Abstract
Mycobacterium kansasii is a slow growing acid-fast non-tuberculosis mycobacterium. It most commonly causes pulmonary disease with tuberculosis-like manifestations. Mycobacterium kansasii-induced skin and soft tissue infections (SSTIs) are very uncommon, especially in the absence of obvious risk factors. In this report, we present a rare case of M. kansasii-associated SSTI complicated by tendonitis and osteomyelitis in an immunocompetent patient. This case highlights the importance of considering non-tuberculosis mycobacteria while investigating chronic, relapsing, non-healing SSTIs and osteomyelitis. Proper pharmacotherapy, along with surgical debridement, is the optimal management to avoid relapse and the production of resistant species.
Introduction
Mycobacterium kansasii is a slow growing acid-fast non-tuberculosis mycobacterium (NTM) that was first described by Buhler and Pollack [1]. It is widely spread in the environment, primarily in aquatic media, tap water and soil [2]. Pulmonary disease is the most common presentation of M. kansasii with tuberculosis-like manifestations; extrapulmonary infection is far less common [2].
Mycobacterium kansasii is a rare cause of skin and soft tissue infection (SSTI), it is either the result of disseminated infection in immunocompromised patients [2] or localized inoculation of the pathogen after penetration of the skin [3]. Osteomyelitis is even less encountered than SSTIs, especially in the absence of obvious risk factors [4]. Only few cases in the medical literature reported M. kansasii-induced osteomyelitis in both healthy and immunocompromised patients [5].
In this report, we present a rare case of M. kansasii cutaneous infection that resulted in tendonitis and osteomyelitis in an immunocompetent patient.
Case Report
A 58-year-old Caucasian female presented to the infectious diseases clinic with a non-healing surgical site (Fig. 1). Three months earlier, she underwent surgical evacuation of an effusion in the right olecranon bursa complicating a fall. Culture of the aspirated serosanguinous fluid was negative for bacteria. Subsequently, she developed wound dehiscence with purulent discharge that failed surgical and antimicrobial therapies, which included 2 weeks of Ciprofloxacin and repeated 10 days of Clindamycin.
Figure 1:
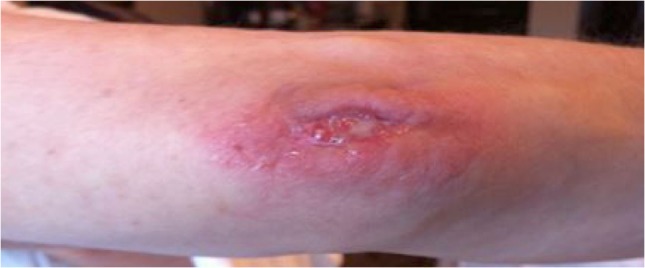
Non-healing surgical site on the right elbow; erythematous skin with signs of purulent discharge.
Physical examination revealed a patient who was alert, well-oriented to person, place and time and showed no signs of distress. Vital signs were: blood pressure 112/72 mm Hg, heart rate 64/minute, respiratory rate 18/minute and body temperature 97.9°F.
Musculoskeletal system evaluation showed a region of erythematous skin over her right elbow with purulent discharge, warmth and tenderness to palpation. The patient had limited right elbow extension due to pain. An MRI of the elbow (Fig. 2) showed extensive inflammation around the triceps insertion, indicating tendonitis, associated with osteomyelitis of the right ulnar olecranon process. The patient underwent surgical debridement. Tissue biopsy showed chronic inflammatory response evident by infiltration with neutrophils, lymphocytes and macrophages with areas of necrosis. Due to lack of information on the prior histopathology of the tissues we could not assess whether these pathological findings were present since the beginning or they were developed by an infection introduced by the surgical evacuation itself. Subsequent cultures grew M. kansasii. The treatment regimen included isoniazid, rifampin and ethambutol, and the patient improved after 6 months of therapy then lost follow-up.
Figure 2:
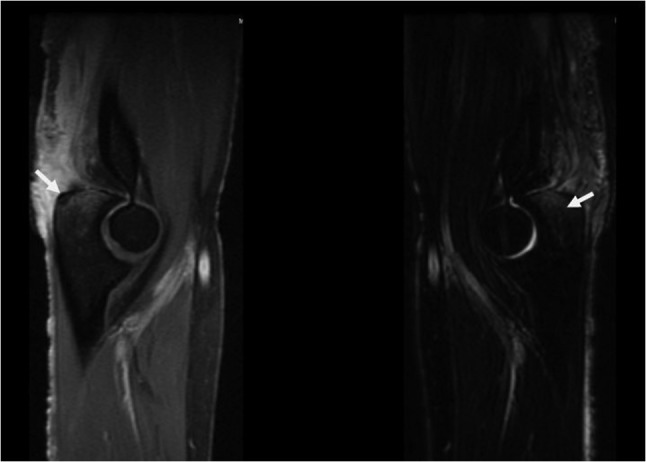
Sagittal T1 (left) and T2 (right) magnetic resonance imaging of the right elbow showing inflammation around the triceps insertion with lytic lesion of the olecranon process of the ulna indicating osteomyelitis. Left arrow points to the lytic lesion in the olecranon process. Right arrow points to T2 hyperintense signal representing bone marrow edema.
Discussion
Mycobacterium kansasii is a slow growing acid-fast photochromogenic bacillus that belongs to NTM. It is the second most common cause of NTM pulmonary disease after Mycobacterium avium complex [6].
Localized SSTIs due to M. kansasii are rare [3]. Rapid growing mycobacteria such as M. marinum account for the majority of NTM related cases of SSTIs [3]. The medical history of patients with M. kansasii-related SSTIs usually includes skin disruptive events, such as trauma, surgery, cosmetic procedures, injections and catheter placement [7]. Such infections are increasingly associated with disseminated disease in immunocompromised patients, like AIDS patients, and are notably less common in immunocompetent individuals [3, 7]. The clinical picture in such cases of NTM related SSTIs is indolent, unresolving cellulitis. It may also present as nodules, subcutaneous abscesses or necrotic ulcers [8]. Such presentation might be challenging and physicians should consider M. kansasii when they approach patients with similar manifestations.
Osteomyelitis is an extremely rare presentation of M. kansasii infection; there are only few reported cases of vertebral ostemoyelitis [5], and even fewer cases of an extra-vertebral osteomyelitis [5]. Skeletal system involvement usually presents as long-standing painful swollen joint or back pain in cases of vertebral osteomyelitis. Similar to the pre-mentioned pattern of SSTIs, such infections were reported in otherwise healthy patients with history of local trauma or local steroid injection, as well as immunocompromised patients with underlying diseases [5]. Septic arthritis may lead to osteomyelitis and should be approached thoroughly to prevent any complications. The possibility of M. kansasii should be considered in all cases of indolent arthritis especially in patients with the following risk factor: chronic systemic steroid use, local steroid injections, local trauma, chronic psoriatic arthritis and/or immunosuppression [8].
Discharge culture is the standard of diagnosing M. kansasii infections [9]. New diagnostic methods using PCR and DNA sequencing are also efficient in recognizing different NTM strains, which is helpful in choosing the appropriate regimen [9]. Due to the risk of misdiagnosis, contamination and sophisticated methods of identifying the causative pathogen, a good communication between clinicians and microbiology team is essential to reach the optimal diagnosis and management [9]. After the confirmation of M. kansasii infection, a drug susceptibility test with rifampin is recommended [9].
The American Thoracic Society (ATS) guidelines recommend the triple regimen for M. kansasii pulmonary disease [9]. The initial drug regimen should include rifampin, which has been shown to yield low failure rates (1.1%) and low long-term relapse rates (<1%) [10]. Other first-line medications include isoniazid and pyridoxine, with treatment duration continuing until sputum culture results are negative for 12 months. Alternative regimen in patients with rifampin-resistent M. kansasii, a 3-drug regimen should be used based on in vitro susceptibilities including clarithromycin or azithromycin, moxifloxacin, ethambutol, sulfamethoxazole or streptomycin. Patients with extrapulmonary and disseminated M. kansasii infections should be treated in a similar manner to those with pulmonary disease. Surgical debridement is also essential to improve the healing process and prevent relapsing, especially in cases of skin and joints involvement.
Due to reports of outbreaks and sporadic cases of NTM infections, ATS recommends preventive measures including avoiding using tap water and ice made by tap water in cleaning medical devices, catheters as well as procedures and surgery [9].
In summary, we present a rare case of M. kansasii-associated SSTI complicated by tendonitis and osteomyelitis in an immunocompetent patient. This case highlights the importance of considering non-tuberculosis mycobacteria while investigating chronic, relapsing, non-healing SSTI and osteomyelitis. Proper pharmacotherapy, along with surgical debridement, is the optimal management to avoid relapse and the production of resistant species.
Conflict of interest statement
None declared.
Funding
No funding was needed for this manuscript.
Ethical approval
No ethical approval was needed for this report.
Consent
The patient signed an informed consent to publish the case and all related figures.
Guarantor
Tarek Turk, Faculty of medicine, Damascus University, Damascus, Syria.
References
- 1.Buhler VB, Pollak A. Human infection with atypical acid-fast organisms. Report of two cases with pathologic findings. Am J Clin Pathol 1953;23:363–74. [DOI] [PubMed] [Google Scholar]
- 2.Lillo M, Orengo S, Cernoch P, Harris RL. Pulmonary and disseminated infection due to mycobactirium kansasii: a decade of experience. Rev Infect Dis 1990;12:760–7. [DOI] [PubMed] [Google Scholar]
- 3.Liao CH, Lai CC, Ding LW, Hou SM, Chiu HC, Chang SC, et al. Skin and soft tissue infection caused by non-tuberculous mycobacteria. Int J Tuberc Lung Dis 2007;11:96–102. [PubMed] [Google Scholar]
- 4.Canueto-Quintero J, Caballero-Granado FJ, Herrero-Romero M, Domínguez-Castellano A, Martín-Rico P, Verdú EV, et al. Epidemiological, clinical, and prognostic differences between the diseases caused by Mycobacterium kansasii and Mycobacterium tuberculosis in patients infected with human immunodeficiency virus: a multicenter study. Clin Infect Dis 2003;37:584–90. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu H, Mizuno Y, Nakamura I, Fukushima S, Endo K, Matsumoto T. Vertebral osteomyelitis caused by non-tuberculous mycobacteria: case reports and review. J Infect Chemother 2013;19:972–7. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009;49:e124–9. [DOI] [PubMed] [Google Scholar]
- 7.Han SH, Kim KM, Chin BS, Choi SH, Lee HS, Kim MS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci 2010;25:304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortoli E. Clinical manifestations of nontuberculous mycobacteria infections. Clin Microbiol Infect 2009;15:906–10. [DOI] [PubMed] [Google Scholar]
- 9.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- 10.Griffith DE. Management of disease due to Mycobacterium kansasii. Clin Chest Med 2002;23:613–21. [DOI] [PubMed] [Google Scholar]


