Abstract
Objective
The role of metabolic condition and diet in regulating circulating levels of adropin, a peptide hormone linked to cardiometabolic control, is not well understood. Here we examined weight loss and diet effects on plasma adropin concentrations.
Methods
The present report includes data from (1) a weight loss trial, (2) an evaluation of acute exercise effects on mixed-meal tolerance test responses, and (3) a meta-analysis to determine normal fasting adropin concentrations.
Results
Plasma adropin concentrations exhibit a distribution with positive skew and kurtosis. The effect of weight loss on plasma adropin concentrations was dependent on baseline plasma adropin concentrations, with an inverse association between baseline and a decline in concentrations after weight loss (Spearman’s ρ=−0.575; P<0.001). When ranked by baseline plasma adropin concentrations, only values in the upper quartile declined with weight loss. Plasma adropin concentrations under the bell-curve correlated negatively with habitual carbohydrate intake and plasma lipids. There was a negative correlation between baseline values and a transient decline in plasma adropin during the MMTT
Conclusions
Plasma adropin concentrations in humans are sensitive to dietary macronutrients, perhaps due to habitual consumption of carbohydrate-rich diets suppressing circulating levels. Very high adropin levels may indicate cardiometabolic conditions sensitive to weight loss.
Keywords: Dietary sugars, Hormones, Metabolism, Adropin, Lipids
INTRODUCTION
Studies using mice suggest the peptide hormone adropin has metabolic (1–3) and vascular functions (4). Circulating adropin concentrations have been measured in several mammalian species using commercially produced enzyme immunoassays (5–22). Human studies screened for associations between plasma adropin concentrations and cardiovascular disease (5, 8, 12, 17), endothelial function (10, 11), type 2 diabetes (7, 9, 14, 17), obesity and aging (6), and exercise response (23). These studies suggest associations between cardiometabolic disorders of obesity and altered circulating adropin concentrations (24, 25).
Adropin expression in a human liver cell line (Hepg2) is suppressed following activation of liver receptor (LXRa), suggesting sensitivity to carbohydrate and lipid metabolism (26). We reported increased plasma adropin concentrations following Roux-en Y gastric bypass (6), changes following sugar consumption (20), and associations between plasma adropin concentrations and fat and carbohydrate intake (16). Metabolic condition and feeding behavior thus influence plasma adropin levels in humans. However, the specific metabolic and dietary factors influencing plasma adropin concentrations in humans remain unclear.
Here we report investigating whether weight loss and improved insulin sensitivity would affect plasma adropin concentrations in humans using plasma samples before and after 6–8% weight reduction (27). We also further investigated associations between plasma adropin concentrations and macronutrient intake previously observed in women participating in a sleep restriction study (16), using habitual intake data froma larger cohort of men and women. We also performed a meta-analysis was used to establish normal values for fasting plasma adropin.
METHODS
Data from three studies were included in this report: (1) a randomized intervention trial to determine whether weight loss and habitual diet affect plasma adropin concentrations (CREG study), (2) a meta-analysis to determine normal plasma adropin concentrations, and (3) an acute exercise study in diabetics evaluating the effects of a single bout of exercise on plasma adropin responses to a mixed-meal tolerance test (MMTT study).
The rationale for examining whether weight loss affects plasma adropin concentrations in humans is based on experiments in mice showing regulation in response to fasting (26) and caloric restriction (28). The rationale for the meta-analysis was based on data from the weight-loss study suggesting a clustering of values below 5 ng/ml. The intent of the MMTT was to examine whether a meal with carbohydrate content similar to the habitual intake of participants with low plasma adropin concentrations would have an inhibitory effect
Caloric Restriction, Exercise, and Glucoregulation in Humans (CREG)
Participants, Intervention
Selection criteria, interventions and primary outcomes were reported previously (27) (ClinicalTrials.gov #NCT00777621). In brief, the study involved sedentary, overweight men and women aged 45–65 years. Subjects were randomized with stratification for sex, and assigned to groups with the established goal of achieving 6–8% weight loss using either calorie restriction (CR), endurance exercise training (EX), or a combination (CREX) to maintain a 20% negative energy balance relative to estimated total energy expenditure. The study was reviewed and approved by the Institutional Review Boards (IRB) of Washington University and Saint Louis University.
Plasma samples used in this study were collected at baseline and follow-up; follow-up samples were collected after 2 wk of weight stability to eliminate confounding effects of negative energy balance. For CREX and EX participants, samples were collected 12–24h after the last exercise bout. Sera were analyzed in a CLIA-certified clinical laboratory for concentrations of total, LDL, and HDL-cholesterol and glycerol-blanked triglyceride concentrations using automated enzymatic/colorimetric assays (Roche/Hitachi Modular Analytics System, Roche Diagnostics Corporation, Indianapolis IN). Plasma glucose was measured using the glucose oxidase method (YSI STAT Plus; YSI Life Sciences, Yellow Springs, OH); insulin was measured using IMMULITE Chemiluminescence Kits (Diagnostics Products Corporation, Los Angeles, CA). Fat mass and fat-free mass were measured by DXA (Lunar iDXA, software version 13.31; GE Healthcare, Madison, WI).
Food diaries
CREG study participants maintained food diaries over 3d prior to weight loss intervention; the sample size is higher (n=62) compared to that used for the weight loss study (n=54) owing to noncompliance of 8 participants. Nutrient intakes were quantified by analyzing the 3-day food diaries (2 weekdays, 1 weekend day) with Food Processor SQL (ESHA Research, Salem, OR).
Response of plasma adropin concentrations following a mixed-meal tolerance test (MMTT)
Participants
Sedentary (0–1 sessions/week of physical activity lasting >30 min; not employed in physically active jobs or hobbies) weight stable, overweight and obese [body mass index (BMI) 25.0–37.0 kg/m2] male (n=2) and female (n=7) subjects aged 48–67y with physician-diagnosed type 2 diabetes (T2D) and HbA1c <10% were recruited for participation. Subjects were non-smokers, not on insulin therapy, had no previous cardiac events, and did not skip breakfast or have other irregular dietary patterns. The study was reviewed and approved by the IRB at the University of Missouri in Columbia, MO.
Intervention
Plasma adropin concentrations during the MMTT were compared before (Pre) and after (Post) a 7-day exercise intervention, allowing within subjects comparisons. Seven days of aerobic exercise training is commonly used to examine the effects of added daily exercise prior to changes in body composition and training adaptations that occur with chronic training (29). Subjects exercised under the supervision of trained personnel for 1h/d at 60% of heart rate reserve (monitored by telemetry) over 7 consecutive days between tests. Exercise involved combining brisk treadmill walking and stationary cycling (29). The last exercise session was completed 14–16 hours prior to the day of the MMTT.
After an overnight fast, blood samples were taken at time zero and subjects then consumed a ~400 kcal mixed meal containing 60% energy as carbohydrates (2/3 as simple sugars) (Table 1). Blood was sampled from the arterialized venous site, collected in EDTA tubes containing aprotinin and dipeptidyl peptidase IV inhibitors; aliquots of plasma and serum were stored at −80°C. Plasma adropin concentrations were measured at baseline (T=0) and then at T=30, 60 and 90 minutes post-meal.
Table 1.
Composition of the breakfast meal (breakfast wrap and orange juice; 60% energy as carbohydrates, 30% as fats, and 10% as protein) used for the MMTT.
| Food | Carbohydrates | Fats | Protein | Energy (kcal) |
|---|---|---|---|---|
|
| ||||
| Flour tortilla | 23.4 g | 3.3 g | 3.7 g | 140.5 |
| Egg beaters | 0.0 g | 0.0 g | 0.2 g | 1.1 |
| Egg yolk | 0.2 g | 1.2 g | 0.7 g | 14.5 |
| Cheese | 1.7 g | 4.3 g | 2.6 g | 60.0 |
| Margarine | 0.0 g | 4.5 g | 0.0 g | 38.6 |
| Orange juice | 34.8 g | 0.0 g | 2.7 g | 147.1 |
|
| ||||
| Total | 60.1 g | 13.3 g | 9.9 g | 401.8 |
Meta-analysis of plasma adropin concentrations
Distribution of plasma adropin concentrations in a mixed population was estimated by pooling data from the current study with published data (6, 16, 20) (ClinicalTrials.gov #NCT01165853; NCT01103921; NCT00935402, and NCT00936130), and data obtained from measurement of plasma adropin concentrations in samples obtained from studies performed at the University of Missouri-Columbia (30–33). The samples used for these measurements were collected at baseline. The original studies were reviewed by the IRB at UC Davis, Syracuse University, St. Luke’s-Roosevelt Hospital Center, Pennington Biomedical Research Center, and the University of Missouri in Columbia.
Measurement of plasma adropin concentrations
Adropin concentrations were determined in the plasma fraction of blood as previously described (6, 16, 20) using a commercially available enzyme immunoassay (s-1385, Peninsula Laboratories, San Carlos, CA) validated previously using plasma from adropin knockout mice, and tested using a spike and recovery of synthetic adropin34–76 (1, 6). The intra- and inter-assay coefficient of variation are <5% and 25–30%, respectively.
Statistical analysis
Data were analyzed using SPSS Statistics Version 23 (IBM). Effects of weight loss on plasma adropin concentrations were assessed using repeated measures ANOVA, including treatment (CR, EX, CREX) as an independent variable and sex and glucose tolerance state as covariates, and using linear regression modeling including plasma adropin data and other metrics. When grouped into quartiles based on ranking plasma adropin concentrations treatment, sex and glucose tolerance status were used as covariates. Associations between changes in plasma adropin concentrations (Δadropin calculated by subtracting the baseline value from final value) and fasting measurements indicating glucose control and lipid metabolism were evaluated using Spearman correlations. Associations between baseline and Δadropin and food intake data were first evaluated by converting all data into z-scores (standard deviations from the mean), and further evaluated by separation into quartiles or tertiles, and ranked by baseline plasma adropin values from lowest to highest.
For the meta-analysis, distribution, skew and kurtosis were determined using SPSS. Effects of sex were assessed using univariate analysis with age, BMI, and glucose tolerance status as covariates.
Associations between plasma adropin concentrations with macronutrient intake were initially analyzed using linear and nonlinear associations using Microsoft Excel. We initially converted macronutrient intake data into z-scores, allowing for comparisons of protein, carbohydrate and fat intake as g/d or relative to total calorie intake as a function of plasma adropin values. Non-linearity appeared to be driven by participants with very high plasma adropin concentrations ;these individuals were treated as outliers (values > 2SD from the mean). Associations between plasma adropin concentrations and nutrient intake were further investigated by separating the tertiles ranked by plasma adropin concentrations; the outliers were not included in the tertiled data, but were treated as a separate group. Comparisons of macronutrient intake between groups were then assessed by ANCOVA with total caloric intake, sex, and glucose tolerance status used as covariates.
Between groups differences were tested using post hoc comparisons (Bonferroni). All of the statistical tests reported were two tailed, with significance accepted at P ≤ 0.05.
RESULTS
Circulating adropin concentrations before and after weight loss (CREG Study)
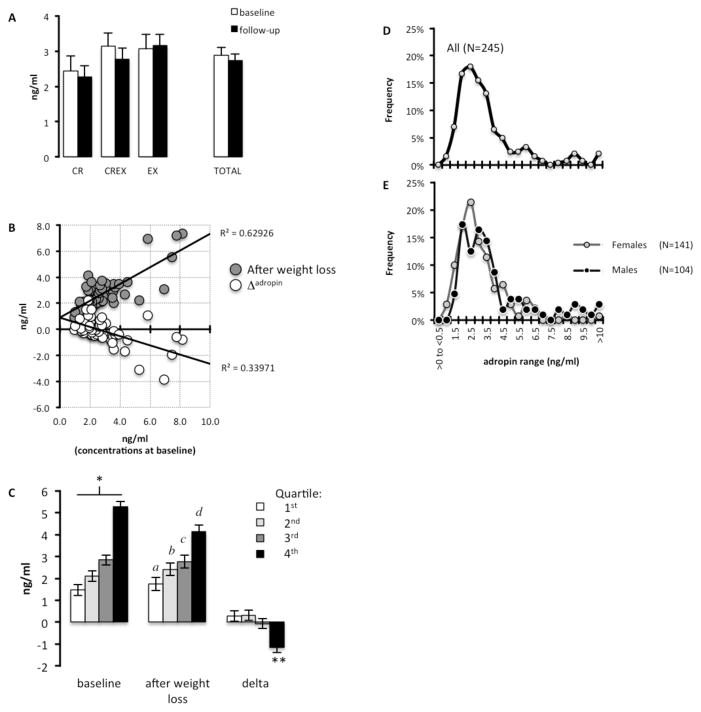
Demographics and metrics of the 54 CREG study participants who completed the weight loss program and for whom plasma adropin concentrations were measured are shown in Table 2; groups were matched for body weight, body composition and weight loss (27). Weight loss and treatment method (CR, EX, CREX) had no significant effect on plasma adropin concentrations (Fig. 1A). However, an analysis of baseline, post-intervention and Δadropin (change in plasma adropin concentrations after weight loss) suggested an effect of weight loss dependent on baseline values (Fig. 1B). There was a strong negative correlation between Δadropin and baseline concentrations (ρ=0.575, P<0.001). The anticipated strong linear correlation between baseline plasma adropin concentrations with values after weight loss was also observed (ρ=0.680, P<0.001). Further analysis using linear regression modeling with Δadropin as the dependent variable and metrics recorded during the study (baseline adropin values, body weight, fat and fat free mass, BMI), demographics and treatment indicated that baseline adropin was the only significant coefficient (R=0.722; B=−0.391; standard error, 0.073; P=0.001).
Table 2. Subject demographics of the CREG participants for whom plasma adropin values were measured for the weight loss study.
Data are mean ± SE.
| Demographic; laboratory measurement | All participants | CR | CREX | EX |
|---|---|---|---|---|
|
| ||||
| Gender (F/M, n) | 42/12 | 13/3 | 16/4 | 13/5 |
| Age (years) | 57.0 ± 0.7 | 57.4 ± 1.4 | 57.2 ± 1.2 | 56.9 ± 1.3 |
| Weeks taken to attain weight loss goals* | 17.0 ± 1.1 | 18.6 ± 1.8 | 13.5 ± 1.7* | 19.4 ± 1.8 |
| BMI (kg/m2)1 | ||||
| Pre treatment | 27.7 ± 0.2 | 27.7 ± 0.4 | 28.3 ± 0.4 | 27.0 ± 0.4 |
| Post treatment | 25.9 ± 0.2 | 25.9 ± 0.4 | 26.3 ± 0.4 | 25.4 ± 0.4 |
| Change | −1.8 ± 0.1 | −1.7 ± 0.2 | −2.0 ± 0.2 | −1.6 ± 0.2 |
| Body weight (kg)1 | ||||
| Pre treatment | 78.7 ± 1.4 | 77.2 ± 2.0 | 81.8 ± 1.8 | 76.5 ± 1.9 |
| Post treatment | 73.5 ± 1.4 | 72.2 ± 2.0 | 75.9 ± 1.8 | 72.0 ± 1.9 |
| Change | −5.2 ± 0.3 | −5.0 ± 0.5 | −5.9 ± 0.5 | −4.5 ± 0.5 |
| Fat mass (kg)1 | ||||
| Pre treatment | 31.8 ± 0.7 | 32.0 ± 1.3 | 33.1 ± 1.1 | 30.3 ± 1.2 |
| Post treatment | 27.6 ± 0.7 | 28.1 ± 1.3 | 28.3 ± 1.1 | 26.4 ± 1.2 |
| Change | −4.3 ± 0.3 | −3.9 ± 0.5 | −4.8 ± 0.4 | −3.9 ± 0.4 |
| Fat-free mass (kg)1 | ||||
| Pre treatment | 46.5 ± 1.2 | 44.9 ± 1.1 | 48.3 ± 1.0 | 45.9 ± 1.1 |
| Post treatment | 45.8 ± 1.2 | 44.0 ± 1.1 | 47.5 ± 1.0 | 45.7 ± 1.0 |
| Change | −0.6 ± 1.2 | −0.8 ± 0.3 | −0.8 ± 0.2 | −0.2 ± 0.3 |
| Fat%1 | ||||
| Pre treatment | 42.2 ± 0.8 | 43.4 ± 1.0 | 42.2 ± 0.9 | 41.2 ± 0.9 |
| Post treatment | 39.1 ± 0.9 | 40.7 ± 1.1 | 38.8 ± 1.0 | 37.9 ± 1.0 |
| Change | −3.2 ± 0.2 | −2.7 ± 0.4 | −3.4 ± 0.4 | −3.3 ± 0.4 |
Sex was used as a covariate in the analysis to adjust for differences in proportion of males and females in each group.
Among group comparison by ANOVA, P<0.05; CREX vs. CR, EX P<0.05.
Figure 1. An inverse association between baseline plasma adropin concentrations and the effect of weight loss.
(A) Plasma adropin concentrations at baseline and after achieving 6–8% weight loss (“follow-up”) by calorie restriction (CR), calorie-restriction plus exercise (CREX) and exercise only (EX). White bars = adropin values at baseline; black bars = adropin values after weight loss.
(B) Scatterplot showing significant associations between baseline plasma adropin concentrations and concentrations after weight loss (follow-up) or the difference in concentration at follow-up from baseline (Δadropin). The data shown have been ln transformed. Grey circles = adropin values after weight loss; white circles = Δadropin
(C) Plasma adropin concentrations quartiled by ranking levels at baseline from low (1st quartile) to high (4th quartile). There was a significant interaction between weight loss and quartile, with the 4th quartile exhibiting a decline in plasma adropin concentrations. * P<0.005 between all quartiles; a P<0.005 vs. 3rd, 4th quartile; b P<0.005 vs. 4th quartile; c P<0.005 vs. 1st quartile; d P<0.005 vs. 1st, 2nd quartile; ** P<0.001 compared to the 1st and 2nd quartile, P<0.05 to 3rd quartile. The data show are ln transformed White bar = 1st quartile; light grey bar = 2nd quartile; dark grey bar = 3rd quartile, black bar = 4th quartile.
(D) Frequency distribution of plasma adropin data pooled from the current and previously published experiments (n=245).
(E) Frequency distribution of plasma adropin data by sex. The values for the x-axis are the same in panels C and D. Grey line with grey circles = females; black line with black circles = males.
We next separated participants into quartiles ranked by baseline plasma adropin concentrations from low (1st quartile) to high (4th quartile) (Fig. 1C). While the effect of weight loss was still not significant, there was a significant interaction between quartile and weight loss (P<0.001). Plasma adropin concentrations in the 4th quartile declined with weight loss, with no change in the 1st to 3rd quartile (Fig. 1C). Demographics in the quartiles were similar (males/females and n for quartile 1, 9/4; 2, 11/3; 3, 11/3; 4, 11/2; mean age for quartile 1, 55.9y; 2, 56.2y; 3, 59.0y; 4, 57.0y; mean BMI adjusted for sex for quartile 1, 27.7 kg/m2; 2, 27.8 kg/m2; 3, 27.5 kg/m2; 4, 27.8 kg/m2). There was also no correlation between Δadropin and measures of glucose control or blood lipids, either at baseline or in response to weight loss (data not shown).
Normal values for fasting plasma adropin concentrations (Meta-analysis)
Inspection of plasma adropin concentrations suggested that plasma adropin concentrations in most individuals are <5 ng/ml (Fig. 1B). This observation was confirmed in a meta-analysis using data from 245 individuals pooled from current and previously published studies (6, 16, 20) (Fig. 1D, Table 3). Plasma adropin concentrations exhibit a unimodal Gaussian distribution profile with positive skew and high kurtosis (concentration in ng/ml; mean, 3.30; median, 2.73; SD, 2.33; skewness, 3.262; kurtosis, 16.23, range 0.57 to 19.95 ng/ml, n=245) (Fig. 1D). As observed previously (6), plasma adropin concentrations are higher in males compared to females (concentrations in ng/ml adjusted for age, BMI and glucose tolerance status for males, 3.8±0.2; females, 2.9±0.2; P<0.01). A more pronounced positive skew and kurtosis may contribute to sex differences, as both are more pronounced in males (mean, 3.86; median, 2.93; SD, 2.93; skewness, 2.91; kurtosis, 11.18, n=104) compared to females (mean, 2.88; median, 2.50; SD, 1.58; skewness, 1.97; kurtosis, 5.866; n=141) (Fig. 1E). While 46 of the 245 participants had been diagnosed with type 2 diabetes (Table 3), there was no significant difference in plasma adropin concentration in participants with or without diabetes (concentrations in ng/ml for diabetic versus non-diabetic, 2.9±0.3 vs. 3.4±0.2).
Table 3.
Demographics of the subjects used for the meta-analysis of plasma adropin concentrations.
| Demographic; laboratory measurement | All subjects (n=245) | Males (n=104) | Females (n=141) | P-value |
|---|---|---|---|---|
|
| ||||
| D2M (n) | 46 | 8 | 38 | |
| Age (years) | ||||
| Mean | 34.9 | 33.3 | 36.1 | n.s |
| SD | 12.8 | 13.6 | 12.2 | |
| Range | 18–67 | 18–70 | 20–67 | |
| BMI (kg/m2) | ||||
| Mean | 30.7 | 28.4 | 32.4 | =0.001 |
| SD | 9.1 | 7.4 | 9.9 | |
| Range | 17.6–71.5 | 17.6–62.6 | 19.4–71.5 | |
| Adropin (ng/ml) | ||||
| Mean | 3.3 | 3.9 | 2.9 | =0.001 |
| SD | 2.3 | 3.0 | 1.6 | |
| Range | 0.6–20.0 | 1.1–20.0 | 0.6–10.9 | |
Negative association between habitual carbohydrate intake and plasma adropin levels (CREG Study)
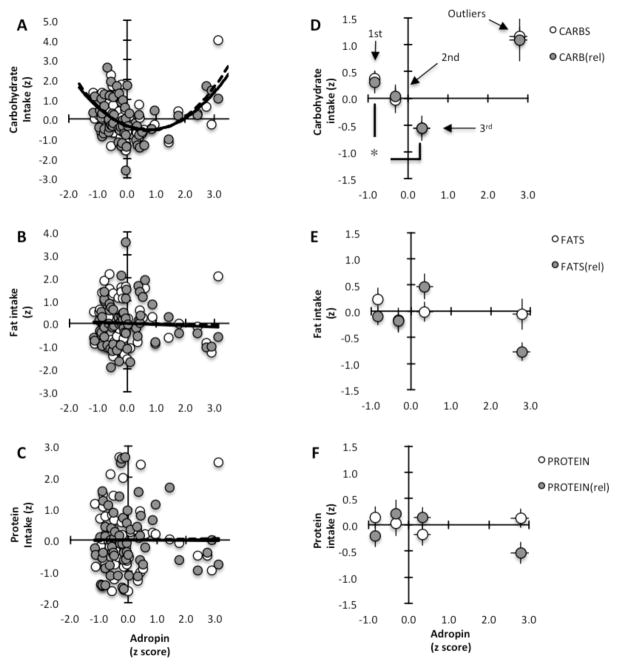
We observed a non-linear association between plasma adropin concentrations and self-reported habitual carbohydrate intake in participants of the CREG study (correlation coefficient between plasma adropin concentrations and carbohydrate intake, 0.5155; for relative carbohydrate intake, 0.4514) (Fig. 2A). In contrast, no associations were evident between plasma adropin concentrations and either fat or protein intake (Fig. 2B, C). When examined as tertiles ranked by plasma adropin concentration, there were significant differences in absolute and relative carbohydrate intake between the 1st and 3rd tertiles (Fig. 2D), with no difference in fat (Fig. 2E) or protein intake (Fig. 2F).
Figure 2. Association between plasma adropin concentration and carbohydrate intake.
The macronutrient intake data (in kJ per person, or expressed relative to other macronutrients) and plasma adropin concentration data were converted into z-scores (±SD from the mean) to allow plotting total and relative intake data on the same graph. White circles = total intake; grey circles = intake relative to other macronutrients; n=62
(A–C) Scatterplots showing a nonlinear association between plasma adropin concentration (x-axis) and carbohydrate intake (y-axis) (A); no associations were evident for fats (B) or protein (C).
(D) Carbohydrate intake of the 58 participants whose plasma adropin concentrations were within 2 SD of the mean separated into tertiles with low-normal (1st tertile, n=19), normal (2nd tertile, n=20) or highnormal (3rd tertile, n=19) plasma adropin concentrations. Carbohydrate intake (in total or relative to other macronutrients) in the 1st and 3rd tertiles was significantly different (* P<0.01). The means of the 4 participants whose plasma adropin concentrations were >2SD from the mean (“outliers”) are also shown.
(E–F) Intake of fats (E) and protein (F) by tertile.
The difference in carbohydrate intake between the 1st and 3rd tertile was due to simple sugars (mono- and disaccharides; 34% difference) and complex carbohydrates (oligo- and polysaccharides; 30% difference); fiber intake was similar between tertiles (Table 4). Analysis of the different fatty acid species for which reliable data were available from the study (saturated, unsaturated) also indicated no significant differences between tertiles. As diet may affect glucose control and lipid profile, we also compared blood chemistries between tertiles (Table 4). There was no evidence for differences in insulin sensitivity between tertiles, however blood lipids (triglycerides, cholesterol, LDL) were significantly lower in the 3rd tertile compared to 1st tertile. In general the tertiles had similar demographics; while people in the 3rd tertile weighed significantly less, their BMI and body composition were normal compared to the other groups (Table 4).
Table 4.
Demographics, blood chemistries and self-reported nutrient intake of CREG study participants separated into tertiles ranked by plasma adropin concentrations (values in bold text).
| Demographic; laboratory measurement | 1st tertile | 2nd tertile | 3rd tertile | Outliers (>2 SD) | P-value |
|---|---|---|---|---|---|
|
| |||||
| Gender (F/M, n) | 14/5 | 13/7 | 18/1 | 3/1 | |
| D2M | 1 | 0 | 1 | 0 | |
| Prediabetes | 10 | 9 | 13 | 3 | |
| Age (years) | 55.9 ± 1.2 | 57.2 ± 1.3 | 57.6 ± 1.1 | 54.7 ± 0.8 | n.s. |
| Weight (kg) 1 | 81.3 ± 1.8 | 79.9 ± 1.8 | 73.8 ± 1.9* | 87.4 ± 4.0 | <0.01 |
| BMI (kg/m2) 1 | 27.7 ± 0.4 | 27.8 ± 0.4 | 27.4 ± 0.4 | 28.8 ± 0.8 | n.s. |
| Fat% | 42.5 ± 1.0 | 42.2 ± 0.9 | 41.5 ± 1.0 | 43.6 ± 2.1 | n.s. |
| SBP | 119.2 ± 2.4 | 115.7 ± 2.4 | 114.2 ± 2.5 | 119.4 ± 5.3 | n.s. |
| DBP | 75.0 ± 1.9 | 74.9 ± 1.9 | 75.8 ± 2.0 | 75.5 ± 4.1 | n.s. |
| Blood chemistries 2 | |||||
| Adropin (ng/ml) 3 | 1.5 ± 0.1 | 2.4 ± 0.1 | 3.7 ± 0.1 | 7.6 ± 0.3 | <0.001 |
| Insulin (μU/ml) | 9.1 ± 1.4 | 8.4 ± 1.4 | 6.5 ± 1.5 | 15.2 ± 2.8 | (0.063) |
| Glucose (mg/d) | 96.9 ± 1.5 | 95.0 ± 1.5 | 93.8 ± 1.6 | 101.6 ± 3.3 | n.s. |
| HOMA-IR | 2.25 ± 0.35 | 1.98 ± 0.36 | 1.51 ± 0.37 | 4.12 ± 0.7** | <0.05 |
| HbA1c | 5.64 ± 0.06 | 5.65 ± 0.06 | 5.68 ± 0.06 | 5.63 ± 0.12 | n.s. |
| Triglycerides (mg/dL) | 135.2 ± 12.9 | 116.2 ± 13.1 | 83.3 ± 13.5*** | 92.5 ± 28.2 | (0.063) |
| Total cholesterol (mg/dL) | 217.3 ± 9.7 | 195.7 ± 9.7 | 174.4 ± 10.1*** | 205.8 ± 21.0 | P<0.05 |
| LDL-C (mg/dL) | 136.4 ± 6.6 | 118.4 ± 6.7 | 104.7 ± 6.9*** | 135.1 ± 14.4 | P<0.05 |
| HDL-C (mg/dL) | 53.9 ± 3.3 | 54.7 ± 3.3 | 61.1 ± 3.4 | 51.9 ± 7.1 | n.s. |
| Nutrient intake 4 | |||||
| Total kcal | 2250 ± 118 | 1915 ± 117 | 2164 ± 128 | 2298 ± 263 | n.s. |
| Carbohydrates (g) | 269 ± 10 | 254 ± 10 | 218 ± 10* | 309 ± 22 | <0.001 |
| Fats (g) | 80 ± 4 | 81 ± 4 | 92 ± 4 | 67 ± 9 | =0.050 |
| Protein (g) | 81 ± 4 | 84 ± 4 | 85 ± 4 | 75 ± 8 | n.s. |
| Carbohydrates by class: | |||||
| Sugars (g) | 96 ± 7 | 87 ± 7 | 76 ± 7 | 103 ± 15 | n.s. |
| Other carbs. (g) | 114 ± 8 | 116 ± 9 | 98 ± 9 | 144 ± 18 | n.s. |
| Fiber (g) | 18 ± 1 | 19 ± 2 | 18 ± 2 | 31 ± 3# | <0.01 |
| Fats by class: | |||||
| Saturated (g) | 26 ± 2 | 27 ± 2 | 30 ± 2 | 18 ± 4## | (0.057) |
| Unsaturated (g) | 52 ± 3 | 52 ± 3 | 60 ± 3 | 47± 6 | n.s. |
Sex included as a covariate in the analysis to adjust for differences in proportion of males and females in each group;
Sex and glucose tolerance status included as covariates in the analysis;
The differences in plasma adropin concentrations between all groups were highly significant (P<0.001);
For total caloric intake, body weight, sex, and glucose tolerance status were used as covariates; For macronutrients total caloric intake, sex and glucose tolerance status were included as covariates;
P<0.05 vs. 1st tertile and outliers;
P<0.05 vs. 3rd tertile;
P<0.05 vs. 1st tertile;
P<0.05 vs. all other tertiles;
P<0.05 vs. 3rd tertile
Reduced plasma adropin concentrations during the MMTT Study
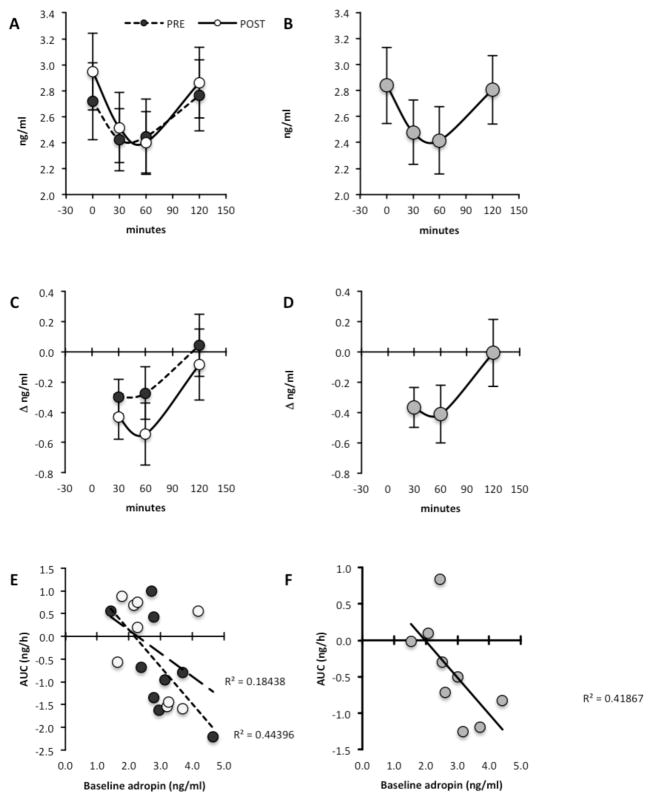
Analysis using repeated measures indicated a significant effect of meal (P<0.01) but not of exercise (mean±SE plasma adropin concentrations at T0, pre-exercise, 2.73±0.30 ng/ml; post-exercise, 2.94±0.30 ng/ml) (Fig 3A, B). Plasma adropin levels at T=30 and T=60 were 13% and 14% lower compared to baseline (mean±SE of the delta in plasma adropin concentrations in ng/ml at T=30, −0.37±0.13 ng/ml; at T=60, −0.41±0.19 ng/ml), returning to normal levels at T=120 (−0.01±0.22 ng/ml) (Fig. 3C, D). The meal effect was predominantly observed in individuals with baseline adropin values >2.5 ng/ml (Fig. 3E, F).
Figure 3. Reduced plasma adropin concentrations following a high carbohydrate (60% energy), low fat (30%) and low protein (10%) meal.
Actual values for the two arms of the study (A, C, E) and least square means of the overall meal effect (B, D, F) are shown. A and B show actual plasma adropin values, while C and D show the change in plasma adropin concentrations relative to baseline (Δadropin). A positive association between the area under the curve (AUC) for plasma adropin concentrations following the meal and baseline values is shown in E and F. White circles = post-exercise; Black circles = preexercise; grey circles = averaged meal effect.
DISCUSSION
Our initial objective was to determine whether weight loss reduces plasma adropin concentrations in humans. A simple association between weight loss and plasma adropin concentrations was not observed. However, further analysis suggested asymmetry in effects of interventions known to alter systemic metabolism on plasma adropin concentration. In individuals with adropin values at the high end of the range, weight loss reduced values (Fig. 1B, C).
A secondary objective of this study was to further investigate the association between diet and plasma adropin concentrations. In lean women (BMI 22–26 kg/m2) aged between 30–45y participating in a sleep-restriction study, we reported a strong positive association between plasma adropin concentrations and fat intake (16). When expressed relative to total calories, there was a positive association between plasma adropin values and fat intake, and a negative association with carbohydrate intake. Here we screened for associations between diet and plasma adropin concentrations using a larger sample size (n=62). Results from this study suggest habitual dietary preferences are a factor determining plasma adropin concentrations. In participants with adropin values under the main peak of the distribution, there was a negative association with carbohydrate consumption. Individuals with low plasma adropin concentrations exhibited higher intake of carbohydrates as simple sugars and complex carbohydrates, with no difference in fiber intake.
The lipid profile between tertiles was also significantly different, with the 3rd tertile having lower blood lipids compared to the 1st tertile (Table 4). An inverse association between fasting triglycerides and plasma adropin concentrations has been reported (6). Whether differences in dietary preferences explain the association between plasma adropin concentrations and lipid profiles is unclear, with further studies needed.
The impact of the high carbohydrate breakfast on plasma adropin values was more pronounced in participants with values >2.5 ng/ml (Fig. 3E, F). While speculative, if plasma adropin values are an indicator of long-term food selection preferences, then the impact of a supervised change in feeding behavior (e.g., a participant who normally consumes diets high in saturated fat consumes a high carbohydrate breakfast) could have proportionately greater impact on plasma adropin concentrations compared to someone who regularly consumes carbohydrate-rich diets. Thus, study participants with adropin values between 3–4 ng/ml and lower self-reported carbohydrate consumption ) would exhibit a decline in plasma adropin values following high carbohydrate meal. On the other hand, people with low plasma adropin concentrations who self-report habitual consumption of high carbohydrate diets will be less responsive when consuming a supervised breakfast that matches their regular diet.
Data from studies using mice suggest inhibitory effects of simple carbohydrates on adropin expression. In silico analysis using the GEO database (34) suggests an 80% reduction of Enho expression (P<0.001) in livers of C57BL/6J mice maintained maintained on a very low fat (1% w/w) high carbohydrate diet (50% sucrose) for 10d relative to chow-fed controls (35) (GEO accession GDS1517). Dietary sugars may therefore suppress adropin synthesis in mice. However, the regulation of plasma adropin concentrations by sugars in humans is not clear. Consumption of glucose as 25% of daily energy requirements for 2–10wk reduces plasma adropin concentration, while consumption of fructose as 25% of daily energy requirements has the opposite effect (20). Plasma adropin concentrations in humans may be affected by multiple factors, including diet composition and secondary effects of diet on metabolic condition.
It is important to note some of the weaknesses of these studies. Most of the participants of the CREG study were women (48 out of 62); whether associations between carbohydrate intake and circulating adropin occur in men is not clear. Relying on self-reported feeding data is also a weakness, as underreporting of total energy intake and macronutrient intakes is common (36). Carbohydrate intake when expressed as a percentage of total energy intake does not appear to be susceptible to bias from underreporting (37). Underreporting is thus unlikely to be responsible for the association between carbohydrate intake and plasma adropin concentrations in the present study. Furthermore, this weakness is offset by replication of the finding in the participants recruited in different clinical settings (St Louis and New York City) in which food intake was objectively measured. Further studies of macronutrient-specific and meal-related effects using healthy non-diabetic individuals are needed, as the impact of altered carbohydrate metabolism on the parameters being investigated is not clear. Finally, the current study did not determine whether plasma adropin concentrations alter eating behavior. Studies using visual analog scales could be useful in determining whether plasma adropin concentrations correlate with altered appetite and/or food preferences.
That plasma adropin concentrations exhibit a normal distribution with positive skew in humans may suggest a ‘normal range’ of plasma adropin concentrations. Important questions raised by this result concern the metabolic conditions of people with values in the extreme low or high ends of the distribution profile. Determining whether plasma adropin values at the low or high end (“hypo-/hyper-adropinemia”) are associated with increased metabolic risk and clearly defined metabolic phenotypes could establish whether adropin has significant physiological roles in humans. The current study suggests that low plasma adropin concentrations may indicate a situation of excess carbohydrate consumption and dyslipidemia. The inverse association between plasma adropin and serum TG was observed previously (6). The current results suggest that the association may not be causative, as carbohydrates appear to have an inhibitory on plasma adropin levels. Further studies examining whether extremely high or low plasma adropin concentrations are associated with dyslipidemias are needed.
We reported an asymmetric impact of sugar consumption and high fat meals on plasma adropin concentrations (20). Combining the results of these studies leads us to propose that obesogenic or leptogenic interventions either increase or reduce the distribution in plasma adropin concentrations at the high end of the range (Fig. 2A). Interventions causing weight loss are thus predicted to reverse the positive skew observed in the distribution of plasma adropin values (Fig. 2B). These results are consistent with hyperadropinemia resulting from a metabolic condition that may be related to changes in systemic lipid metabolism. The exact nature of this metabolic condition requires further analysis. However, we can use published data from animal studies to suggest two hypotheses. First, if adropin regulates fuel selection in skeletal muscle in humans as observed in mice (2, 3), then abnormalities in carbohydrate and/or lipid metabolism are possible in situations of hyperadropinemia. Consistent with this theory, the changes in plasma adropin concentrations in response to sugars were linked to systemic lipid metabolism (20). A second possibility is vascular; adropin enhances vascular function in mouse models (4). Elevated plasma adropin concentrations have been observed with heart failure (5), while myocardial infarction in a rat model increases adropin expession (12).
CONCLUSIONS
These results provide further evidence supporting a link between circulating adropin concentrations, dietary macronutrient intake and systemic lipid metabolism in humans. Further investigation will be required to determine how carbohydrate intake affects plasma adropin concentrations, as the response may be specific for different sugar species (20). Further studies focusing on individuals with plasma adropin concentrations at either end of the spectrum may also provide important information on the role of this peptide in human physiology.
What is already known about this subject?
Studies in mice suggest adropin is a nutritionally regulated peptide hormone involved in cardiometabolic control.
Human participants of a sleep-restriction study exhibit associations between plasma adropin concentrations and dietary macronutrient intake.
What does this study add?
Plasma adropin concentrations exhibit a distribution with kurtosis and positive skew, with most people having values <5ng/ml.
Weight loss partially reverses skewness, lowering plasma concentrations in the upper quartile while not significantly affecting values under the bell-curve.
We confirm our previous results in a larger cohort, reporting an inverse association between habitual carbohydrate intake and plasma adropin concentrations.
Acknowledgments
Funding: AAB acknowledges the support of a Proof of Principle Award from Novo Nordisk’s Diabetes Innovation Award Program and the Lottie Caroline Hardy Charitable Trust. Support for the CREG study was provided by National Institutes of Health grants K01-DK-080886 and DK-56341 (Nutrition and Obesity Research Center) and UL1-RR-024992 (Clinical Translational Science Award). Dr. Havel’s and Dr. Stanhope’s laboratory received funding from NIH grants: R01-HL-075675, R01-HL-091333. R01-HL-107256, R01-HL-121324, 1R01- HL-121324-02S1 and a Multi-campus Award from the University of California, Office of the President. Dr Thyfault is supported by NIH grant R01DK088940. Dr Kanaley was supported by NIH-R21-DK084467 and grants provided by the University of Missouri Research Council.
We thank Mr James Graham for performing the adropin assays.
Footnotes
Disclosure: The authors have nothing to disclose.
Author contributions – P.J.H., K.L.S., M-P S-O, JPT and EPW contributed materials for the collection of data, assisted with data interpretation, and reviewed the manuscript. JRS and MLK collected data, assisted with data analysis, and reviewed the manuscript. AAB contributed materials for collection of data, analyzed and interpreted data, and prepared the first draft of the manuscript. AAB also had full access to the data and takes responsibility for the integrity and accuracy of the analysis.
References
- 1.Ganesh Kumar K, Zhang J, Gao S, Rossi J, McGuinness OP, Halem HH, et al. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity. 2012;20(7):1394–402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao S, McMillan RP, Jacas J, Zhu Q, Li X, Kumar GK, et al. Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes. 2014;63(10):3242–52. doi: 10.2337/db14-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao S, McMillan RP, Zhu Q, Lopaschuk GD, Hulver MW, Butler AA. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Molecular metabolism. 2015;4(4):310–24. doi: 10.1016/j.molmet.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta M, et al. Adropin is a novel regulator of endothelial function. Circulation. 2010;122(11 Suppl):S185–92. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 5.Lian W, Gu X, Qin Y, Zheng X. Elevated plasma levels of adropin in heart failure patients. Internal medicine. 2011;50(15):1523–7. doi: 10.2169/internalmedicine.50.5163. [DOI] [PubMed] [Google Scholar]
- 6.Butler AA, Tam CS, Stanhope KL, Wolfe BM, Ali MR, O’Keeffe M, et al. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. The Journal of clinical endocrinology and metabolism. 2012;97(10):3783–91. doi: 10.1210/jc.2012-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aydin S, Kuloglu T, Aydin S. Copeptin, adropin and irisin concentrations in breast milk and plasma of healthy women and those with gestational diabetes mellitus. Peptides. 2013;47:66–70. doi: 10.1016/j.peptides.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Celik A, Balin M, Kobat MA, Erdem K, Baydas A, Bulut M, et al. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovascular therapeutics. 2013;31(3):174–8. doi: 10.1111/1755-5922.12025. [DOI] [PubMed] [Google Scholar]
- 9.Celik E, Yilmaz E, Celik O, Ulas M, Turkcuoglu I, Karaer A, et al. Maternal and fetal adropin levels in gestational diabetes mellitus. Journal of perinatal medicine. 2013;41(4):375–80. doi: 10.1515/jpm-2012-0227. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Molero-Ramirez H, Tan HL, Bandla HP. Circulating adropin concentrations in pediatric obstructive sleep apnea: potential relevance to endothelial function. The Journal of pediatrics. 2013;163(4):1122–6. doi: 10.1016/j.jpeds.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topuz M, Celik A, Aslantas T, Demir AK, Aydin S, Aydin S. Plasma adropin levels predict endothelial dysfunction like flow-mediated dilatation in patients with type 2 diabetes mellitus. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2013;61(8):1161–4. doi: 10.231/JIM.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 12.Aydin S, Kuloglu T, Aydin S, Kalayci M, Yilmaz M, Cakmak T, et al. Elevated adropin: a candidate diagnostic marker for myocardial infarction in conjunction with troponin-I. Peptides. 2014;58:91–7. doi: 10.1016/j.peptides.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Demircelik B, Cakmak M, Nazli Y, Gurel OM, Akkaya N, Cetin M, et al. Adropin: a new marker for predicting late saphenous vein graft disease after coronary artery bypass grafting. Clinical and investigative medicine Medecine clinique et experimentale. 2014;37(5):E338–44. doi: 10.25011/cim.v37i5.22014. [DOI] [PubMed] [Google Scholar]
- 14.Qiu X, He JR, Zhao MG, Kuang YS, Xu SQ, Zhang HZ, et al. Relationship between human cord blood adropin levels and fetal growth. Peptides. 2014;52:19–22. doi: 10.1016/j.peptides.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Sayin O, Tokgoz Y, Arslan N. Investigation of adropin and leptin levels in pediatric obesity- related nonalcoholic fatty liver disease. Journal of pediatric endocrinology & metabolism : JPEM. 2014;27(5–6):479–84. doi: 10.1515/jpem-2013-0296. [DOI] [PubMed] [Google Scholar]
- 16.St-Onge MP, Shechter A, Shlisky J, Tam CS, Gao S, Ravussin E, et al. Fasting plasma adropin concentrations correlate with fat consumption in human females. Obesity. 2014;22(4):1056–63. doi: 10.1002/oby.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Fang J, Chen L, Zhao Z, Luo Y, Lin C, et al. Low serum adropin is associated with coronary atherosclerosis in type 2 diabetic and non-diabetic patients. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2014;52(5):751–8. doi: 10.1515/cclm-2013-0844. [DOI] [PubMed] [Google Scholar]
- 18.Yildirim B, Celik O, Aydin S. Adropin: a key component and potential gatekeeper of metabolic disturbances in policystic ovarian syndrome. Clinical and experimental obstetrics & gynecology. 2014;41(3):310–2. [PubMed] [Google Scholar]
- 19.Yu HY, Zhao P, Wu MC, Liu J, Yin W. Serum adropin levels are decreased in patients with acute myocardial infarction. Regulatory peptides. 2014;190–191:46–9. doi: 10.1016/j.regpep.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Butler AA, St-Onge MP, Siebert EA, Medici V, Stanhope KL, Havel PJ. Differential Responses of Plasma Adropin Concentrations To Dietary Glucose or Fructose Consumption In Humans. Sci Rep. 2015;5:14691. doi: 10.1038/srep14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zapata RC, Salehi R, Ambrose DJ, Chelikani PK. Effects of prepartum fat supplementation on plasma concentrations of glucagon-like peptide-1, peptide YY, adropin, insulin, and leptin in periparturient dairy cows. Journal of dairy science. 2015;98(10):6876–85. doi: 10.3168/jds.2014-9283. [DOI] [PubMed] [Google Scholar]
- 22.Aydin S. Presence of adropin, nesfatin-1, apelin-12, ghrelins and salusins peptides in the milk, cheese whey and plasma of dairy cows. Peptides. 2013;43:83–7. doi: 10.1016/j.peptides.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Fujie S, Hasegawa N, Sato K, Fujita S, Sanada K, Hamaoka T, et al. Aerobic exercise training- induced changes in serum adropin level are associated with reduced arterial stiffness in middle- aged and older adults. American journal of physiology Heart and circulatory physiology. 2015;309(10):H1642–7. doi: 10.1152/ajpheart.00338.2015. [DOI] [PubMed] [Google Scholar]
- 24.Aydin S. Three new players in energy regulation: preptin, adropin and irisin. Peptides. 2014;56:94–110. doi: 10.1016/j.peptides.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Xie W, Zheng XL, Yin WD, Tang CK. A novel peptide adropin in cardiovascular diseases. Clin Chim Acta. 2016;453:107–13. doi: 10.1016/j.cca.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell metabolism. 2008;8(6):468–81. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss EP, Albert SG, Reeds DN, Kress KS, Ezekiel UR, McDaniel JL, et al. Calorie Restriction and Matched Weight Loss From Exercise: Independent and Additive Effects on Glucoregulation and the Incretin System in Overweight Women and Men. Diabetes care. 2015;38(7):1253–62. doi: 10.2337/dc14-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhla A, Hahn S, Butschkau A, Lange S, Wree A, Vollmar B. Lifelong caloric restriction reprograms hepatic fat metabolism in mice. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(8):915–22. doi: 10.1093/gerona/glt160. [DOI] [PubMed] [Google Scholar]
- 29.Mikus CR, Fairfax ST, Libla JL, Boyle LJ, Vianna LC, Oberlin DJ, et al. Seven days of aerobic exercise training improves conduit artery blood flow following glucose ingestion in patients with type 2 diabetes. Journal of applied physiology. 2011;111(3):657–64. doi: 10.1152/japplphysiol.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heden TD, Liu Y, Kearney ML, Kanaley JA. Weight classification does not influence the short- term endocrine or metabolic effects of high-fructose corn syrup-sweetened beverages. Appl Physiol Nutr Metab. 2014;39(5):544–52. doi: 10.1139/apnm-2013-0407. [DOI] [PubMed] [Google Scholar]
- 31.Nyhoff LM, Heden TD, Leidy HJ, Winn NC, Park YM, Thyfault JP, et al. Prior exercise does not alter the incretin response to a subsequent meal in obese women. Peptides. 2015;71:94–9. doi: 10.1016/j.peptides.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heden TD, Liu Y, Sims LJ, Whaley-Connell AT, Chockalingam A, Dellsperger KC, et al. Meal frequency differentially alters postprandial triacylglycerol and insulin concentrations in obese women. Obesity. 2013;21(1):123–9. doi: 10.1002/oby.20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmstrup M, Fairchild T, Keslacy S, Weinstock R, Kanaley J. Multiple short bouts of exercise over 12-h period reduce glucose excursions more than an energy-matched single bout of exercise. Metabolism: clinical and experimental. 2014;63(4):510–9. doi: 10.1016/j.metabol.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic acids research. 2002;30(1):207–10. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flowers MT, Groen AK, Oler AT, Keller MP, Choi Y, Schueler KL, et al. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. Journal of lipid research. 2006;47(12):2668–80. doi: 10.1194/jlr.M600203-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van’t Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. The British journal of nutrition. 2009;101(Suppl 2):S73–85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- 37.Livingstone MB, Black AE. Markers of the validity of reported energy intake. The Journal of nutrition. 2003;133(Suppl 3):895S–920S. doi: 10.1093/jn/133.3.895S. [DOI] [PubMed] [Google Scholar]