Abstract
Purpose/Aim
Data from chronic stroke studies have reported reduced blood flow and vascular endothelial function in the stroke affected limb. It is unclear whether these differences are present early after stroke. First, we investigated whether vascular endothelial function in the stroke affected limb would be different from healthy adults. Second, we examined whether between-limb differences in vascular endothelial function existed in the stroke affected arm compared to the non-affected arm. Last, we tested whether reduced vascular endothelial function was related to pro-inflammatory markers that are present early after stroke.
Materials and Methods
Vascular endothelial function was assessed by flow-mediated dilation (FMD) in the brachial artery within 72 hours post-stroke. All participants withheld medications from midnight until after the procedure. Ultrasound scans and blood draws for pro-inflammatory markers occurred on the same day between 7:30 am and 9:00 am.
Results
People with acute stroke had significantly lower FMD (4.2% ± 4.6) than control participants (8.5% ± 5.2, p = 0.037). Stroke participants had between limb differences in FMD (4.2% ± 4.6 stroke affected vs 5.3% ± 4.4 non-affected, p = 0.02) whereas the control participants did not. Of the pro-inflammatory markers only vascular cell adhesion molecule-1(VCAM-1) had a significant relationship to FMD (stroke affected limb, r = −0.62, p = 0.03; non-affected limb, −0.75, p = 0.005), but not tumor necrosis factor alpha nor interleukin-6.
Conclusions
Vascular endothelial function is reduced starting in the early stage of stroke recovery. People with higher levels of VCAM-1 had a lower FMD response.
Keywords: flow-mediated dilation, vascular endothelial function, inflammatory markers, acute stroke
Introduction
The vascular endothelium has an integral role regulating vasomotor tone. Vascular endothelial dysfunction results when this regulatory function is compromised and has been associated with increased cardiovascular risk.(1, 2) Vascular endothelial dysfunction has been reported in people with cardiovascular disease, hypercholesterolemia, and chronic stroke.(1, 3–5) Individuals with chronic stroke have reduced femoral artery diameter and blood flow in the stroke-affected leg compared to the non-affected leg at rest(4, 6–8) and during exercise.(8) We reported that these vascular adaptations are observed during the subacute period of stroke recovery.(9) Our findings showed that the brachial artery of the stroke-affected arm had reduced vascular endothelial function when compared to the non-stroke affected arm. However, an 8-week aerobic exercise program improved vascular endothelial function by approximately 19% in both brachial arteries and the adaptations remained at the one-month follow up. However, the stroke affected arm still had a lower FMD when compared to the non-stroke affected arm. These are unique unilateral adaptations that are not observed in healthy young and older adults and can influence performance of activities of daily living and quality of life.(10),(11)
Nitric oxide (NO) is a known regulator of vascular endothelial function.(12) Elevated levels of pro-inflammatory markers may interfere with signaling and reduce NO release, which would result in decreased vascular endothelial function.(13) Previous work suggests that individuals without hypertension prior to an acute stroke who present with elevated levels of pro-inflammatory markers (vascular cell adhesion molecule 1; VCAM-1, tumor necrosis factor-α; TNF-α, and interleukin-6; IL-6) had a new onset of hypertension.(14) Furthermore, elevated levels of pro-inflammatory markers have been reported after stroke(15–17) and have been strongly associated with poor outcomes (i.e., early neurological decline).(14, 17) Therefore, it is plausible to suggest that pro-inflammatory markers may interfere with NO bioavailability and have a negative effect on peripheral vascular endothelial function starting in the acute stroke setting. Gaining insight into these vascular changes may be useful information to support activity early after stroke, which could minimize vascular decline. However, the current literature has not established a clear timeline early after stroke when the impairment in vascular endothelial function occurs or why these unilateral adaptations occur.
The purpose of this study was to characterize vascular endothelial function in people with acute stroke. First, we examined whether vascular endothelial function measured using flow-mediated dilation (FMD) would be different from age- and gender- matched controls. We hypothesized that FMD would be reduced in the brachial artery in the stroke affected limb when compared to the control group. Next, we examined whether between-limb differences in FMD were observed in the stroke affected arm when compared to the non-affected side. We hypothesized that the stroke affected arm would have a reduced FMD when compared to the non-stroke affected arm. Last, to explore a possible mechanistic hypothesis, we tested whether reduced FMD was related to pro-inflammatory markers in those with an acute stroke. Specifically, we hypothesized that reduced FMD would be moderately associated with elevated levels of pro-inflammatory markers, VCAM-1, TNF-α, and IL-6.
Materials and Methods
Participants
Between March 2013 and April 2015, people admitted to XX hospital with acute stroke were recruited into the study. Patients were admitted to an acute stroke unit and were screened for the following inclusion criteria: 1) diagnosis of unilateral stroke from neuroimaging, 2) the ability to consent within 72 hours of admission to the stroke unit, and 3) between 30–80 years of age. Individuals were not enrolled if the following exclusion criteria were present: 1) acute renal failure, 2) ischemic cardiovascular event or coronary artery bypass surgery less than 3 months prior to stroke, 3) severe peripheral artery disease, 4) diagnosis of congestive heart failure, and 5) unable to maintain position of the upper extremity to access the brachial artery during ultrasound scanning. The control group was recruited after the enrollment of the stroke participants to ensure age- and sex-matched. Control participants were recruited from a database at the XX Center on Aging. We provided age and sex to the person recruiting to ensure balance between the 2 groups. Individuals were included in the study if they were free from cardiovascular disease and hypertension (no diagnosis and not taking anti-hypertensive medications). They traveled to the XX Lab at XX Medical Center for a single visit. The study was approved by the Human Subjects Committee at the University of XX Medical Center. Institutionally approved written consent was obtained prior to enrollment from all participants or the surrogate decision maker (individuals with stroke only).
Flow Mediated Dilation (FMD)
Vascular endothelial function assessed by FMD using Doppler ultrasound was obtained from bilateral brachial arteries in the upper extremities for all participants enrolled in the study.(9) Participants refrained from food or caffeine for 12 hours prior to the ultrasound scan and control participants were asked not participate in vigorous activity for 24 hours of the ultrasound scan. All participants withheld medications from midnight until after the procedure. Similar to previously published work, vasoactive medications were withheld.(18) For consistency, all ultrasound scans occurred on the same day between 7:30 am and 9:00 am. Each participant rested supine for 20 minutes prior to the ultrasound examination. Heart rate (HR) was monitored continuously using a 3-lead electrocardiogram (ECG). Resting supine blood pressure (BP) was taken after the 20-minute rest period.
We have previously reported on our methodology to assess vascular compliance using non-invasive FMD techniques.(9) Briefly, the arm was placed on a custom stabilizing device to allow for optimal scanning of the brachial artery and to avoid arm movements during the ultrasound imaging. The brachial artery was identified longitudinally at the same reference point, 2–3 cm proximal to the antecubital fossa using an ultrasound system and a 7.5 MHz linear array transducer (Siemens Medical Solutions, Malvern, Pennsylvania). Once a satisfactory image of the brachial artery was obtained, the transducer was stabilized using a custom-designed holder. An insonation angle of 60° was used. Baseline diameter and blood flow velocity was recorded continuously for one minute. The pneumatic cuff, (D.E. Hokanson, Bellevue, Washington) which was placed distal to the olecranon process, was then inflated to supra-systolic pressure (220 mmHg) and maintained for 5 minutes. Twenty seconds prior to cuff deflation, recording of diameter and blood flow velocity began. At 5 minutes, the cuff was deflated and recording continued for a total of 3 minutes. All images were stored on a computer and analyzed off-line using specialized software (Brachial Analyzer, Medical Imaging Applications, Coralville, Iowa). In our previous work, we stated that “flow-mediated dilation (FMD) was calculated as the peak increase in arterial diameter from the baseline value,”(9) which is a measure of vascular endothelial function.
Blood Analysis
Blood draws were performed at the same time of day as the ultrasound scans. We collected blood samples to assess levels of the pro-inflammatory markers, VCAM-1, TNF-α, and IL-6. Approximately 10 mLs of venous blood were obtained from each participant on the same day as the FMD procedure. Blood was immediately stored on ice to be transported to the laboratory for processing and storage. Full blood was spun down in a centrifuge to obtain and separate plasma. Centrifuge was cooled to 4 degrees Celsius and samples spun at 3,400 rotations per minute for 15 minutes. Plasma was then aliquoted into three separate tubes to limit the number of freeze/thaw cycles. All plasma samples were frozen for storage at −80 degrees Celsius within one hour of collection from the participant. Enzyme-linked immunosorbent assays (ELISAs) were used to quantify VCAM-1, TNF-α, and IL-6 after all samples were collected from each participant. Procedures were performed according to manufacturer’s recommendations.
Analysis
Statistical Analysis
Based on the previously reported difference in mean values for FMD between healthy controls (8.1±6.0%) and people with stroke (> 4 weeks post-stroke; 0.06±4.9%),(19) we determined that a sample size of n=11 in each group would be sufficient to provide statistical power (two-tailed, alpha = 0.05) at 85%. We overenrolled (n = 15) to account for effect sizes less than expected and to ensure reliable FMD scores.
Data analysis was performed with SPSS Version 22 (IBM, Armonk, NY) for Windows. Values are expressed as mean ± SD. One-way ANOVA was used to detect differences between those with acute stroke and control group. Paired t-test was used to assess whether between limb differences were present in the acute stage of stroke recovery. Pearson correlation was used for correlation analyses between FMD and pro-inflammatory markers. A significant p-value was ≤ 0.05.
Results
We enrolled 30 participants into the study, 15 participants with an acute stroke, and 15 age- and gender-matched controls. Twenty-eight participants (n =14 acute stroke, n = 14 control) had complete data sets for the Doppler ultrasound and FMD technique. Participant demographics (n = 28) are reported in Table 1.
Table 1.
Participant Demographics
| Characteristics; n = 28 | Stroke Group | Control Group | P-value |
|---|---|---|---|
| Male/Female | 7/7 | 6/8 | |
| Age, years | 61.4 (11.0) | 63.2 (11.4) | 0.66 |
| Body Mass Index | 30.1 (5.7) | 27.7 (4.7) | 0.15 |
| NIHSS | 7.6 (7.6) | N/A | |
| Type of Stroke | |||
| Hemorrhagic | 4 | N/A | |
| Ischemic | 10 | N/A | |
| Stroke Affected Side | |||
| Left | 6 | N/A | |
| Right | 8 | N/A | |
| Race/Ethnicity | |||
| White/Caucasian | 12 | 13 | |
| African American | 2 | 0 | |
| Asian | 0 | 1 | |
| Hispanic | 0 | 0 | |
| Non-Hispanic | 14 | 14 | |
| Diabetes | |||
| None | 11 | 13 | |
| Type II | 3 | 1 | |
| Medications | |||
| Blood Thinner | 2 | 2 | |
| Anti-hypertensive | 8 | 0 | |
| Cholesterol | 6 | 9 |
Data are expressed as mean (SD) unless stated otherwise stated. SD= standard deviation, NIHSS = National Institutes of Health Stroke Scale, N/A = not applicable
Flow-mediated Dilation
We chose to analyze the stroke-affected limb to the control participant’s left limb as there were no between limb differences for the control group (8.5% ± 5.2 vs 8.2% ± 5.5, p = 0.54). We report no significant difference between participants with acute stroke (stroke-affected limb) and control participants left limb (F(1,28) = 0.72; p = 0.39). However, in 3 individuals with stroke, we found a higher FMD response in the stroke- affected limb. We report FMD values (19.0 % ± 1.7) in the stroke-affected limb, which were greater than values observed in the non- affected limb. In our previous work in subacute stroke,(9) we have not observed this large FMD response in the stroke affected limb. In acute stroke, values above 17% for FMD have not been reported.(20) We report these individuals (n = 3) to have had a mild stroke (NIH Stroke Scale on admission to the stroke unit < 5, Range 3–5), atrial fibrillation, normal body mass index, normotensive (not taking anti-hypertensive medications) and length of stay was 3 days. We acknowledge that the information provided is descriptive, but we have no other mechanistic or medical rationale for this large FMD response in the stroke-affected limb.
We tested whether these 3 data points were considered to be outliers using the outlier labeling rule(21) and found that 2 data points met the criteria to be considered outliers. We then excluded these 2 individuals from subsequent analyses. Using one-way ANOVA, we found that the participants with stroke had a lower mean %FMD in the affected limb (4.2% ± 4.6) when compared to the control group (8.5% ± 5.2; F(1,26) = 4.0, p = 0.04). When comparing the non-stroke affected limb to the control group, we report the between group differences approached significance (p = 0.06). We used paired t-tests to examine whether between limb differences were present during acute stroke. We found that the stroke-affected limb had a significantly lower FMD than the non-affected limb (4.2% ± 4.6 vs 5.3% ± 4.4, p = 0.02).
Flow-mediated Dilation and Pro-inflammatory Markers in Acute Stroke
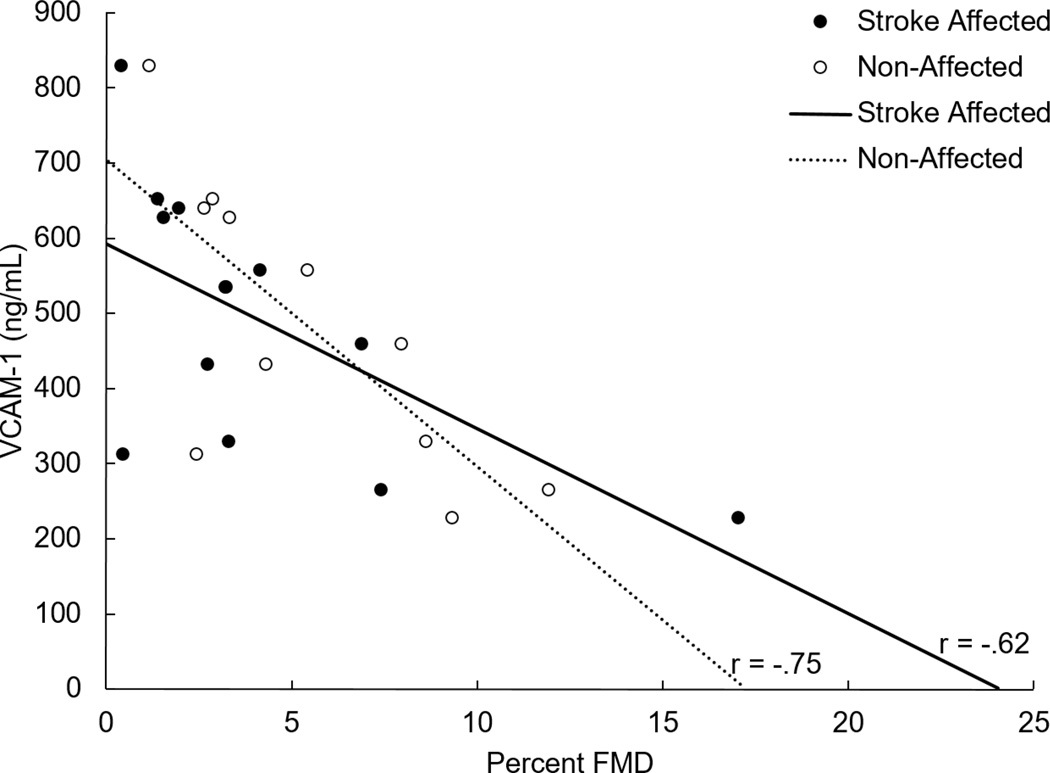
Fasting values for the pro-inflammatory markers are presented in Table 2. We found that only VCAM-1 was significantly related to FMD in the stroke-affected limb (r = −0.62, p = 0.03) and to the non-affected limb (−0.75, p = 0.005) see Figure 1). TNF-α and IL-6 showed a weak, but non-significant association to FMD values in both limbs.
Table 2.
Pro-inflammatory Markers for Stroke Participants
| n = 14 | Pro-inflammatory marker | Concentration, ng * mL-1 (SD) |
|---|---|---|
| VCAM-1 | 499.4 (219.6) | |
| TNF-A | 2.0 (6.5) | |
| IL-6 | 4.8 (6.3) | |
| * n=12 | Pro-inflammatory marker | Concentration, ng * mL-1 (SD) |
| VCAM-1 | 489.5 (184.0) | |
| TNF-A | 2.3 (7.0) | |
| IL-6 | 4.8 (6.7) | |
Pro-inflammatory markers for stroke participants included in the final FMD analysis
Figure 1.
VCAM-1 Concentration and Limb Percent FMD in Stroke
Discussion
There is limited evidence regarding vascular endothelial function in acute stroke using previously published methodological and physiological recommendations.(22) To better understand vascular endothelial function early after stroke (within 72 hours of stroke) we examined whether FMD would be different from age- and gender-matched controls with no cardiovascular disease or hypertension. Our primary finding was that people with acute stroke had a lower brachial artery FMD than healthy individuals. We did report between-limb differences in brachial artery FMD but only after the outliers (n = 2) were removed from the data analysis. To better understand a potential mechanism for reduced vascular endothelial function, we studied whether pro-inflammatory markers would be negatively related to brachial artery FMD. Our findings suggest that only VCAM-1 had a strong and significant negative relationship to brachial artery FMD. No significant relationship was found between FMD and TNF-alpha or IL-6.
Flow-Mediated Dilation: Acute Stroke and Controls
Blood flow distribution is governed by a delicate balance between the parasympathetic and sympathetic activity.(23) There is also a specific role of peripheral mechanisms (i.e. metabolic demand) and communication between the vascular endothelium and the smooth muscle in the vessel wall.(24, 25) Alterations in either central or peripheral regulation after stroke may interrupt normal vascular function.
Although we have observed unilateral changes resting blood flow and arterial diameter in the femoral artery of the hemiparetic leg in chronic stroke,(6, 7) it is unclear when these changes occur or the mechanisms involved. Vascular endothelial function via FMD in the brachial artery has been reported to be reduced in chronic(3) stroke when compared to controls. A recent study examined people within 3 months of stroke and compared brachial artery FMD to control patients without stroke.(26) They reported significantly lower FMD values in the right brachial artery for the people with stroke (8.4±4.4% vs 12.6±4.9%). However, the control patients were not age and gender- matched. For our study, individuals in the control group were both age- and gender-matched and were free of hypertension and cardiovascular disease. Our findings support recent work that FMD is significantly lower in acute stroke when compared to the healthy individuals free of hypertension and cardiovascular disease. Following recommendations for FMD methodology, both groups refrained from vitamins, medications and fasted overnight.(22) For the stroke participants, vasoactive drugs were not given. However, we cannot rule out some effect of the medications especially those that may have a longer half-life on the FMD response in people following an acute stroke. Therefore, we cannot rule out that some medications may influence vascular endothelial function.
We also examined whether between-limb differences in FMD were observed in the stroke-affected arm when compared to the non-affected side. Our prior work has suggested that between limb differences are present in the femoral artery diameter and blood flow in people with chronic stroke(6, 7) and in in subacute stroke, brachial artery FMD was lower in the stroke-affected limb when compared to the less affected side.(9) In 2004, Ivey et al used strain gauge plethysmography to examine vascular endothelial function in the lower limbs in people with chronic stroke.(5) They reported findings similar to our previous work(6, 7) regarding between limb differences. Ivey and colleagues explored whether these alterations were a function of reduced lean muscle mass using dual energy X-ray absorptiometry.(5) The authors found only a small percentage of the variance was explained by lean muscle tissue in both limbs, which suggests other systemic mediators such as hypertension(14), muscle fiber type(5, 27) or as we have demonstrated circulating VCAM-1 may influence vascular health.
Flow-mediated Dilation and Pro-inflammatory Markers in Acute Stroke
The bioavailability of NO is important for a healthy vascular system and vessel compliance. A reduction in the bioavailability of NO may negatively affect vascular endothelial function.(12) The presence of pro-inflammatory markers in circulation has been purported to interfere with vascular function and can have a negative effect on the bioavailability of NO.(28–30) There is evidence to support that elevated levels of pro-inflammatory markers are present within 24 hours after the stroke insult(15–17) and are strongly associated with poor outcomes (i.e. larger lesion size, early neurological decline).(14, 17) Individuals with acute stroke without a history of chronic hypertension prior to stroke onset and elevated levels of pro-inflammatory markers post-stroke (TNF-alpha, IL-6 and VCAM-1) exhibited new-onset hypertension.(14) This suggests that elevated levels of pro-inflammatory markers may influence peripheral mechanisms such as cell adhesion regulated processes and lower NO release in the vessel that contributes to decreased vascular endothelial function.(13) While it is known that these pro-inflammatory markers are increased early after stroke,(15–17) our findings suggest that TNF-alpha and IL-6 are not related to brachial artery vascular endothelial function using FMD. We did not gather information regarding symptom onset. Therefore, it is possible we may have missed the ideal timeframe to assess the relationship between FMD and TNF-alpha and IL-6 especially if they peak at earlier stroke onset. VCAM-1 has been found to be involved with impaired endothelial dependent dilation in cardiovascular risk factors and disease(31, 32) and in cerebral artery vasoreactivity after stroke.(33) Our work extends these previous findings and demonstrates that VCAM-1 is related to peripheral vascular endothelial function early after stroke. Although future work is needed, VCAM-1 may be a better marker of vascular health following stroke.
There are several strengths of this study. We used FMD procedures that followed published methodological recommendations.(22) Further, the FMD procedure and blood draws were completed on the same day, following an overnight fast, and within a narrow timeframe (within 72 hours of stroke and between the hours of 7:30 – 9:00 am). While medications were withheld overnight, one study suggested that all vasoactive drugs be withheld for 24 hours.(20) Therefore, we cannot rule out the effect of some medications on the FMD response. To our knowledge, this study is the first to examine the relationship of pro-inflammatory markers to peripheral vascular endothelial function in acute stroke. Future work needs to examine the role of vascular adhesion molecules on long-term vascular health and whether pharmacological targets or lifestyle interventions could be beneficial for improving vascular endothelial function. Our prior work has shown that an 8-week aerobic exercise intervention improved both systolic blood pressure and brachial artery FMD in subacute stroke(9) but the long-term effects on cardiovascular health or future stroke remain unknown. Our study is limited by the small sample size and the results should be interpreted with caution. We also did not assess endothelial independent vasodilation, which could provide additional information related to vascular health.
Conclusion
People following an acute stroke demonstrate a lower FMD response than healthy adults without cardiovascular disease and hypertension. People during the acute stage of recovery also demonstrate between-limb differences suggesting that there may be vascular changes occurring early after stroke. The brachial artery FMD response had a strong, negative relationship with VCAM-1 but this relationship was not found with other pro-inflammatory markers. Future work is needed to determine other potential mediators for changes in vascular endothelial function and how this may affect long-term vascular health in people after stroke.
Acknowledgments
Funding: SAB is supported in part by K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. JFS and AEM were supported in part by Award Number T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. AEM was also supported in part by award number 14PRE20040026 from the American Heart Association. ASA was supported in part by funding from King Saud University. This project was supported by an Institutional Clinical and Translational Science Award, NIH/NCATS Grant Number UL1TR000001. This project was supported in part by the Kansas Intellectual and Developmental Disabilities Research Center (P30 HD002528). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. REACH laboratory space is supported by the Georgia Holland Endowment Fund.
We would like to thank Jason Gorup, Alison Boydston, and Janice Sandt for their assistance with data collection and entry. We also want to thank the participants for their time and effort on the study.
Footnotes
Declaration of Interests: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. PubMed PMID: 17452608. [DOI] [PubMed] [Google Scholar]
- 2.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160. doi: 10.1016/s0735-1097(03)00994-x. PubMed PMID: 14522472. [DOI] [PubMed] [Google Scholar]
- 3.Stenborg A, Terent A, Lind L. Endothelium-dependent vasodilatation in forearm is impaired in stroke patients. J Intern Med. 2006;259(6):569–575. doi: 10.1111/j.1365-2796.2006.01635.x. PubMed PMID: 16704557. [DOI] [PubMed] [Google Scholar]
- 4.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, et al. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88(2):145–151. doi: 10.1161/01.res.88.2.145. PubMed PMID: 11157665. [DOI] [PubMed] [Google Scholar]
- 5.Ivey FM, Gardner AW, Dobrovolny CL, Macko RF. Unilateral impairment of leg blood flow in chronic stroke patients. Cerebrovasc Dis. 2004;18(4):283–289. doi: 10.1159/000080353. PubMed PMID: 15331874. [DOI] [PubMed] [Google Scholar]
- 6.Billinger SA, Gajewski BJ, Guo LX, Kluding PM. Single limb exercise induces femoral artery remodeling and improves blood flow in the hemiparetic leg poststroke. Stroke. 2009;40(9):3086–3090. doi: 10.1161/STROKEAHA.109.550889. PubMed PMID: 19520990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billinger SA, Kluding PM. Use of Doppler Ultrasound to Assess Femoral Artery Adaptations in the Hemiparetic Limb in Chronic Stroke. Cerebrovascular Diseases. 2009;27:552–558. doi: 10.1159/000214218. [DOI] [PubMed] [Google Scholar]
- 8.Landin S, Hagenfeldt L, Saltin B, Wahren J. Muscle metabolism during exercise in hemiparetic patients. Clin Sci Mol Med. 1977;53(3):257–269. doi: 10.1042/cs0530257. PubMed PMID: 913049. [DOI] [PubMed] [Google Scholar]
- 9.Billinger SA, Mattlage AE, Ashenden AL, Lentz AA, Harter G, Rippee MA. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther. 2012;36(4):159–165. doi: 10.1097/NPT.0b013e318274d082. Epub 2012/11/01. PubMed PMID: 23111686; PubMed Central PMCID: PMC3508075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. American journal of physiology Heart and circulatory physiology. 2005;288(4):H1747–H1755. doi: 10.1152/ajpheart.00966.2004. PubMed PMID: 15576435. [DOI] [PubMed] [Google Scholar]
- 11.Bleeker MW, De Groot PC, Rongen GA, Rittweger J, Felsenberg D, Smits P, et al. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005;99(4):1293–1300. doi: 10.1152/japplphysiol.00118.2005. PubMed PMID: 15932956. [DOI] [PubMed] [Google Scholar]
- 12.Lerman A, Burnett JC., Jr Intact and altered endothelium in regulation of vasomotion. Circulation. 1992;86(6 Suppl):III12–III19. Epub 1992/12/11. PubMed PMID: 1424046. [PubMed] [Google Scholar]
- 13.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9. PubMed PMID: 12234944. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Yanez M, Castellanos M, Blanco M, Garcia MM, Nombela F, Serena J, et al. New-onset hypertension and inflammatory response/poor outcome in acute ischemic stroke. Neurology. 2006;67(11):1973–1978. doi: 10.1212/01.wnl.0000247064.53130.91. PubMed PMID: 17159103. [DOI] [PubMed] [Google Scholar]
- 15.Hassan A, Gormley K, O'Sullivan M, Knight J, Sham P, Vallance P, et al. Endothelial nitric oxide gene haplotypes and risk of cerebral small-vessel disease. Stroke. 2004;35(3):654–659. doi: 10.1161/01.STR.0000117238.75736.53. PubMed PMID: 14963277. [DOI] [PubMed] [Google Scholar]
- 16.Nadar SK, Lip GY, Lee KW, Blann AD. Circulating endothelial cells in acute ischaemic stroke. Thromb Haemost. 2005;94(4):707–712. doi: 10.1160/TH04-12-0795. PubMed PMID: 16270621. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos M, Castillo J, Garcia MM, Leira R, Serena J, Chamorro A, et al. Inflammation-mediated damage in progressing lacunar infarctions: a potential therapeutic target. Stroke. 2002;33(4):982–987. doi: 10.1161/hs0402.105339. PubMed PMID: 11935048. [DOI] [PubMed] [Google Scholar]
- 18.Chen PL, Wang PY, Sheu WH, Chen YT, Ho YP, Hu HH, et al. Changes of brachial flow-mediated vasodilation in different ischemic stroke subtypes. Neurology. 2006;67(6):1056–1058. doi: 10.1212/01.wnl.0000237526.32692.67. PubMed PMID: 17000977. [DOI] [PubMed] [Google Scholar]
- 19.Pretnar-Oblak J, Sabovic M, Sebestjen M, Pogacnik T, Zaletel M. Influence of atorvastatin treatment on L-arginine cerebrovascular reactivity and flow-mediated dilatation in patients with lacunar infarctions. Stroke. 2006;37(10):2540–2545. doi: 10.1161/01.STR.0000239659.99112.fb. PubMed PMID: 16931784. [DOI] [PubMed] [Google Scholar]
- 20.Santos-Garcia D, Blanco M, Serena J, Arias S, Millan M, Rodriguez-Yanez M, et al. Brachial arterial flow mediated dilation in acute ischemic stroke. Eur J Neurol. 2009;16(6):684–690. doi: 10.1111/j.1468-1331.2009.02564.x. Epub 2009/02/25. PubMed PMID: 19236459. [DOI] [PubMed] [Google Scholar]
- 21.Hoaglin DC, Iglewicz B, Tukey JW. Performance of Some Resistant Rules for Outlier Labeling. J Am Stat Assoc. 1986;81(396):991–999. PubMed PMID: ISI:A1986F049000018. [Google Scholar]
- 22.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American journal of physiology Heart and circulatory physiology. 2011;300(1):H2–H12. doi: 10.1152/ajpheart.00471.2010. Epub 2010/10/19. PubMed PMID: 20952670; PubMed Central PMCID: PMC3023245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson JW, Fadel PJ, Mitchell JH. New insights into central cardiovascular control during exercise in humans: a central command update. Experimental physiology. 2006;91(1):51–58. doi: 10.1113/expphysiol.2005.032037. PubMed PMID: 16239250. [DOI] [PubMed] [Google Scholar]
- 24.Mohrman DE, Heller LJ. Cardiovascular Physiology. Sixth. New York: Lange Medical Books/McGraw-Hill; 2006. p. 256. [Google Scholar]
- 25.Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001;82(6):832–839. doi: 10.1053/apmr.2001.23305. PubMed PMID: 11387591. [DOI] [PubMed] [Google Scholar]
- 26.Sunbul M, Ozben B, Durmus E, Kepez A, Pehlivan A, Midi I, et al. Endothelial dysfunction is an independent risk factor for stroke patients irrespective of the presence of patent foramen ovale. Herz. 2013;38(6):671–676. doi: 10.1007/s00059-013-3759-5. Epub 2013/02/16. PubMed PMID: 23412553. [DOI] [PubMed] [Google Scholar]
- 27.De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004;30(2):209–215. doi: 10.1002/mus.20085. PubMed PMID: 15266637. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari R, Bachetti T, Agnoletti L, Comini L, Curello S. Endothelial function and dysfunction in heart failure. Eur Heart J. 1998;19(Suppl G):G41–G47. PubMed PMID: 9717055. [PubMed] [Google Scholar]
- 29.Blum A, Miller H. Role of cytokines in heart failure. Am Heart J. 1998;135(2 Pt 1):181–186. doi: 10.1016/s0002-8703(98)70080-8. PubMed PMID: 9489963. [DOI] [PubMed] [Google Scholar]
- 30.Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83(3):376–382. doi: 10.1016/s0002-9149(98)00872-8. PubMed PMID: 10072227. [DOI] [PubMed] [Google Scholar]
- 31.Krieglstein CF, Granger DN. Adhesion molecules and their role in vascular disease. Am J Hypertens. 2001;14(6 Pt 2):44S–54S. doi: 10.1016/s0895-7061(01)02069-6. Epub 2001/06/20. PubMed PMID: 11411765. [DOI] [PubMed] [Google Scholar]
- 32.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105(5):576–582. doi: 10.1161/hc0502.103333. Epub 2002/02/06. PubMed PMID: 11827922. [DOI] [PubMed] [Google Scholar]
- 33.Tchalla AE, Wellenius GA, Travison TG, Gagnon M, Iloputaife I, Dantoine T, et al. Circulating vascular cell adhesion molecule-1 is associated with cerebral blood flow dysregulation, mobility impairment, and falls in older adults. Hypertension. 2015;66(2):340–346. doi: 10.1161/HYPERTENSIONAHA.115.05180. Epub 2015/06/10. PubMed PMID: 26056332. [DOI] [PMC free article] [PubMed] [Google Scholar]