Abstract
Portal vein thrombosis (PVT) in a setting of liver metastasis is not easy to treat as it may be portal vein tumor thrombus (PVTT). A 77-year-old male patient was diagnosed as ascending colon carcinoma, underwent right hemicolectomy in 1991 with a recurrence in July 2009. In August 2009, he underwent computed tomography (CT) scan of the abdomen which showed evidence of superior mesenteric vein thrombosis with no liver metastasis. He was started with anticoagulation and decision was to treat long term. He was admitted with mesenteric artery ischemic symptoms in February 2012 on anticoagulation. CT scan abdomen and pelvis in February 2012 showed tumor thrombus involving the superior mesenteric vein, portal vein, and splenic vein with hepatic metastasis. His tumor marker chorioembryonic antigen was 34 µg/L. He was continued on anticoagulation. A repeat CT scan abdomen after 2 years (in January 2014) showed, increase in size of hepatic metastasis, extensive thrombus involving the superior mesenteric vein, portal vein, and splenic vein with collaterals. Mesentery was congested due to extensive superior mesenteric vein thrombus. He finally succumbed in June 2014. It is very important to differentiate PVT from PVTT as the prognosis is different. PVTT progresses despite of long-term anticoagulation with poor prognosis.
Keywords: anticoagulation, colon, ischemia, malignancy, portal vein, thrombosis, tumour thrombosis
Portal vein thrombosis (PVT)1 2 3 4 in a setting of liver metastasis is not easy to treat. It is at times difficult to say whether the thrombus is PVT or a portal vein tumor thrombus (PVTT).
We describe an elderly male patient, treated as PVT which was very resistant to treatment. He had liver metastasis with colonic malignancy and thrombosis was very resistant to treatment.
Case Report
A 77-year-old male patient, diagnosed as ascending colon carcinoma, underwent right hemicolectomy in 1991. He had no other history of note.
In August 2009, he had a recurrence of colonic carcinoma and was treated with chemotherapy. He was followed up in medical oncology clinic. In August 2009, he underwent computed tomography (CT) abdomen scan which showed evidence of superior mesenteric vein (SMV) thrombosis with no liver metastasis. He was started on anticoagulation.
His blood tests before starting anticoagulation showed hemoglobin level of 10.5 g/dL, total white blood cells at 4.2 × 109/L, and platelets at 149 × 109/L. His renal and liver functions were normal.
His workup for thrombophilia screen was as follows: protein C 37% (normal range, 70–150%) and protein S 51% (normal range, 65–130%) were low.
Antithrombin III, antiphospholipid, activated protein C resistance was normal and lupus anticoagulant was absent. There was increase in chorioembryonic antigen (CEA) from 3 µg/L in May 2009 to 14 µg/L in November 2009. He was continued on warfarin with prothrombin time international normalized ratio (PT/INR) maintained between 2 and 3 seconds.
In April 2010, his colonic malignancy was progressive and CEA increased to 27 µg/L. Computed tomography (CT) scan of the abdomen showed thrombus within the SMV and progression proximally into the main portal vein. This was suspicious of tumor thrombus.
In February 2012, on anticoagulation, he was admitted with severe abdominal pain in and diagnosed as mesenteric ischemia. CT scan abdomen and pelvis showed progression of mesenteric lymphadenopathy, tumor thrombus involving the SMV, branches of the SMV, lower segment of the main portal vein and in the splenic vein. It was also noted that there was a heterogeneous hypodense lesion in segment 2/3 of the liver, suspicious of hepatic metastasis. His tumor marker CEA was 24 µg/L in July 2011 which increased to > 34 µg/L in February 2012. He was continued on anticoagulation.
In January 2014, as his symptoms of abdominal pain were worsening, he underwent CT scan for the abdomen which showed that hepatic metastases were larger in size. There was extensive thrombosis in portal vein (Fig. 1), SMVs (Fig. 2), and splenic veins (Fig. 3) with associated collaterals. The mesentery was congested related to SMV thrombosis (Fig. 4). His anticoagulation was continued with very close monitoring of PT/INR.
Fig. 1.
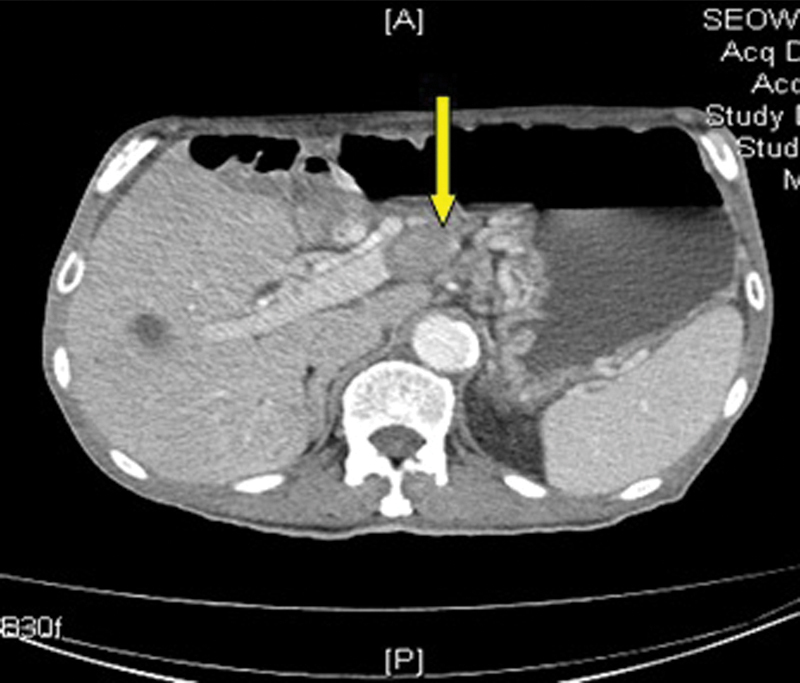
Liver metastasis with portal vein thrombosis. Yellow arrow points to portal vein thrombus.
Fig. 2.
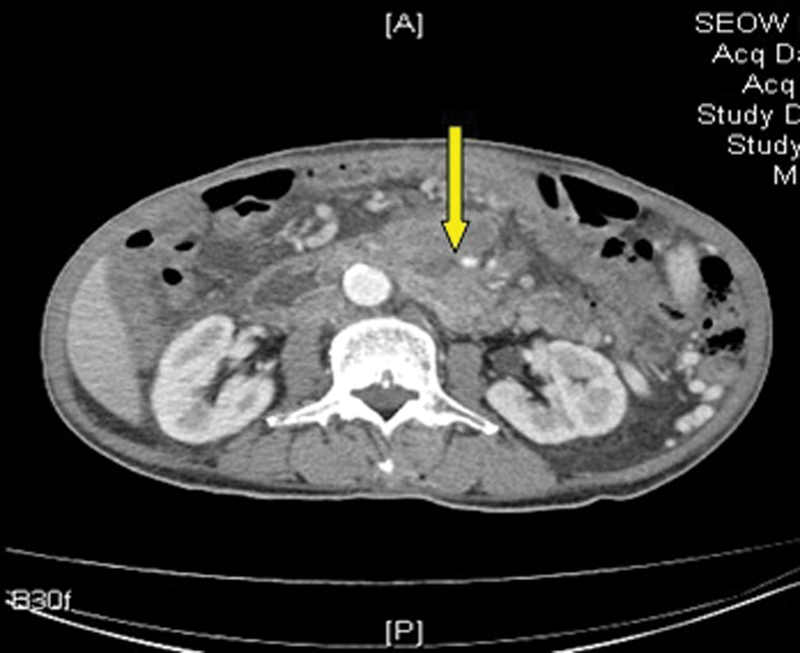
Superior mesenteric vein thrombosis. Yellow arrow points to superior mesenteric vein thrombus.
Fig. 3.
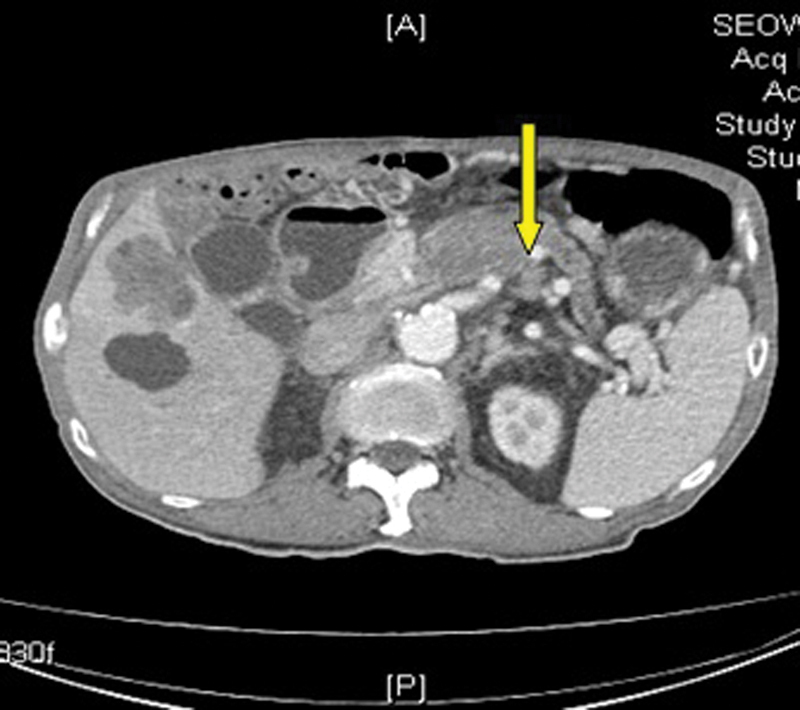
Liver metastasis with splenic vein thrombosis. Yellow arrow points to splenic vein thrombus.
Fig. 4.
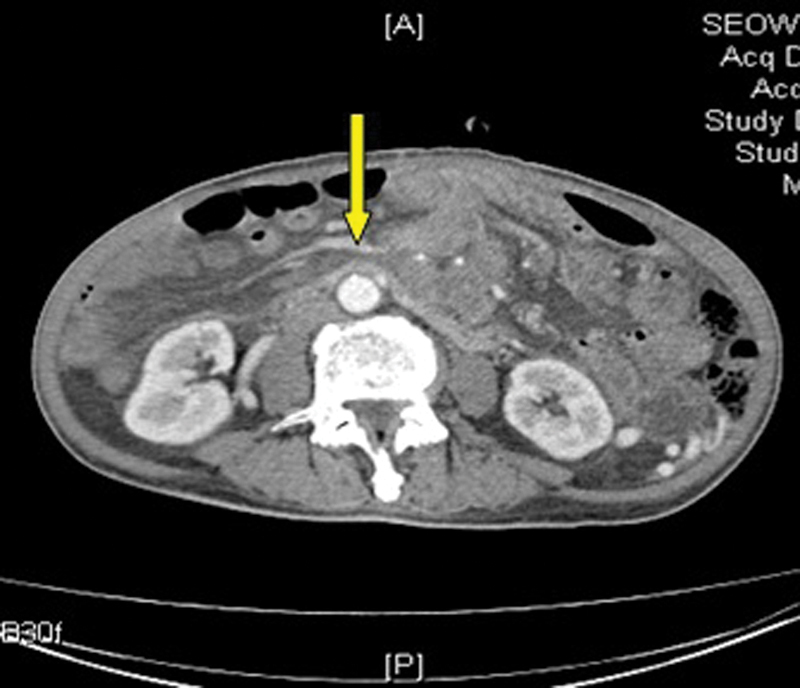
Mesenteric congestion with superior mesenteric vein thrombosis. Yellow arrow points to mesenteric congestion.
He was followed up with palliative physicians and was finally discontinued from anticoagulation in May 2014 due to frequent falls. He finally succumbed and passed away in June 2014.
Discussion
PVT can occur in both cirrhotic and noncirrhotic patients and it can be an acute or a chronic episode. PVT in noncirrhotic patients can be due to intra-abdominal inflammatory lesions, injury to the portal venous system or intra-abdominal malignancy or metastasis (also known as malignancy with PVT or PVTT.1
Its incidence in patients without cirrhosis is not well described in the literature, but accounts for approximately 5 to 10% of all cases of portal hypertension in the Western hemisphere.1 2
In cirrhotic patients, it has been well established that the prevalence of PVT increases with the severity of the cirrhosis. In those with well-compensated cirrhosis, the incidence of thrombosis reportedly varies between 0.6 and 16%. The incidence is higher in patients with advanced hepatic dysfunction, particularly those with hepatocellular cancer, in whom it reaches about 35%.2
Most of the time, noncirrhotic patients noted to have PVT without any bleeding risk factors are better managed with anticoagulation. Limited data are available for the management of PVT in cirrhotic patients.
Transient PVT has been reported in 23% of the patients with acute pancreatitis and 57% in those with pancreatic necrosis. The management of these patients primarily depends on treatment of primary cause.3 4
Spontaneous recanalization (in 16.7% of the patients) without treatment has also been documented. It has been said in multiple literature that, frequently, spontaneous PVT resolution is associated with a self-limiting underlying pathology or minimal thrombus extension.5
PVTT is commonly associated with hepatocellular carcinoma (HCC), because HCC is a hypervascular tumor with shunt formation from the hepatic artery to the portal vein. Microscopic invasion of the portal vein, hepatic vein, and intrahepatic biliary duct are reportedly present at rates of 22.5, 7.5, and 40.0%, respectively.5
Colorectal liver metastases are usually accompanied by microscopic tumor invasion into the intrahepatic portal vein, and the incidence of macroscopic tumor thrombus in the trunk of the portal vein is estimated to be 2.8%.5 6 Most reported cases of PVTT from colorectal cancer had concomitant metastatic nodules in the liver parenchyma, and the PVTT was continuous with the liver nodules, similar to PVTT in HCC.1 5 6
Differentiating benign from malignant PVT is particularly important in patients with cirrhosis and HCC who are being considered for liver transplantation since malignant PVT is a contraindication to liver transplantation.6
Findings that suggest a malignant PVT include the following6:
Elevated α fetoprotein
Portal vein diameter > 23 mm
Enhancement of endoluminal material during the arterial phase of contrast injection
Arterial-like pulsatile flow seen with Doppler ultrasound
Disruption of the vessel walls
Biopsy of the thrombus can be used to differentiate benign from malignant PVT in cases where the diagnosis is unclear.6
Transient PVT has been reported in 23% of the patients with acute pancreatitis and 57% in those with pancreatic necrosis. Spontaneous recanalization (in 16.7% of the patients) without treatment has also been documented.3 It has been said in multiple literature that, frequently, spontaneous PVT resolution is associated with a self-limiting underlying pathology or minimal thrombus extension.3 4 7
Conclusion
It is very important to differentiate PVT from PVTT as the prognosis is different.
Anticoagulation is important in the treatment of PVTT but the primary malignancy needs to be treated aggressively. PVTT progresses despite of long-term anticoagulation with poor prognosis.
References
- 1.Squizzato A, Ageno W, Cattaneo A, Brumana N. A case report and literature review of portal vein thrombosis associated with cytomegalovirus infection in immunocompetent patients. Clin Infect Dis. 2007;44(2):e13–e16. doi: 10.1086/509641. [DOI] [PubMed] [Google Scholar]
- 2.DeLeve L D Valla D C Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver Hepatology 20094951729–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sameer P, Riddhi S, Prashant K. Portal vein thrombosis. Am J Med. 2010;123(2):111–119. doi: 10.1016/j.amjmed.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Chawla Y, Duseja A, Dhiman R K. Review article: the modern management of portal vein thrombosis. Aliment Pharmacol Ther. 2009;30(9):881–894. doi: 10.1111/j.1365-2036.2009.04116.x. [DOI] [PubMed] [Google Scholar]
- 5.Dörffel T, Wruck T, Rückert R I, Romaniuk P, Dörffel Q, Wermke W. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000;21(2):126–133. doi: 10.1097/00006676-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto N, Sugano N, Morinaga S. et al. Massive portal vein tumor thrombus from colorectal cancer without any metastatic nodules in the liver parenchyma. Rare Tumors. 2011;3(4):e47. doi: 10.4081/rt.2011.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cedrone A, Rapaccini G L, Pompili M. et al. Portal vein thrombosis complicating hepatocellular carcinoma. Value of ultrasound-guided fine-needle biopsy of the thrombus in the therapeutic management. Liver. 1996;16(2):94–98. doi: 10.1111/j.1600-0676.1996.tb00711.x. [DOI] [PubMed] [Google Scholar]


