Abstract
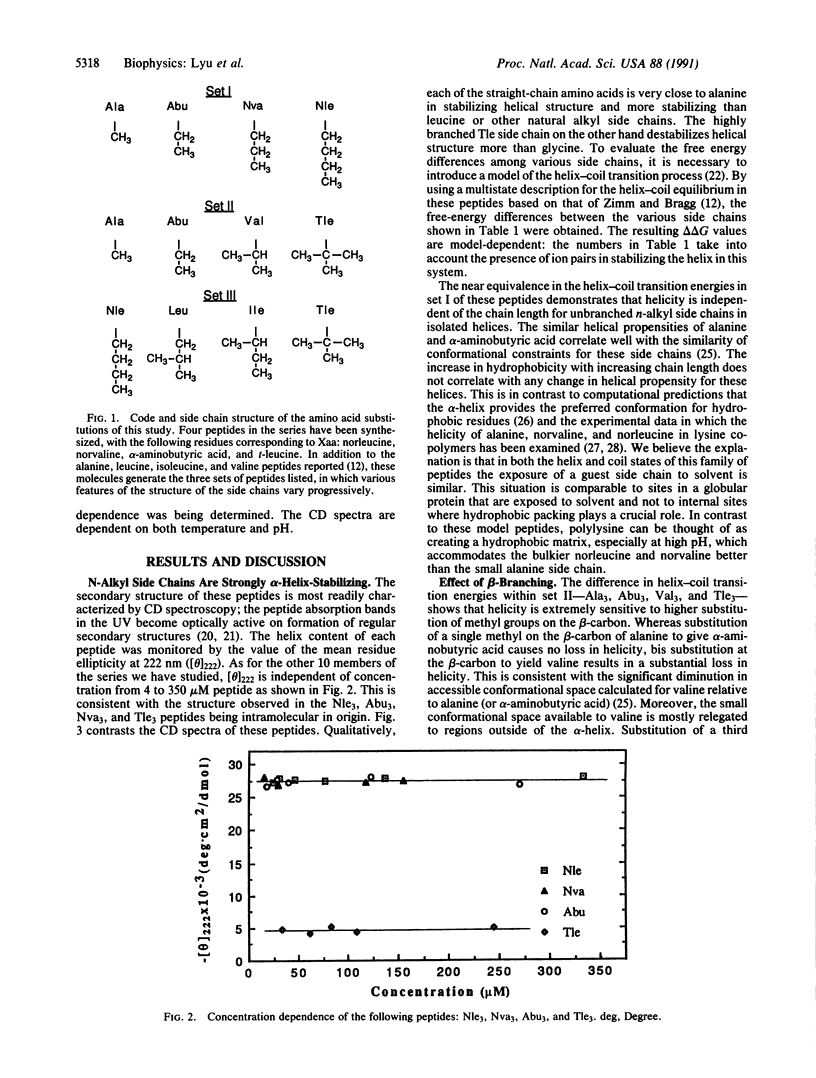
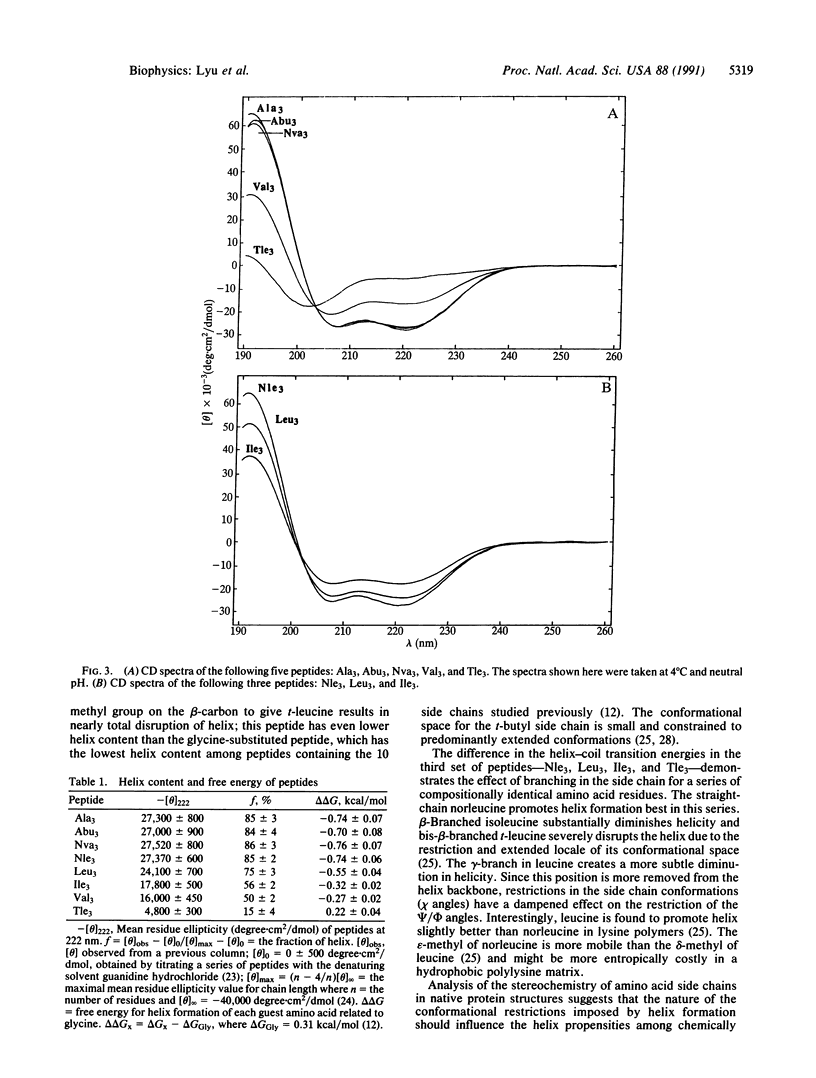
Knowledge of the role of individual side chains in forming different secondary structures such as the alpha-helix would be useful for prediction of protein structure from sequence or de novo protein design. Experimental and theoretical studies on natural and synthetic peptides and proteins indicate that individual side chains differ in their helix-forming potential. Four aliphatic side chains occur in the standard complement of amino acids: alanine and leucine are helix stabilizing, whereas isoleucine and valine are weakly destabilizing. We have synthesized a series of helical peptides containing unnatural aliphatic side chains having two to four carbons to explore some of the factors involved in alpha-helix stabilization and the basis for selection of the natural set. We find that linear side chains with two, three, or four carbons are as strongly helix stabilizing as the single methyl in alanine and that all linear side chains are stronger helix promoters than leucine. In addition, a t-butyl side chain is significantly more helix destabilizing than the sec-butyl side chain of isoleucine, the isopropyl side chain of valine, or even the unrestricted side chain of glycine. These results provide experimental evidence that restriction in conformational freedom of a side chain imposed by alpha-helix formation is a major component of the role of a side chain in stabilizing helical structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfmann H. A., Labitzke R., Wagner K. G. Conformational properties of L-leucine, L-isoleucine, and L-norleucine side chains in L-lysine copolymers. Biopolymers. 1977 Aug;16(8):1815–1826. doi: 10.1002/bip.1977.360160815. [DOI] [PubMed] [Google Scholar]
- Barlow D. J., Thornton J. M. Ion-pairs in proteins. J Mol Biol. 1983 Aug 25;168(4):867–885. doi: 10.1016/s0022-2836(83)80079-5. [DOI] [PubMed] [Google Scholar]
- Barskaya T. V., Ptitsyn O. B. Thermodynamic parameters of helix-coil transition in polypeptide chains. II. Poly-L-lysine. Biopolymers. 1971 Nov;10(11):2181–2197. doi: 10.1002/bip.360101112. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Kaplan L. J. Derivative sspectroscopy applied to tyrosyl chromophores. Studies on ribonuclease, lima bean inhibitors, insulin, and pancreatic trypsin inhibitor. Biochemistry. 1973 May 8;12(10):2011–2024. doi: 10.1021/bi00734a027. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Dill K. A. Origins of structure in globular proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6388–6392. doi: 10.1073/pnas.87.16.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Secondary structure of proteins through circular dichroism spectroscopy. Annu Rev Biophys Biophys Chem. 1988;17:145–166. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr, Tinoco I., Jr Circular dichroism of polypeptide solutions in the vacuum ultraviolet. J Am Chem Soc. 1972 Jun 14;94(12):4389–4392. doi: 10.1021/ja00767a084. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Polypeptide chain folding through a highly helical intermediate as a general principle of globular protein structure formation. FEBS Lett. 1978 May 1;89(1):10–14. doi: 10.1016/0014-5793(78)80511-0. [DOI] [PubMed] [Google Scholar]
- Lyu P. C., Liff M. I., Marky L. A., Kallenbach N. R. Side chain contributions to the stability of alpha-helical structure in peptides. Science. 1990 Nov 2;250(4981):669–673. doi: 10.1126/science.2237416. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Baldwin R. L. Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqusee S., Robbins V. H., Baldwin R. L. Unusually stable helix formation in short alanine-based peptides. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5286–5290. doi: 10.1073/pnas.86.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor M. J., Islam S. A., Sternberg M. J. Analysis of the relationship between side-chain conformation and secondary structure in globular proteins. J Mol Biol. 1987 Nov 20;198(2):295–310. doi: 10.1016/0022-2836(87)90314-7. [DOI] [PubMed] [Google Scholar]
- Merutka G., Lipton W., Shalongo W., Park S. H., Stellwagen E. Effect of central-residue replacements on the helical stability of a monomeric peptide. Biochemistry. 1990 Aug 14;29(32):7511–7515. doi: 10.1021/bi00484a021. [DOI] [PubMed] [Google Scholar]
- Moult J., James M. N. An algorithm for determining the conformation of polypeptide segments in proteins by systematic search. Proteins. 1986 Oct;1(2):146–163. doi: 10.1002/prot.340010207. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990 Nov 2;250(4981):646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S., Marqusee S., Ridgeway T., Laue T. M., Baldwin R. L. Relative helix-forming tendencies of nonpolar amino acids. Nature. 1990 Mar 15;344(6263):268–270. doi: 10.1038/344268a0. [DOI] [PubMed] [Google Scholar]
- Piela L., Nemethy G., Scheraga H. A. Conformational constraints of amino acid side chains in alpha-helices. Biopolymers. 1987 Aug;26(8):1273–1286. doi: 10.1002/bip.360260805. [DOI] [PubMed] [Google Scholar]
- Ponder J. W., Richards F. M. Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol. 1987 Feb 20;193(4):775–791. doi: 10.1016/0022-2836(87)90358-5. [DOI] [PubMed] [Google Scholar]
- Presta L. G., Rose G. D. Helix signals in proteins. Science. 1988 Jun 17;240(4859):1632–1641. doi: 10.1126/science.2837824. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Shoemaker K. R., Fairman R., Schultz D. A., Robertson A. D., York E. J., Stewart J. M., Baldwin R. L. Side-chain interactions in the C-peptide helix: Phe 8 ... His 12+. Biopolymers. 1990 Jan;29(1):1–11. doi: 10.1002/bip.360290104. [DOI] [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., Brems D. N., Marqusee S., York E. J., Chaiken I. M., Stewart J. M., Baldwin R. L. Nature of the charged-group effect on the stability of the C-peptide helix. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2349–2353. doi: 10.1073/pnas.82.8.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., York E. J., Stewart J. M., Baldwin R. L. Tests of the helix dipole model for stabilization of alpha-helices. Nature. 1987 Apr 9;326(6113):563–567. doi: 10.1038/326563a0. [DOI] [PubMed] [Google Scholar]



