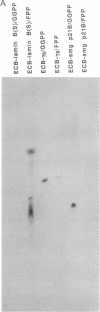
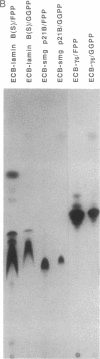
Abstract
A protein geranylgeranyltransferase (PGT) that catalyzes the transfer of a 20-carbon prenyl group from geranylgeranyl pyrophosphate to a cysteine residue in protein and peptide acceptors was detected in bovine brain cytosol and partially purified. The enzyme was shown to be distinct from a previously characterized protein farnesyltransferase (PFT). The PGT selectively geranylgeranylated a synthetic peptide corresponding to the C terminus of the gamma 6 subunit of bovine brain G proteins, which have previously been shown to contain a 20-carbon prenyl modification. Likewise, a peptide corresponding to the C terminus of human lamin B, a known farnesylated protein, selectively served as a substrate for farnesylation by the PFT. However, with high concentrations of peptide acceptors, both prenyl transferases were able to use either peptide as substrates and the PGT was able to catalyze farnesyl transfer. Among the prenyl acceptors tested, peptides and proteins with leucine or phenylalanine at their C termini served as geranylgeranyl acceptors, whereas those with C-terminal serine were preferentially farnesylated. These results suggest that the C-terminal amino acid is an important structural determinant in controlling the specificity of protein prenylation.
Full text
PDF

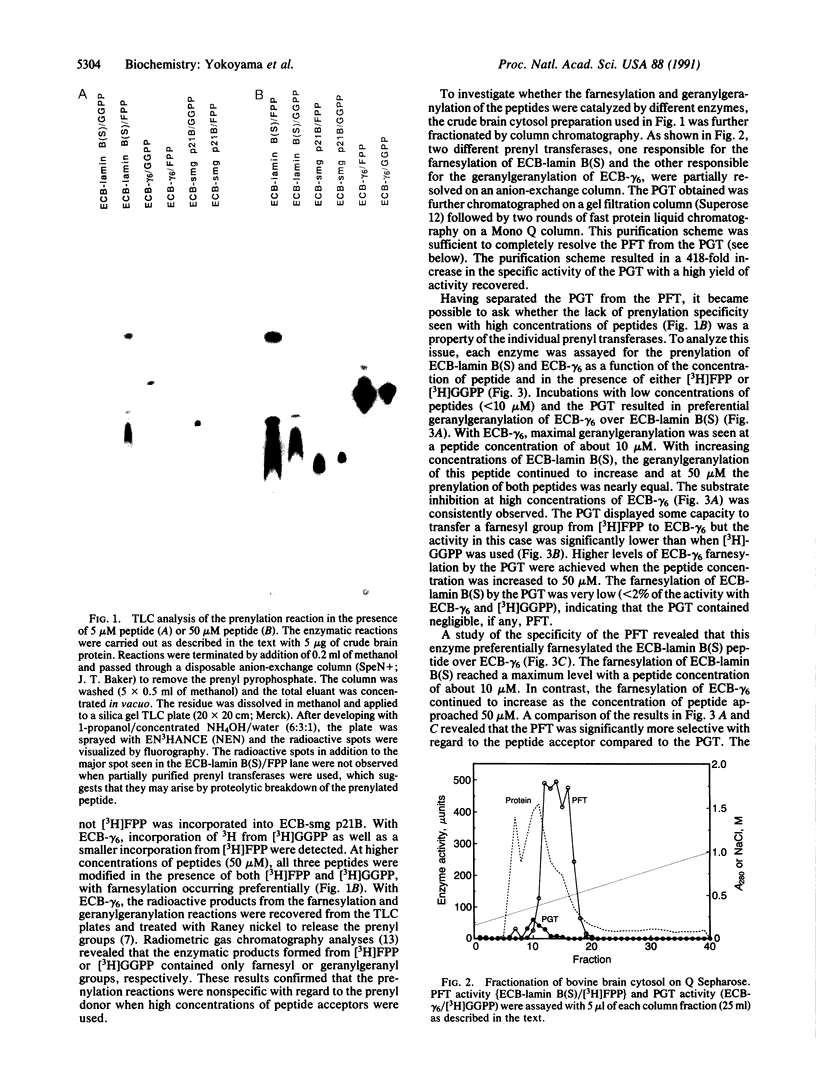
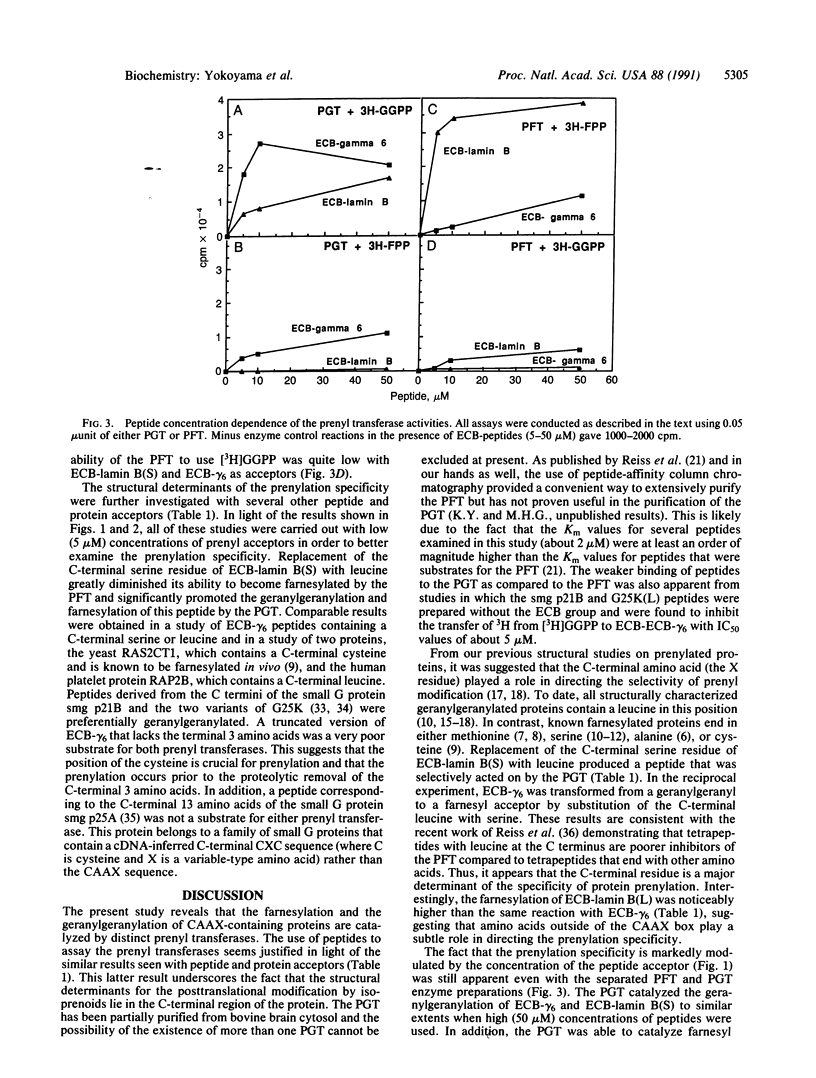

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- Buss J. E., Quilliam L. A., Kato K., Casey P. J., Solski P. A., Wong G., Clark R., McCormick F., Bokoch G. M., Der C. J. The COOH-terminal domain of the Rap1A (Krev-1) protein is isoprenylated and supports transformation by an H-Ras:Rap1A chimeric protein. Mol Cell Biol. 1991 Mar;11(3):1523–1530. doi: 10.1128/mcb.11.3.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes R. J., Stimmel J. B., Clarke S., Stock J., Broach J. R. RAS2 protein of Saccharomyces cerevisiae is methyl-esterified at its carboxyl terminus. J Biol Chem. 1989 Jul 15;264(20):11865–11873. [PubMed] [Google Scholar]
- Farnsworth C. C., Gelb M. H., Glomset J. A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science. 1990 Jan 19;247(4940):320–322. doi: 10.1126/science.2296721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989 Dec 5;264(34):20422–20429. [PMC free article] [PubMed] [Google Scholar]
- Finegold A. A., Johnson D. I., Farnsworth C. C., Gelb M. H., Judd S. R., Glomset J. A., Tamanoi F. Protein geranylgeranyltransferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4448–4452. doi: 10.1073/pnas.88.10.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Goodman L. E., Judd S. R., Farnsworth C. C., Powers S., Gelb M. H., Glomset J. A., Tamanoi F. Mutants of Saccharomyces cerevisiae defective in the farnesylation of Ras proteins. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9665–9669. doi: 10.1073/pnas.87.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hrycyna C. A., Clarke S. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Oct;10(10):5071–5076. doi: 10.1128/mcb.10.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y., Sakurai A., Tamura S., Takahashi N. Structure of rhodotorucine A, a novel lipopeptide, inducing mating tube formation in Rhodosporidium toruloides. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1077–1083. doi: 10.1016/0006-291x(78)91505-x. [DOI] [PubMed] [Google Scholar]
- Kawata M., Farnsworth C. C., Yoshida Y., Gelb M. H., Glomset J. A., Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8960–8964. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai R. K., Perez-Sala D., Cañada F. J., Rando R. R. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J. 1990 Dec;4(15):3319–3328. doi: 10.1096/fasebj.4.15.2123808. [DOI] [PubMed] [Google Scholar]
- Manne V., Roberts D., Tobin A., O'Rourke E., De Virgilio M., Meyers C., Ahmed N., Kurz B., Resh M., Kung H. F. Identification and preliminary characterization of protein-cysteine farnesyltransferase. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7541–7545. doi: 10.1073/pnas.87.19.7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr R. S., Blair L. C., Thorner J. Saccharomyces cerevisiae STE14 gene is required for COOH-terminal methylation of a-factor mating pheromone. J Biol Chem. 1990 Nov 25;265(33):20057–20060. [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kawata M., Kondo J., Teranishi Y., Takai Y. Molecular cloning of smg p21B and identification of smg p21 purified from bovine brain and human platelets as smg p21B. Biochem Biophys Res Commun. 1990 Jan 30;166(2):1010–1016. doi: 10.1016/0006-291x(90)90911-6. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kondo J., Hishida T., Teranishi Y., Takai Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11071–11074. [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S., Innis M. A., Clark R., McCormick F., Ullrich A., Polakis P. Molecular cloning and expression of a G25K cDNA, the human homolog of the yeast cell cycle gene CDC42. Mol Cell Biol. 1990 Nov;10(11):5977–5982. doi: 10.1128/mcb.10.11.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y., Goldstein J. L., Seabra M. C., Casey P. J., Brown M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990 Jul 13;62(1):81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Stradley S. J., Gierasch L. M., Brown M. S., Goldstein J. L. Sequence requirement for peptide recognition by rat brain p21ras protein farnesyltransferase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):732–736. doi: 10.1073/pnas.88.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling H. C., Breunger E., Epstein W. W., Crain P. F. Prenylated proteins: the structure of the isoprenoid group. Science. 1990 Jan 19;247(4940):318–320. doi: 10.1126/science.2296720. [DOI] [PubMed] [Google Scholar]
- Rine J., Kim S. H. A role for isoprenoid lipids in the localization and function of an oncoprotein. New Biol. 1990 Mar;2(3):219–226. [PubMed] [Google Scholar]
- Robishaw J. D., Kalman V. K., Moomaw C. R., Slaughter C. A. Existence of two gamma subunits of the G proteins in brain. J Biol Chem. 1989 Sep 25;264(27):15758–15761. [PubMed] [Google Scholar]
- Schaber M. D., O'Hara M. B., Garsky V. M., Mosser S. C., Bergstrom J. D., Moores S. L., Marshall M. S., Friedman P. A., Dixon R. A., Gibbs J. B. Polyisoprenylation of Ras in vitro by a farnesyl-protein transferase. J Biol Chem. 1990 Sep 5;265(25):14701–14704. [PubMed] [Google Scholar]
- Schafer W. R., Trueblood C. E., Yang C. C., Mayer M. P., Rosenberg S., Poulter C. D., Kim S. H., Rine J. Enzymatic coupling of cholesterol intermediates to a mating pheromone precursor and to the ras protein. Science. 1990 Sep 7;249(4973):1133–1139. doi: 10.1126/science.2204115. [DOI] [PubMed] [Google Scholar]
- Shinjo K., Koland J. G., Hart M. J., Narasimhan V., Johnson D. I., Evans T., Cerione R. A. Molecular cloning of the gene for the human placental GTP-binding protein Gp (G25K): identification of this GTP-binding protein as the human homolog of the yeast cell-division-cycle protein CDC42. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9853–9857. doi: 10.1073/pnas.87.24.9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R. C., Clarke S. Identification of a C-terminal protein carboxyl methyltransferase in rat liver membranes utilizing a synthetic farnesyl cysteine-containing peptide substrate. J Biol Chem. 1990 Sep 25;265(27):16248–16254. [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Evans T., Howald W. N., Gelb M. H., Glomset J. A., Clarke S., Fung B. K. Membrane-binding domain of the small G protein G25K contains an S-(all-trans-geranylgeranyl)cysteine methyl ester at its carboxyl terminus. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):286–290. doi: 10.1073/pnas.88.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]