Abstract
Advances in engineering of cells and culture formats have led to the development of a new generation of 3D cultures that can recapitulate a variety of multi-cell type, morphogenetic behaviors that largely were only observable in in vivo settings. Ultimately, these systems are likely to be assimilated into and forever change the landscape of biomedical research.
Keywords: Organotypic, in vitro models, three-dimensional, organ-on-chip, organoids
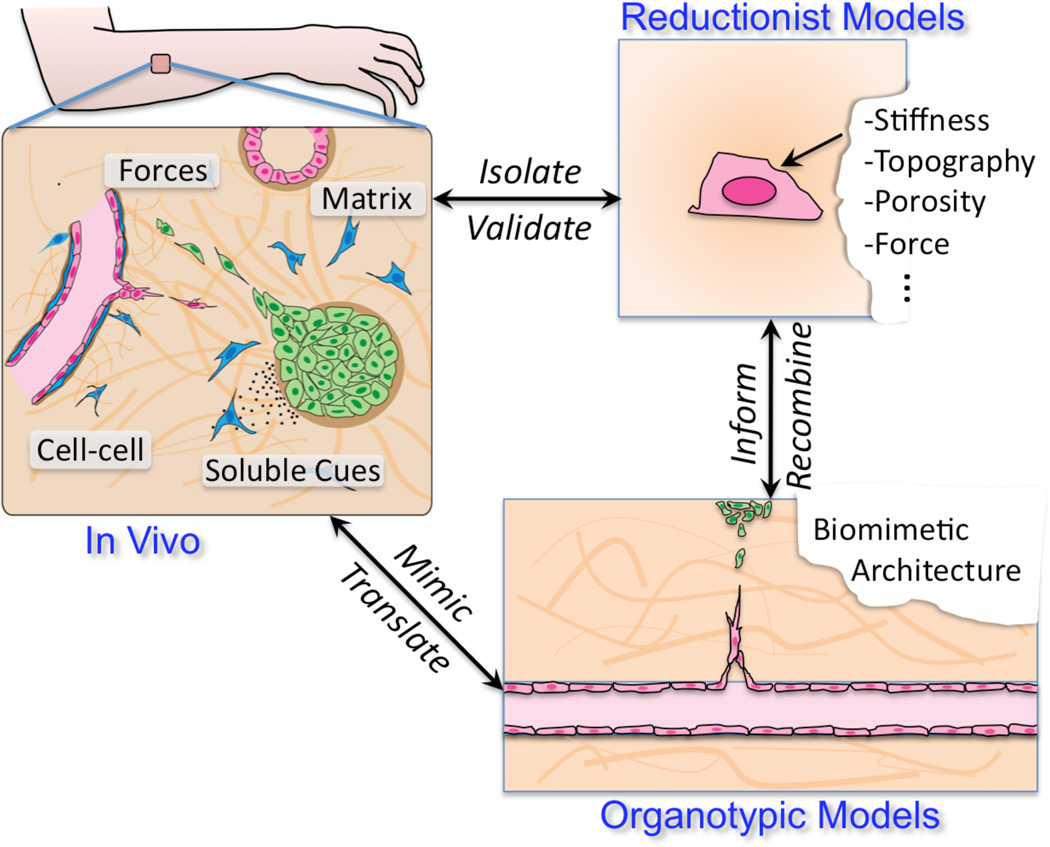
As with all sciences, biology advances through our ability to experiment, in this case with living systems. Modern biomedical research relies essentially on two experimental test beds – animals and cultured cells. The knowledge revolution of the past half century that we know as cell biology largely rests on the dissemination of cultured cells – HeLa cells first, then other immortalized lines, and now a wide array of primary cells and stem-like cells – as accepted experimental systems to understand cell structure and function. As an apt adjunct to animal systems, which capture the full complexity of biology but with limited ability to quickly isolate detailed mechanisms, experimental manipulation of cells in culture is straightforward and has revolutionized our molecular understanding of cells. However, despite amazing advances, our ability to translate cell biological insights has been mitigated because culture on plastic dishes is so different from the in vivo microenvironment. Cells not only change their behavior in this non-physiologic environment, but cells are unable to remodel the matrix and re-organize freely as they would during development and homeostasis. As such, many key functions are lost or unobservable in cell culture. These limitations compel us to consider whether innovative platforms that allow us to examine cells cultured in more biomimetic contexts can be developed to bridge the gap between traditional cell culture and the whole organism, and what impact would such systems will have on our biomedical research enterprise (Figure 1).
Figure 1.
In vivo and in vitro models have co-evolved synergistically to provide distinct approaches to understanding living systems. New biomimetic models offer the potential to provide a third approach to the ecosystem, reconstituting more complex behaviors in culture.
In vivo, local tissue structure defines the cellular environment, constraining how cells interact with surrounding extracellular matrix (ECM), neighboring cells, soluble growth factors, and physical forces. These “microenvironmental” cues cooperate to regulate cell behavior. Thus, while it is no surprise that culture on plastic dishes results in decompensated cell signaling, gene expression, phenotype, and function, attempting to fully reconstruct a tissue environment for in vitro applications would be excessive. The real challenge is in identifying which factors to incorporate in order to appropriately model different in vivo processes in cell culture, and then establishing what such systems would and would not be able to recapitulate.
In recent years, a number of ex vivo experimental models have been developed to capture a variety of higher level behaviors that historically were largely reserved for animal models. Some of these models are methodologically ‘simple’, natural extensions of classical 3D cultures that have been used to generate mammary acini, hanging drop embryoid bodies, or spheroid cultures, though with remarkable new morphogenetic capabilities. For example, single intestinal stem cells embedded within extracellular matrix gels have been shown to give rise to self-organizing structures characteristic of cryptvilli of the intestine [1]. Similarly, spheroid cultures of neuronal stem cells have been developed to recapitulate layering and morphogenesis of the developing brain [2]. Other systems in contrast involve substantial engineering and incorporation of synthetic materials, pre-fabricated architectures, and/or microfluidics in order to model specific biological processes. For example, using a device containing two microfluidic channels separated by a porous elastic membrane, Ingber and colleagues were able to model the interface between lung alveolar air, epithelium, capillary endothelium, and blood [3]. Using pumps to control air and blood flow and mechanical actuators to mimic the stretching forces of breathing on the epithelial-endothelial interface, the model has been used to recapitulate injury and inflammation, and has inspired a cadre of organ-on-chip efforts spanning from cardiac muscle to liver tissue [4,5]. Incorporation of human cells and human iPS-derived cell types into some of these systems have suggested the possibility that these biomimetic systems have the potential to close the gap between traditional animal models and human physiology and disease [6,7]. Indeed, DARPA, NIH and NCATS, and the popular press have embraced the idea that these systems will ultimately replace preclinical testing of therapeutics in animals [8–10]. How can the research community come together to realize such high expectations, separate reality from hype, and ultimately benefit with a bevvy of experimentally tractable systems that model human physiology and disease?
Classically, biological experimental systems were used not as models – systems used to predict the behavior of another system – but as an end unto themselves. Describing anatomical structures in HeLa cells or wing formation in Drosophila was valued for its own knowledge. In contrast, the 3D biomimetic systems that are now being developed are explicitly valued for their ability to model specific processes mostly in human biology. It stands to reason that a large part of establishing such models will be to define what the models can or cannot recapitulate. It is important to note that unlike in vivo systems, these models are necessarily and intentionally simplifications to capture a narrow range of behavior, physiology, or time. For example, while intravital recordings of developing vasculature of the avian ovum or zebrafish can capture vasculogenesis (when endothelial cells assemble to form networks spontaneously), angiogenesis (when existing vessels sprout and branch to form new vessels), or tumor cell trafficking, different biomimetic culture systems have been established to capture each of these events separately (for example, see [11–13]). Thus, a key feature of these models in their current state of evolution is that they are best adopted when fit for a specific purpose, and holding expectations that such models would have universal applicability would be unrealistic.
Thus, key questions remain about how and when different models can or should be used. If mini-brains can recapitulate some aspects of neuronal organization, will they show aberrations with known genetically caused brain malformations? Will they respond predictively to neurochemical modulators? Will they predict neurological side effects of test compounds? Can they model aging? If the lung-on-chip can model inflammation, can it also recapitulate effects of cystic fibrosis? Will it respond similarly to biomechanical injury? If we take the lessons learned from cell culture and animal models, the key to answering these questions is not to wait for the group that first described these models to test all of these conditions. In fact, the only path to establishing these models, continually improving them, or deciding to abandon them is to make the models widely accessible to as many scientists that are willing to study them. There are many reasons why only a handful of cell lines and animal models dominated the research community, but perhaps the foremost were ease of adoption and the ability to share insights and advances amongst scientists. This poses a major challenge for many of these engineered organotypic models: They do not reproduce themselves; many of the systems are assembled as artisan pieces with many parameters that can affect the model so it can be difficult to teach; many different biomimetic systems or variations would be expected to emerge in order to highlight different biological events, and this customization inherently may limit wider adoption of each specific system; and it remains unclear which models should scientists aggregate around versus leave under investigated.
Despite these hurdles, the eventual incorporation of these synthetic, biomimetic culture systems into biomedical research laboratories is inevitable. The confluence of technological advances in both engineering and biological communities appears to be a virtual perfect storm that will push to continue to establish engineered 3D organotypic cultures. On the biological side, the coming together of iPSC technologies and stem cell biology to advance access to human cell types is arriving, and the application of genomic editing technologies into the fray offers the possibility to both model human genetic diseases and to mechanistically implicate molecular players in these culture systems. On the engineering side, a suite of technologies have been established that can be used to build different types of systems for organ-on-chip applications, including the development of biomaterials that can begin to mimic and decouple aspects of extracellular matrix, the application of micro- and nano-fabrication tools such as microfluidics to support cell-based systems, the advancement of 3D printing and other technologies to organize cells in 3-dimensions, microscopy advances to observe living cells in 3D contexts, and the use of insights gained by tissue engineers to assemble cell and extracellular matrix. The dire need for better models of human physiology and disease than either traditional cell culture or animals also provides a pull to advance these systems. Lastly, while ultimately these systems may become a primary platform for preclinical testing, their development will play a major role in our basic understanding of life’s design principles. Analogous with the in vitro reconstitution of subcellular processes, the iterative effort that leads to the synthetic reconstitution of multi-cell type, morphogenetic events will reveal the key components and subsystems necessary to generate such behaviors. Thus, one can only presume that these efforts will lead to a more complete understanding of how cells organize and stabilize within their surroundings, and will at a minimum become a mainstay approach alongside standard reductionist and animal models to deepen our understanding of life.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 2.Lancaster MA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh D, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 5.Esch EW, et al. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14:248–260. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinson JT, et al. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349:982–986. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutherland ML, et al. The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem Cell Res Ther. 2013;4(Suppl 1):I1. doi: 10.1186/scrt361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tissue Chip for Drug Screening. [Accessed: 14-Aug-2016];National Center for Advancing Translational Sciences. [Online]. Available: http://www.ncats.nih.gov/tissuechip.
- 10.Chips that Mimic Organs Could Be More Powerful Than Animal Testing. [Accessed: 14-Aug-2016];WIRED. [Online]. Available: http://www.wired.com/2016/06/chips-mimic-organs-powerful-animal-testing/
- 11.Nguyen D-HT, et al. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A. 2013;110:6712–6717. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moya ML, et al. In vitro perfused human capillary networks. Tissue Eng Part C Methods. 2013;19:730–737. doi: 10.1089/ten.tec.2012.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zervantonakis IK, et al. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci U S A. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]