Type Mesoporous Titanium Nanovehicle for Tumor Sonodynamic-Chemotherapy. ACS Appl Mater Interfaces. 2015;7(51):28554–28565. doi: 10.1021/acsami.5b09937. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Wang M, Xie HY, Zhou L, Meng XQ, Shi J, Zheng S. Role of Reactive Oxygen Species in Mediating Hepatic Ischemia-reperfusion Injury and Its Therapeutic Applications in Liver Transplantation. Transplant Proc. 2007;39(5):1332–1337. doi: 10.1016/j.transproceed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Koh EK, Ryu BK, Jeong DY, Bang IS, Nam MH, Chae KS. A 60-Hz Sinusoidal Magnetic Field Induces Apoptosis of Prostate Cancer Cells through Reactive Oxygen Species. Int J Radiat Biol. 2008;84(11):945–955. doi: 10.1080/09553000802460206. [DOI] [PubMed] [Google Scholar]
- 16.Kalbacova M, Roessler S, Hempel U, Tsaryk R, Peters K, Scharnweber D, Kirkpatrick JC, Dieter P. The Effect of Electrochemically Simulated Titanium Cathodic Corrosion Products on ROS Production and Metabolic Activity of Osteoblasts and Monocytes/macrophages. Biomaterials. 2007;28(22):3263–3272. doi: 10.1016/j.biomaterials.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Ehrensberger MT, Tobias ME, Nodzo SR, Hansen LA, Luke-Marshall NR, Cole RF, Wild LM, Campagnari AA. Cathodic Voltage-controlled Electrical Stimulation of Titanium Implants as Teatment for Methicillin-resistant Staphylococcus aureus Periprosthetic Infections. Biomaterials. 2015;41:97–105. doi: 10.1016/j.biomaterials.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Wang XP, Wu JG, Xiao DQ, Zhu JG, Cheng XJ, Zheng T, Zhang BY, Lou XJ, Wang XJ. Giant Piezoelectricity in Potassium-Sodium Niobate Lead-Free Ceramics. J Am Chem Soc. 2014;136(7):2905–2910. doi: 10.1021/ja500076h. [DOI] [PubMed] [Google Scholar]
- 19.Wu JG, Xiao DQ, Zhu JG. Potassium-Sodium Niobate Lead-Free Piezoelectric Materials: Past, Present, and Future of Phase Boundaries. Chem Rev. 2015;115(7):2559–2595. doi: 10.1021/cr5006809. [DOI] [PubMed] [Google Scholar]
- 20.Mei SL, Wang HY, Wang W, Tong LP, Pan HB, Ruan CS, Ma QL, Liu MY, Yang HL, Zhang L, Cheng YC, Zhang YM, Zhao LZ, Chu PK. Antibacterial Effects and Biocompatibility of Titanium Surfaces with Graded Silver Incorporation in Titania Nanotubes. Biomaterials. 2014;35(14):4255–4265. doi: 10.1016/j.biomaterials.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Birol H, Damjanovic D, Setter N. Preparation and Characterization of (K0.5Na0.5) NbO3 Ceramics. J Eur Ceram Soc. 2006;26(6):861–866. [Google Scholar]
- 22.Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, Vunjak-Novakovic G. Electrical Stimulation of Human Embryonic Stem Cells: Cardiac Differentiation and the Generation of Reactive Oxygen Species. Exp Cell Res. 2009;315(20):3611–3619. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayir H. Reactive Oxygen Species. Crit Care Med. 2005;33(12):S498–S501. doi: 10.1097/01.ccm.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 24.Ning CY, Wang XL, Li LH, Zhu Y, Li M, Yu P, Zhou L, Zhou ZN, Chen JQ, Tan GX, Zhang Y, Wang YJ, Mao CB. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem Res Toxicol. 2015;28(9):1815–1822. doi: 10.1021/acs.chemrestox.5b00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gharibi R, Yeganeh H, Rezapour-Lactoee A, Hassan ZM. Stimulation of Wound Healing by Electroactive, Antibacterial, and Antioxidant Polyurethane/Siloxane Dressing Membranes: In Vitro and in Vivo Evaluations. ACS Appl Mater Interfaces. 2015;7(43):24296–24311. doi: 10.1021/acsami.5b08376. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Zhang W, Qiao Y, Jiang X, Liu X, Ding C. Antibacterial Activity and Increased Bone Marrow Stem Cell Functions of Zn-incorporated TiO2 Coatings on Titanium. Acta Biomater. 2012;8(2):904–915. doi: 10.1016/j.actbio.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 27.Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, Chung SJ, Kim DD, Sung JH. Evidence Supporting Antioxidant Action of Adipose-derived Stem Cells: Protection of Human Dermal Fibroblasts from Oxidative Stress. J Dermatol Sci. 2008;49(2):133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Wong VW, Rustad KC, Glotzbach JP, Sorkin M, Inayathullah M, Major MR, Longaker MT, Rajadas J, Gurtner GC. Pullulan Hydrogels Improve Mesenchymal Stem Cell Delivery into High-Oxidative-Stress Wounds. Macromol Biosci. 2011;11(11):1458–1466. doi: 10.1002/mabi.201100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prockop DJ, Oh JY. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol Ther. 2012;20(1):14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou J, Han ZP, Jing YY, Yang X, Zhang SS, Sun K, Hao C, Meng Y, Yu FH, Liu XQ, Shi YF, Wu MC, Zhang L, Wei LX. Autophagy Prevents Irradiation Injury and Maintains Stemness through Decreasing ROS Generation in Mesenchymal Stem Cells. Cell Death Dis. 2013;4:e844. doi: 10.1038/cddis.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


