Abstract
Cell invasion through the basement membrane (BM) occurs during normal embryonic development and is a fundamental feature of cancer metastasis. The underlying cellular and genetic machinery required for invasion has been difficult to identify, due to a lack of adequate in vivo models to accurately examine invasion in single cells at subcellular resolution. Recent evidence has documented a functional link between cell cycle arrest and invasive activity. While cancer progression is traditionally thought of as a disease of uncontrolled cell proliferation, cancer cell dissemination, a critical aspect of metastasis, may require a switch from a proliferative to an invasive state. Here we review evidence that BM invasion requires cell cycle arrest and discuss the implications of this concept with regards to limiting the lethality associated with cancer metastasis.
Keywords: cell invasion, cell cycle arrest
Linking Cell Invasion and Cell Cycle Regulation
The basement membrane (BM, see Glossary), or basal lamina, is a specialized form of extracellular matrix (see Glossary) and a metazoan innovation [1, 2] that likely helped support the evolution of the three-dimensional body plan [3, 4]. Structurally composed of polymeric laminin and cross-linked type IV collagen networks, the BM is a thin, dense, sheet-like material that provides structural support for epithelial and endothelial tissues and functions as a barrier limiting cellular movement [5]. However, specific cell types, notably those involved in embryogenesis and cancer, have evolved the ability to actively breach or cross BM barriers by adopting an invasive phenotype [5, 6] (Fig. 1).
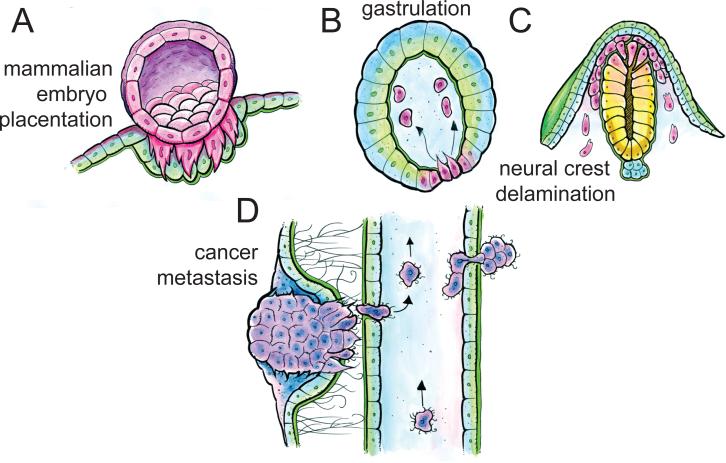
Figure 1. Cell invasion occurs during development and cancer metastasis.
(A-C). During development, cells acquire invasive phenotypes to facilitate mammalian embryo placentation (A), gastrulation in many organisms (B), and in neural crest delamination (C). (D) During cancer metastasis, cancer cells are invasive at multiple steps, including escape from the primary tumor, intra- and extravasation from the bloodstream, and establishment of a secondary tumor at a distant site.
Cell invasion is a morphogenetic behavior that results in the penetration of tissue barriers including the BM and, in vertebrates, the interstitial type I collagen from the stroma [7, 8]. Acquisition of invasive behavior requires both dynamic restructuring of the actin and microtubule cytoskeleton, along with changes in transcriptional and epigenetic states [9-11]. Cells can invade individually or collectively by maintaining cell-cell adhesions, led by highly protrusive “leader cells” [12, 13]. Invasive cells adopt either a mesenchymal or amoeboid morphology. Molecularly, mesenchymal and amoeboid invasion are defined quite differently, based on reliance of proteolytic versus ROCK/actomyosin-dependent mechanisms, respectively [13-15]. While these different invasive behaviors are usually segregated in the cancer literature, it is becoming more apparent that many invasive cells, particularly during cancer progression, are quite plastic and can adopt different morphogenetic programs based on their local environment [13, 14, 16]. For those cells that utilize a mesenchymal invasion program, the switch from epithelial to mesenchymal cell morphology is often referred to as epithelial to mesenchymal transition (EMT, see glossary), and occurs across a range of cell types throughout organismal development [17]. While defining EMT during cancer progression is more challenging [18], similar transitions between cell morphology have been documented [19]. For the purpose of this review, we refer to this morphogenetic switch in cancer as EMT.
At a cell biological level, invasion requires adhesion to and degradation of extracellular matrix components [8, 20]. While, in many systems, BM invasion is often associated with migration through the stroma, it is important to note that cells require unique genetic control mechanisms to remove the BM, independent of the genetic networks that regulate cell migration [6, 21]. The molecular and genetic mechanisms underpinning invasive cellular behavior have been challenging to elucidate. This is largely due to the difficulty of modeling this dynamic, complex behavior in vitro using artificial substrates. Fortunately, recent insights from traditional model systems including C. elegans [10, 11, 22], Drosophila [23], zebrafish [24], chick [25], and mouse [26-28] have begun to illuminate how cells breach BM in vivo.
C. elegans anchor cell (AC) invasion (see Glossary) into the vulval epithelium during nematode larval development has proved particularly useful in decoupling invasion and migration to examine invasive cellular behavior [29] (Fig. 2A). The AC, a specialized somatic gonadal cell, initiates uterine-vulval attachment by invading through the BMs separating these developing tissues [29]. As the non-motile AC maintains adhesion to neighboring uterine cells, examination of this invasive event permits separation of invasion from migratory behavior. Furthermore, researchers can visualize C. elegans AC invasion through a fluorescently labelled BM using live cell imaging [30].
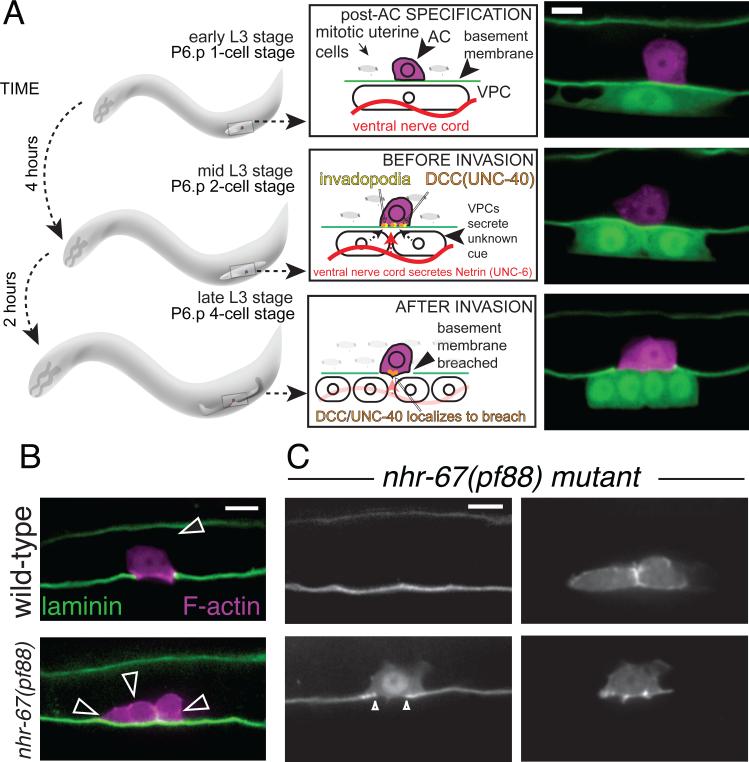
Figure 2. C. elegans anchor cell (AC) invasion into the vulval epithelium is a tractable in vivo model to examine invasion at single cell resolution in real time.
(A) During the third larval stage of C. elegans development, the AC invades in a highly stereotyped fashion. Shortly after the AC is specified (top), the invasive AC localizes invadopodia along the basolateral surface in response to extracellular cues (netrin, red, from the ventral nerve cord, and an unknown cue from the vulval cells) from the microenvironment [11] (middle). Next, the AC breaches the BM, contacting the vulval precursor cells (VPCs) and initiating the uterine-vulval connection (bottom). Spinning disc confocal images depict the AC (magenta, expressing zmp-1>mCherry) and BM, visualized by laminin::GFP (green), and 1° VPCs (green, expressing egl-17>GFP). (B) A single transcription factor, the nuclear hormone receptor, nhr-67/tlx, mediates AC invasion by maintaining the AC in a G1 cell cycle arrested state (top, left). Loss of nhr-67/tlx results in mitotic ACs that fail to invade (bottom). (C) Induced expression of cki-1 restores G1/G0 arrest and rescues invasion (middle) [9]. Scale bar, 5 μm. Images in (C) from [9].
Recent data from C. elegans AC invasion have linked cell cycle control with BM invasion [9], suggesting that invasive behavior may be functionally coupled to the proliferative states of various cell types. Specifically, the AC must be in the G1/G0 phase of the cell cycle in order to invade [9]. However, it is unclear whether G1/G0 cell cycle arrest (see Glossary) represents a general principle of all invading cells. Here, we review the potential conservation of cell cycle arrest in the invasive cascade across Metazoa, in normal and pathological states. Whether metastatic invasive cells also require discrete cell cycle control is an open question with important implications for future therapeutics designed to regulate invasive behavior during pathogenic processes.
Cell cycle regulation of invasion during development
Invasive behavior is a critical component of metazoan development. This section reviews literature that suggests that the acquisition of invasive behavior during development is specifically regulated in a cell cycle-dependent fashion. During mammalian embryo implantation (Fig. 1A), cytotrophoblasts, the first embryonic cell type to exhibit highly specialized functions, differentiate into extravillous trophoblasts, which then invade into the uterine lining, as the first step of placentation [31]. This differentiation event is regulated by several transcription factors [32] that control the expression of downstream effectors of trophoblast invasion, including adhesion molecules [33] and MMPs [34] and is required for the adoption of the invasive phenotype. To differentiate, extravillous trophoblasts exit the cell cycle in the G1 phase and upregulate cyclin dependent kinase inhibitors (CKIs, see Glossary) such as p16INK4a, p21CIP1 and p27KIP1 [35]. Whether cell cycle arrest is required for these trophoblast cells to adopt an invasive phenotype is currently unknown.
EMT is often associated with invasiveness and appears to be regulated in a cell cycle-dependent fashion [36-40]. EMT-associated cell behaviors in development and cancer progression demonstrate a strong association between loss of proliferation through downregulaton of mitotic cyclin/CDK activity and upregulation of CKIs [36, 40] (Fig. 3 and Table 1). In some animals, gastrulation proceeds through EMT-initiated cellular movements that include endomesodermal cells adopting an invasive phenotype and passing through a BM. In sea urchin (Lytechinus variegatus) gastrulation, primary mesenchyme cells cross the BM (Fig. 1B) and divide only after invading into the blastocoel [41, 42]. Similarly, during chick gastrulation, cells undergo an EMT associated with BM removal [25]. Whether invasive gastrulation movements such as these in urchins and chick, require cell cycle arrest is currently unknown.
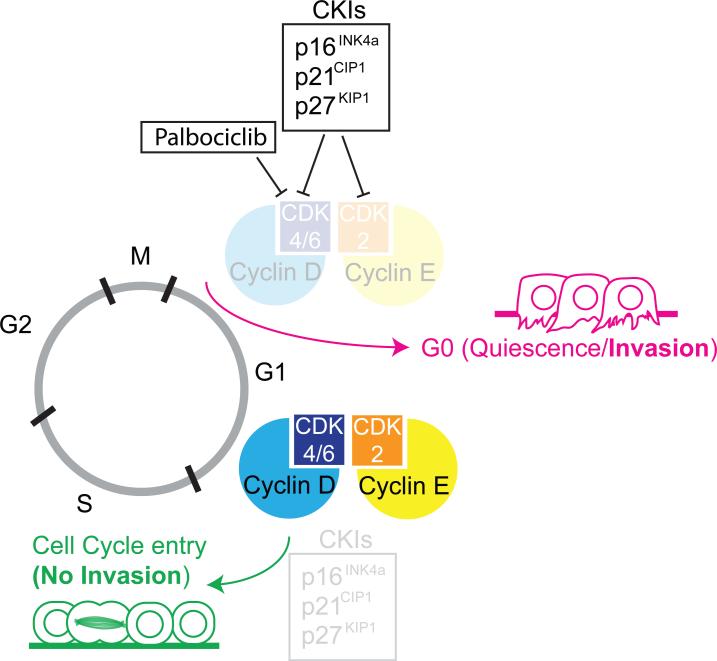
Figure 3. Cell cycle state and invasive activity.
The activity of cyclin-dependent kinase inhibitors (CKIs: p21CIP1/p27KIP1/p16INK4a) inhibit G1/S phase cyclins and cyclin dependent kinases (Cyclin D/CDK4/6 and Cyclin E/CDK2), inducing G1/G0 cell cycle arrest and promote quiescence and invasion. Reduced activity of CKIs results in increased levels of G1/S phase cyclins and CDKs, promoting cell cycle entry, preventing invasive behavior. New cancer therapeutics such as Palbociclib limit tumor growth by inducing G1/G0 cell cycle arrest by directly inhibiting CDK4/6 activity.
Table 1.
Evidence for cell cycle regulation of cancer cell invasion.
| Organ of Origin | Cancer Subtype | In vivo Assays | In vitro Assays | Findings | Ref |
|---|---|---|---|---|---|
| Melanoma | IHC of tumors for MITF and p27KIP1, tumor formation assays using SKMel28 cells. | Matrigel invasion assays performed on cell lines generated for this paper | Cell cycle arrest is associated with metastatic potential | [64] | |
| Histopathology of patient samples and Mouse xenografts of melanoma cell lines for MITF and Ki67. | Gene expression profiling and western blots performed on primary melanoma cells. | Invasive cells spend more time in G1 and there is a switch between proliferation and invasion mediated by transcriptional changes. | [65] | ||
| RNA-seq and IHC of individual human melanoma cells. | - | A sub-population of malignant melanoma cells show a non-cycling and chemoresistant signature based on transcriptome profile. | [66] | ||
| Epithelial | Multiple Types | In situ hybridization for Snail in mouse embryos, along with staining for cell death and BrdU incorporation. | Caspase 3 activity assay and western blotting of MDCK and MCA3D cells. | Proliferation and metastatic behaviors are not necessarily linked. | [36] |
| Basal Cell Carcinoma | IHC of patient tumor samples for Ki67 and p16INK4a. | - | Invasive cells are nonproliferative, express p16INK4a, and likely are in G1/G0. | [67] | |
| Epidermoid Carcinoma | - | Matrigel invasion, FACS, expression reporters, IF, ChIP of A431 cells. | After undergoing EMT, A431 cells, repress the cell cycle by blocking Cyclin D and become more invasive. | [68] | |
| Squamous Cell Carcinoma | Primary mouse keratinocytes, mouse xenograft and inducible SCC mice. Lineage tracing and metastases probed in vivo using immunofluoresence. FACS and RNA-seq performed on tumor cells. | Primary Keratinocytes and tumor cells: IHC, immunofluorescence. | TGFβ suppresses proliferation and promotes invasion in squamous cell carcinoma stem cells, through regulation of p21CIP1, leading to chemotherapeutic resistance. | [87] | |
| Others | Gastric Adenocarcinoma | - | Live cell imaging of MKN45 cells expressing FUCCI invading into a gelfoam based histoculture prep. | Invading cancer cells are predominantly in G0/G1. | [57] |
| Lung | Mouse xenografts of H460 cells, quantifying tumor growth and brachyury expression by IHC. | Matrigel invasion, IF, ChIP, FACS sorting and western blots on A549 and H460 cells. | Brachyury blocks the cell cycle by repressing p21CIP1 rendering cancer cells less sensitive to chemotherapy. | [69] | |
| Hepatocellular Carcinoma | - | AH130 cells: FACS sorting, cell cycle synchronization and in vitro invasion assays. | Cells invade in the G1 phase of the cell cycle. | [72] | |
| Breast | Microarray analysis of cells collected by invasion into microneedles or chemotaxis, and intravital imaging of MTLn3 cells as rat xenograft or PyMT mouse tumor model. | - | Invasive cells upregulate genes associated with cell cycle arrest (i.e., p21CIP1, p16INK4a), and downregulate those associated with proliferation. | [73-75] | |
| Quantification of spontaneous metastases in PyMT tumor and mouse xeongraft models. | In vitro assays including matrigel invasion and immunofluorescence (e.g. p21), using MCF-7, MCF10A and MDA-MB-231 cells. | Loss of p21CIP1 or over-expression of Cyclin E prevent metastasis by preventing state-switching between invasion and proliferation. | [76] | ||
| IHC of primary tumors for Ki67 and Cyclin D1 and Cyclin E. | FACS sorting, Matrigel invasion and live cell imaging of MDA-MB-231/435/468 cells | Decreased Cyclin D1/E makes cells more invasive, increased Cyclin D1/E makes cells more proliferative. In patient tissue samples, more Cyclin D1/E correlates to less invasion, and vice versa. | [77,78] | ||
| Quantification of spontaneous and experimental metastases from MDA-MB-231 xenograft tumors in mice. | - | Cell proliferation and invasive behavior show an inverse correlative relationship, mediated by Arg/Abl2 and CSFR1 via TGFβ signaling. | [79,80] | ||
| Colon | Carcinoma | IHC of patient samples for Ki67, p16Ink4a. | - | The invasive fronts of colon cancers are non-proliferative and express p16Ink4a. Cell cycle exit appears to be required for invasive behavior. | [81-84,86] |
| Colorectal | IHC of patient samples | ChIP on DLD-1, HCT-116, LS174T and SW480 Cells. | The proportion of cells expressing p16Ink4a at the invasive front of the tumor inversely correlates to long-term patient survival. | [85] | |
| Pancreatic | - | Matrigel invasion and gelatin degradation by secreted media performed on gamma irradiated Panc-1, Suit-2 and Hs766T cells. | Gamma irradiated cells with lower proliferative ability exhibit increased invasive potential. | [98] | |
| - | Microarray analysis, FACS and IF on AsPC-1, PANC-1 and COLO-357 cells. | The CDK4/6 inhibitor Palbociclib increases EMT and invasiveness. | [104] | ||
Note On Abbreviations. IHC: immunohistochemistry, ChIP: Chromatin Immunoprecipitation, IF: Immunofluorescence, FACS: Fluorescence-activated cell sorting, PyMT: Polyoma middle T, BrdU: Bromodeoxyuridine.
During vertebrate development, neural crest cells, a population of specialized migratory cells, give rise to melanocytes, craniofacial structures, including cartilage and bone, as well as smooth muscle, and peripheral and enteric neurons and glia. Neural crest cells undergo an EMT-like behavior as they delaminate (see Glossary), crossing the nascent BM that lies over the dorsal portion of the neural tube [43] (Fig. 1C). Trunk neural crest appears to delaminate at the G1/S phase transition [44], while the delamination of the cranial neural crest does not appear to be cell cycle-dependent [45]. Live-cell imaging with FUCCI has revealed that the majority of cranial neural crest cells following delamination are quiescent during their initial migration and show altered cell cycle dynamics dependent on their destination, with some cells rapidly proliferating and others exiting the cell cycle [46]. Unfortunately, the BM has never been visualized during live neural crest migration, making it difficult to draw conclusions related to cell cycle state as individual neural crest cells cross BM barriers.
Development and Cancer: two sides of the same coin
Cancer cells hijack developmental regulatory programs and signaling pathways to execute the suite of behaviors required for metastasis. Thus, the same morphogenetic cell biological behaviors and molecular cues that are required for developmental processes such as gastrulation and neural crest delamination during embryogenesis are also utilized by tumor cells to proliferate, communicate with the surrounding microenvironment, and adopt an invasive phenotype [47]. For processes like cell invasion, which are challenging to study in the complex in vivo environments where they occur, insights gained from the study of simple developmental systems such as C. elegans AC invasion have been helpful in elucidating general principles underlying invasive behavior.
The single AC normally exists in a post-mitotic, cell-cycle arrested state [9], where, in response to extracellular cues, F-actin and actin regulators are recruited to the basolateral surface of the AC, generating dynamic, F-actin rich, protrusive, membrane-associated, punctate structures called invadopodia (Fig. 2A) [11]. Through coordination by netrin signaling, a single invadopodium breaches the underlying BM, connecting the uterine and vulval tissues [11, 29, 30, 48]. Intrinsically, AC invasion is under the control of the conserved AP-1 transcription factor, fos-1a, which regulates the activity of the matrix metalloproteinase (MMP), zmp-1 [22]. Loss of fos-1a results in the failure of the AC to breach the BM.
What evidence exist that cancer cells and the C. elegans AC share conserved genetic programs mediating invasive behavior? First, human orthologs of pro-invasive genes that function during AC invasion (i.e., AP-1/Fos, EVI1, Netrin1 and integrins) have been shown to regulate invasion in mammalian cells [49-52]. Second, RNAi screens in C. elegans have identified novel pro-invasive genes (i.e., NLK and the CCT complex), which, when depleted in both breast and colon cancer cell lines resulted in inhibition of invasion [10]. Third, the AC utilizes invadopodia to breach the underlying BM [11, 53, 54] (Fig. 2A). Based on data from cancer cell lines and a wealth of in vitro experimental data, invadopodia have been implicated in invasive behavior, but their significance in vivo has been controversial due to the difficulties associated with resolving subcellular structures with adequate temporal resolution in complex environments where invasion occurs in vertebrates. However, recent data from C. elegans demonstrates a functional requirement for G1/G0 cell cycle arrest in the acquisition of an invasive phenotype in vivo [9].
A targeted RNA interference (RNAi) screen identified the conserved NR2E1 class nuclear hormone receptor transcription factor, nhr-67/tlx, as a novel regulator of AC invasion. Loss of nhr-67/tlx results in the normally post-mitotic AC entering the cell cycle, proliferating, and failing to invade (Fig. 2B). Live-cell imaging revealed that mitotic ACs do not localize invadopodia, nor do they express pro-invasive genes, including MMPs and F-actin regulators, suggesting that the entire invasion program is altered in cycling ACs. In support of the G1 phase of the cell cycle being critical for invasive activity, AC-specific expression of the cyclin-dependent kinase inhibitor, cki-1 (p21CIP1/p27KIP1 homolog), induces G1/G0 arrest in nhr-67-deficient ACs, and restores invadopodia formation and MMP expression, thereby rescuing invasion (Fig. 2C) [9].
If the dichotomy between invasion and proliferation were solely based on the incompatibility of cell invasive behavior and active cell division, then one could imagine that a pause in any phase of the cell cycle prior to mitosis could be permissive to invasive activity. Induced cell cycle arrest, however, in the S or G2 phase fails to rescue the invasive activity of mitotic nhr-67-deficient ACs, implicating the G1 phase of the cell cycle as critical for the acquisition of an invasive phenotype [9]. Taken together, data from mammalian embryo placentation and EMT-like behaviors during gastrulation and neural crest delamination indicate that cell cycle arrest may be important for acquisition of an invasive phenotype. These data are strongly supported by genetic and live-cell imaging data from C. elegans AC invasion that clearly defines a relationship between G1/G0 cell cycle arrest and invasion [9]. The following section will highlight the cancer biology literature that also suggests that cell cycle regulation may be required during metastatic progression.
Cell cycle regulation of BM invasion during cancer metastasis
Metastatic processes, particularly cell invasion, remain poorly understood in vivo [55], due to the difficulty of studying complex biological processes occurring deep within organisms. BM invasion is required at multiple steps in the course of metastasis: at the primary tumor, during intra- and extravasation of blood vessels, and at new tissue compartments, where they can form secondary tumors [5, 6, 8, 20] (Fig. 1D). In vitro models of invasion using native BM, such as rat peritoneal BM [56] have not yet been used to assess the cell cycle state of invasive cells. Other biological membranes, such as gelatin [57] exist, but the most prevalent model used for invasion assays is Matrigel, an extracellular matrix-like secretion of EHS sarcoma cells [58]. While Matrigel contains many of the same proteins as BM (e.g. laminin, type IV collagen and nidogen), cellular invasion through Matrigel does not necessarily correlate to invasiveness in vivo [59]. Specifically, Matrigel has been shown to be more permissive to invasion and lacks the intricate network of covalently crosslinked type IV collagen found in endogenous BM [5, 8, 59, 60]. Thus, the relationship between BM invasion and cell cycle state during cancer metastasis is poorly understood.
As cancer is primarily characterized by uncontrolled cell proliferation [61], it may initially seem counterintuitive that there exists a switch between cell proliferation and BM invasion. Nonetheless, there is mounting evidence that such a switch exists. Recent theoretical work using in silico metabolic modeling predicts that cancer cells that are able to switch to a less proliferative state when presented with physical barriers and fewer metabolic resources are more likely to not only survive but to spread more efficiently to distant sites as compared to more proliferative tumor cells [62]. Importantly, this in silico model of invasive versus proliferative cancer cell behavior is based on experimental data, and provides a framework for others to test the interplay between proliferation and invasion that likely occurs in many cancers. Here, we review the potential link between cell cycle arrest and invasive behavior. Due to the evolving nature of the field, we have based our assessment of the cell cycle state of cancer cells during invasion on multiple experimental methodologies (Table 1). The strongest experimental evidence stems from in vivo histopathological data. We have also included studies that utilize matrix invasion assays coupled with careful analysis of cell cycle state. As simultaneous studies of cell cycle and BM invasion remain challenging in living specimens, we have discussed work using measures of metastatic potential as a proxy for invasiveness. Much of the available data at present remains descriptive and correlative, thus, our ability to make definitive statements about the cell cycle state of tumor cells actively invading BM is limited by the available cancer literature.
Evidence of a switch between proliferation and invasion stems from studies performed in a wide variety of cancer types (Table 1). For cancers of epithelial origin, the ability of cancer cells to undergo EMT has long been associated with invasiveness and other metastatic characteristics (reviewed in [19]). In epithelial cell lines, as in developmental systems, the developmental bHLH transcription factors Snail and Slug have been shown to induce an EMT-like state [63]. Upon Snail-mediated induction of EMT in mammalian embryogenesis and cancer, cell cycle progression is impaired and the cell cycle is arrested in G1/G0 through increased expression of p21CIP1 [36]. In melanoma, the bHLHe32 transcription factor MITF controls the switch between proliferative and invasive states [64]. Tumor samples with high MITF expression revealed enhanced invasiveness but decreased tumor size and growth rate [65, 66]. In basal cell carcinomas, immunohistochemistry of tumors has shown that the invasive cell population is nonproliferative and expresses markers associated with G1/G0 cell cycle arrest [67]. Also, epidermoid carcinoma cell lines undergo EMT and reduce of Cyclin D1 levels, thereby enhancing invasiveness in vitro [68].
Cell cycle arrest may also be required for invasive behavior in lung cancer where pro-metastatic cell cycle arrest is mediated by the expression of p21CIP1 and modulated by the T-box transcription factor, Brachyury [69], which also drives EMT in many contexts during embryonic development [70]. Reuse of developmental transcription factors in cancer is common [61]: in osteosarcomas, the transcription factor RUNX1 drives the expression of MMP9, a pro-metastatic gene, and is specifically expressed in the G1 phase of the cell cycle [71]. Additionally, hepatocellular carcinoma cells invade during the G1 phase of the cell cycle [72]. Thus, it appears that EMT-like behavior from multiple cancer types may be linked to G1/G0 cell cycle arrest.
Perhaps, the strongest association between invasive cells and the G1/G0 cell cycle state is seen in breast cancer metastasis [73-80]. Recent, correlative evidence from polyoma virus middle T oncogene (MMTV-PyMT) organoid culture showed that proliferation is not required for the acquisition of an invasive leader cell phenotype, delineated by the presence of cytokeratin (K14)+ cells, which are found at the invasive front in all major subtypes of human breast cancer [12]. Additionally, microarray analysis of invasive mouse xenograft tumor cells isolated by invasion into microneedles uncovered gene expression associated with the G1/G0 phase of the cell cycle (i.e., p21CIP1), as well as an upregulation of genes associated with cell invasive behavior (i.e., β-catenin, and F-actin regulators such as cdc42) [73-75]. There also exists strong in vivo evidence for a proliferative to invasive switch, where direct perturbations of the cell cycle in mouse models of breast cancer revealed that the loss of p21CIP1 decreases the ability of breast cancer cells to metastasize [76]. Specifically, p21CIP1 null pyMT mammary tumors were hyperproliferative but less invasive both in vitro and in vivo [76]. As p21CIP1 functions to regulate the activity of Cyclin E (Fig. 3), human breast cancer cell lines expressing a constitutively active Cyclin E construct were also hyperproliferative and 10-fold less invasive than control tumors in a mouse xenograft model [76].
Clinically, this also appears to be the case, as primary tumors show an inverse relationship between levels of G1 and S phase cyclins (Cyclin D1 and E, Fig. 3) and the infiltration or invasiveness of the tumor [76-78]. Together, these data suggest that manipulation of CKIs and/or their target cyclins could limit invasive activity during breast cancer progression. Changes in the CKI/Cyclin/CDK axis may not be unique to breast cancer, as histopathological studies have revealed that the invasive fronts of colonic adenocarcinomas do not express the proliferative marker, Ki-67 [81-84] but do express p16INK4a [85, 86] (Fig. 3), indicating that invasive cells are disproportionately arrested in G1/G0. Additionally, it was recently shown in a squamous cell carcinoma mouse model that TGFβ signaling positively regulates transcription of p21CIP1, leading to slower cycling of the invasive stem cells and increased resistance to chemotherapy [87]. Traditionally, CKIs (p21CIP1/p27KIP1/p16INK4a) are classified as tumor suppressors, as they limit cellular growth [88-90]. However, since manipulating the CKI/Cyclin/CDK axis, which is required for cell cycle entry and exit, has profound effects on the invasive capacity of tumor cells, this raises the possibility that at least some “tumor suppressors” might function as activators of metastasis (Fig. 3).
The alternative hypothesis: invasion and proliferation are not exclusive behaviors
Contrary to the hypothesis posed above, the majority of cancer literature assumes a positive correlation between cell proliferation and cell invasive activity. However, in most cases where this relationship is examined, the positive correlation obtained is based on in vitro assays, which do not simultaneously assess BM integrity and cell cycle state. In the case of in vivo, or histopathological work, often, population level effects rather than single-cell state changes are assessed. Indeed two studies in oral squamous cell carcinoma indicated that proliferation is linked with increased invasion, based on the Ki-67 index at the invasive fronts of patient biopsies [91, 92]. Similarly, a recent in vivo study using intravital imaging of an HCT116 colorectal cancer cell line, with a FUCCI cell cycle biosensor, showed that that majority of cells infiltrating the stroma were primarily in the S/G2/M phases of the cell cycle [93]. This is in direct contrast to a study using invasive gastric adenocarcinoma cells in a gelfoam based invasion assay, where cells were observed to be predominantly in the G1/G0 phase of the cell cycle [57].
One plausible explanation for how enhanced proliferation and invasion may be linked, as proposed in the studies mentioned above, is that highly proliferative tumors could initiate invasive behavior non-cell autonomously by recruiting stromal cells to facilitate dissemination and intravasation. Indeed, cancer associated fibroblasts (CAFs) and tumor associated macrophages are well known to mediate metastasis [94-96]. In this scenario, the cell cycle state of metastatic tumor cells would be irrelevant if tumor associated immune cells were to mediate BM invasion of the cancer cells as well as subsequent stromal infiltration. This could also serve as an alternative explanation for the results shown in squamous cell carcinoma [91, 92] and colorectal cancer cell lines [93], where CAFs have been shown to be critical in facilitating metastasis [96, 97]. For example, in vitro live cell imaging through matrix has shown that SCC cells can either follow directly behind CAFs or utilize tracks made by CAFs during their collective invasion, but are unable to invade without CAF assistance [96]. Although increased proliferation is a characteristic of CAFs, the cell cycle state of individual CAFs or macrophages during tumor dissemination and intravasation is currently unknown. Since cancer does not represent a single disease, but rather a myriad of many different disorders [61], it is possible that some cancers develop the ability to invade BM and proliferate simultaneously, either through the cooption of the host cells’ invasive abilities, or through the acquisition of invasion on their own as the result of currently unknown genetic and/or epigenetic mechanisms. Regardless, these conflicts in the literature highlight the importance that should be placed on the development of new models to directly assess BM invasion and cell cycle state in both cancer cells and the surrounding microenvironment at the onset of metastatic behavior.
Therapeutic implications of cell cycle regulation of invasive behavior
Traditional antineoplastic chemotherapeutics kill rapidly dividing cells. However, since invasive cells appear to exist in quiescent G1/G0 arrest (Table 1), these invasive, metastatic cell populations remain when the bulk of the tumor is killed by classical antineoplastics. For example, sublethal irradiation, which blocks the G1/S phase checkpoint, increases the metastatic potential of gliomas [98]. Further research is necessary to determine if cell cycle arrest triggered by sublethal doses of DNA damaging antineoplastic treatments can drive metastatic behaviors as well.
In 2015, the first antineoplastic chemotherapeutic drug to directly target the cell cycle, Palbociclib (PD-0332991) was approved by the FDA for use in breast cancer treatment [99] and is currently being used in clinical trials to target other cancers [100]. Palbociclib and other drugs are inhibitors of CDK4/6, the G1/S phase transition checkpoint [101] (Fig. 3). Experimental inhibition of the G1/S phase transition through genetic mechanisms such as overexpression of p21CIP1 [102, 103] or high levels of p16INK4a [85] have led to increased metastatic characteristics. As Palbociclib similarly blocks cell cycle progression at the G1/S phase transition, this raises the possibility that it may also drive invasive behavior. Notably, in pancreatic cancer cell lines, Palbociclib is sufficient to induce EMT and drive an increase in Matrigel invasion [104]. Therefore, while antineoplastic chemotherapeutic drugs targeting the G1 phase of the cell cycle show great promise, more work must be done to ensure that treatment regimens do not inadvertently drive metastatic progression by facilitating invasive cell behavior by inducing G1/G0 cell cycle arrest.
Concluding Remarks
We have reviewed literature that demonstrates that a broad array of cancers switch between invasive and proliferative states, with evidence ranging from correlative Matrigel invasion assays to histopathological studies of primary tumor samples (Table 1). Together, these data argue that cell cycle arrest may be a requirement for the acquisition of invasive activity. Given recent functional data from a developmental invasion event in C. elegans, we suggest that G1/G0 phase cell cycle arrest may be required broadly to properly execute invasive behavior.
In spite of this mounting evidence, our mechanistic understanding of the relationship between cell cycle control and cell invasion remains limited due to a lack of tools to accurately visualize BM in vitro or in vivo while assaying cell cycle state (see Outstanding Questions). Future use of microfluidics to analyze cells at single resolution in vitro [105] paired with advanced imaging modalities, including light sheet microscopy [106] and dynamic cell cycle biosensors [107, 108] will hopefully provide a more accurate assessment of cell cycle state during invasion. These same advanced imaging techniques, particularly light sheet [109] and two photon microscopy [110], allow for long term vital imaging [27, 111-113] at cellular and subcellular resolution in vivo. Going forward, better in vivo models are needed. Genome editing combined with improved microscopy should allow simultaneous visualization of labeled BM and invasive cells at single cell resolution. These new models could be paired with functional cell cycle perturbations, across multiple cancer cell types, revealing if the many disparate observations illustrated here represent a deeply conserved evolutionary principle underlying cell invasive behavior between organisms that last shared a common ancestor over 500 million years ago.
Outstanding Questions.
Can individual cells switch between invasive and proliferative states and if so, what are the autonomous and environmental signals that dictate the ability to transition between these states?
Why is the G1/G0 state associated with cell invasive behavior? This review highlights the many cases in different cancers that show correlation between quiescence in G1/G0 and increased invasiveness.
How can we ensure that cancer therapeutics which promote G1/G0 cell cycle arrest do not inadvertently select for invasive cellular behavior?
Can we create better in vivo models that will allow for single cell visual analyses paired with cell cycle perturbations and live imaging of basement membrane invasion? This will allow for further exploration of this dichotomy between proliferative and invasive cellular states.
Trends.
Cell invasive behavior is critical during development and is dysregulated in disease states, including cancer metastasis.
The ability to adopt an invasive phenotype and breach a mechanical barrier such as the basement membrane may be regulated in a cell cycle-dependent fashion. This underlies a dichotomy between cell proliferation and cell invasion.
Invasion occurs primarily in a G1/G0 cell cycle-arrested state, and expression of pro-invasive genes driving EMT and F-actin cytoskeletal reorganization are associated with this cell cycle state.
Changes in the activity of cyclin dependent kinase inhibitors (CKIs) and their target cyclins and cyclin dependent kinases (CDKs) not only mediate the decision to enter or exit the cell cycle, but may be critical to acquiring an invasive phenotype.
Therapeutics which cause G1/G0 arrest, such as palbociclib, show great promise, but further research is required to ensure that these drugs do not inadvertently drive metastatic cancer progression.
Acknowledgements
The authors would like to thank Mathieu Boissan, R. Antonio Herrera and Taylor Medwig for their feedback, as well as the two anonymous reviewers, whose comments greatly improved the manuscript. We also thank Taylor Medwig for confocal micrographs used in Figure 1. D.Q.M. is supported by the Carol M. Baldwin Foundation for Breast Cancer Research and by the National Institute of Health, National Cancer Institute R00-CA154870.
Glossary
- Basement Membrane
A dense highly crosslinked sheet of polymeric laminin and type IV collagen forming the substrate for endo- and epithelia and providing a barrier function for most cells
- Basement Membrane Invasion
The process by which cells remove basement membrane allowing contact between cell layers or passage through the basement membrane
- C. elegans anchor cell invasion
An in vivo model system used to examine the process of basement membrane invasion, as a specialized somatic gonad cell, the anchor cell (AC), breaches the underlying basement membrane to initiate uterine-vulval contact, allowing worms to passage eggs to the external environment
- Cyclin dependent kinase inhibitors (CKIs)
A family of conserved eukaryotic proteins (p16INK4a, p21CIP1, p27KIP1 in vertebrates) that inhibit the activity of G1/S phase cyclins (Cyclins D and E) and cyclin dependent kinases (CDK4/6 and CDK2)
- Delamination
The process by which cells leave an epithelium to migrate elsewhere, or form a new epithelial layer. This process is often coupled with epithelial to mesenchymal transition (EMT)
- Epithelial to mesenchymal transition (EMT)
The morphogenetic process by which cells switch from an epithelial to mesenchymal morphology occurring during development and cancer metastasis
- Extracellular Matrix
The scaffolding of proteins supporting and surrounding metazoan cells
- G1/G0 cell cycle arrest
A quiescent cellular state that occurs following mitosis, where a cell either temporarily pauses, prior to entering S phase (G1) or permanently arrests (G0). As these cellular states can be difficult to distinguish, we will refer to this arrest point as G1/G0 arrest for the purposes of this review
- Invasion
The morphogenetic process by which cells penetrate the basement membrane and, in vertebrates, remodel the extracellular matrix-derived stroma
- Migration
The process by which cells move from place to place
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matus DQ, et al. Cell division and targeted cell cycle arrest opens and stabilizes basement membrane gaps. Nature communications. 2014;5:13. doi: 10.1038/ncomms5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozbek S, et al. The evolution of extracellular matrix. Molecular biology of the cell. 2010;21:4300–4305. doi: 10.1091/mbc.E10-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doljanski F. The sculpturing role of fibroblast-like cells in morphogenesis. Perspect Biol Med. 2004;47:339–356. doi: 10.1353/pbm.2004.0048. [DOI] [PubMed] [Google Scholar]
- 5.Rowe R, Weiss S. Breaching the basement membrane: who, when and how? Trends in Cell Biology. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Kelley LC, et al. Traversing the basement membrane in vivo: A diversity of strategies. The Journal of cell biology. 2014;204:291–302. doi: 10.1083/jcb.201311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 8.Glentis A, et al. Assembly, heterogeneity, and breaching of the basement membranes. Cell adhesion & migration. 2014;8:236–245. doi: 10.4161/cam.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matus DQ, et al. Invasive Cell Fate Requires G1 Cell-Cycle Arrest and Histone Deacetylase-Mediated Changes in Gene Expression. Developmental cell. 2015;35:162–174. doi: 10.1016/j.devcel.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matus DQ, et al. In Vivo Identification of Regulators of Cell Invasion Across Basement Membranes. Science Signaling. 2010;3:9. doi: 10.1126/scisignal.2000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagedorn EJ, et al. The netrin receptor DCC focuses invadopodia-driven basement membrane transmigration in vivo. The Journal of cell biology. 2013;201:903–913. doi: 10.1083/jcb.201301091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung KJ, et al. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Wolf K, et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. The Journal of cell biology. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabeh F, et al. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. The Journal of cell biology. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeger A, et al. Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 2015;25:556–566. doi: 10.1016/j.tcb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Revenu C, Gilmour D. EMT 2.0: shaping epithelia through collective migration. Current opinion in genetics & development. 2009;19:338–342. doi: 10.1016/j.gde.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675–686. doi: 10.1016/j.tcb.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahai E. Mechanisms of cancer cell invasion. Current opinion in genetics & development. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Schaeffer D, et al. Cellular migration and invasion uncoupled: increased migration is not an inexorable consequence of epithelial-to-mesenchymal transition. Molecular and cellular biology. 2014;34:3486–3499. doi: 10.1128/MCB.00694-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherwood DR, et al. FOS-1 Promotes Basement-Membrane Removal during Anchor-Cell Invasion in. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava A, et al. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiler C, et al. Smooth muscle tension induces invasive remodeling of the zebrafish intestine. PLoS biology. 2012;10:e1001386. doi: 10.1371/journal.pbio.1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakaya Y, et al. RhoA and microtubule dynamics control cell–basement membrane interaction in EMT during gastrulation. Nature Cell Biology. 2008;10:765–775. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 26.Hiramatsu R, et al. External Mechanical Cues Trigger the Establishment of the Anterior-Posterior Axis in Early Mouse Embryos. Developmental cell. 2013;27:131–144. doi: 10.1016/j.devcel.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Kedrin D, et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philippar U, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Developmental cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Developmental cell. 2003;5:21–31. doi: 10.1016/s1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 30.Ziel JW, et al. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nature Cell Biology. 2009;11:183–189. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Red-Horse K, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janatpour MJ, et al. Id-2 regulates critical aspects of human cytotrophoblast differentiation, invasion and migration. Development (Cambridge, England) 2000;127:549–558. doi: 10.1242/dev.127.3.549. [DOI] [PubMed] [Google Scholar]
- 33.Damsky CH, et al. Integrin switching regulates normal trophoblast invasion. Development (Cambridge, England) 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 34.Librach CL, et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. The Journal of cell biology. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genbacev O, et al. A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am J Pathol. 2000;157:1337–1351. doi: 10.1016/S0002-9440(10)64648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega S, et al. Snail blocks the cell cycle and confers resistance to cell death. Genes & development. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran DD, et al. Temporal and spatial cooperation of Snail1 and Twist1 during epithelial-mesenchymal transition predicts for human breast cancer recurrence. Mol Cancer Res. 2011;9:1644–1657. doi: 10.1158/1541-7786.MCR-11-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grassi ML, et al. Proteomic analysis of ovarian cancer cells during epithelial-mesenchymal transition (EMT) induced by epidermal growth factor (EGF) reveals mechanisms of cell cycle control. J Proteomics. 2016 doi: 10.1016/j.jprot.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Iordanskaia T, Nawshad A. Mechanisms of transforming growth factor beta induced cell cycle arrest in palate development. J Cell Physiol. 2011;226:1415–1424. doi: 10.1002/jcp.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders LR, McClay DR. Sub-circuits of a gene regulatory network control a developmental epithelial-mesenchymal transition. Development (Cambridge, England) 2014;141:1503–1513. doi: 10.1242/dev.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyons DC, et al. Morphogenesis in sea urchin embryos: linking cellular events to gene regulatory network states. Wiley Interdiscip Rev Dev Biol. 2012;1:231–252. doi: 10.1002/wdev.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clay MR, Halloran MC. Rho activation is apically restricted by Arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development (Cambridge, England) 2013;140:3198–3209. doi: 10.1242/dev.095448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burstyn-Cohen T, Kalcheim C. Association between the cell cycle and neural crest delamination through specific regulation of G1/S transition. Developmental cell. 2002;3:383–395. doi: 10.1016/s1534-5807(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 45.Theveneau E, et al. Ets-1 confers cranial features on neural crest delamination. PLoS One. 2007;2:e1142. doi: 10.1371/journal.pone.0001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridenour DA, et al. The neural crest cell cycle is related to phases of migration in the head. Development (Cambridge, England) 2014;141:1095–1103. doi: 10.1242/dev.098855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aiello NM, Stanger BZ. Echoes of the embryo: using the developmental biology toolkit to study cancer. Dis Model Mech. 2016;9:105–114. doi: 10.1242/dmm.023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, et al. UNC-6 (netrin) stabilizes oscillatory clustering of the UNC-40 (DCC) receptor to orient polarity. The Journal of cell biology. 2014;206:619–633. doi: 10.1083/jcb.201405026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnston IM, et al. Regulation of a multigenic invasion programme by the transcription factor, AP-1: re-expression of a down-regulated gene, TSC-36, inhibits invasion. Oncogene. 2000;19:5348–5358. doi: 10.1038/sj.onc.1203927. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, et al. Netrin-1 signaling mediates NO-induced glial precursor migration and accumulation. Cell Res. 2010;20:238–241. doi: 10.1038/cr.2010.7. [DOI] [PubMed] [Google Scholar]
- 51.Dumartin L, et al. Netrin-1 Mediates Early Events in Pancreatic Adenocarcinoma Progression, Acting on Tumor and Endothelial Cells. Gastroenterology. 2010;138:1595–1606. e1598. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 52.White DE, Muller WJ. Multifaceted roles of integrins in breast cancer metastasis. Journal of mammary gland biology and neoplasia. 2007;12:135–142. doi: 10.1007/s10911-007-9045-5. [DOI] [PubMed] [Google Scholar]
- 53.Hagedorn EJ, et al. ADF/cofilin promotes invadopodial membrane recycling during cell invasion in vivo. The Journal of cell biology. 2014;204:1209–1218. doi: 10.1083/jcb.201312098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohmer LL, et al. A Sensitized Screen for Genes Promoting Invadopodia Function In Vivo: CDC-42 and Rab GDI-1 Direct Distinct Aspects of Invadopodia Formation. PLoS Genet. 2016;12:e1005786. doi: 10.1371/journal.pgen.1005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schoumacher M, et al. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. The Journal of cell biology. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yano S, et al. Invading cancer cells are predominantly in G0/G1 resulting in chemoresistance demonstrated by real-time FUCCI imaging. Cell Cycle. 2014;13:953–960. doi: 10.4161/cc.27818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benton G, et al. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. International journal of cancer. 2011;128:1751–1757. doi: 10.1002/ijc.25781. [DOI] [PubMed] [Google Scholar]
- 59.Noel AC, et al. Invasion of reconstituted basement membrane matrix is not correlated to the malignant metastatic cell phenotype. Cancer research. 1991;51:405–414. [PubMed] [Google Scholar]
- 60.Vukicevic S, et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- 61.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Hecht I, et al. The motility-proliferation-metabolism interplay during metastatic invasion. Sci Rep. 2015;5:13538. doi: 10.1038/srep13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye X, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carreira S, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes & development. 2006;20:3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoek KS, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer research. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 66.Tirosh I, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svensson S, et al. Invade or proliferate? Two contrasting events in malignant behavior governed by p16(INK4a) and an intact Rb pathway illustrated by a model system of basal cell carcinoma. Cancer research. 2003;63:1737–1742. [PubMed] [Google Scholar]
- 68.Mejlvang J, et al. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Molecular biology of the cell. 2007;18:4615–4624. doi: 10.1091/mbc.E07-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang B, et al. The embryonic transcription factor Brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis. 2013;4:e682. doi: 10.1038/cddis.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner DA, et al. Brachyury cooperates with Wnt/beta-catenin signalling to elicit primitive-streak-like behaviour in differentiating mouse embryonic stem cells. BMC Biol. 2014;12:63. doi: 10.1186/s12915-014-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baniwal SK, et al. Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis. Mol Cancer. 2010;9:258. doi: 10.1186/1476-4598-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwasaki T, et al. Cell-cycle-dependent invasion in vitro by rat ascites hepatoma cells. International journal of cancer. 1995;63:282–287. doi: 10.1002/ijc.2910630223. [DOI] [PubMed] [Google Scholar]
- 73.Wang W, et al. Coordinated Regulation of Pathways for Enhanced Cell Motility and Chemotaxis Is Conserved in Rat and Mouse Mammary Tumors. Cancer research. 2007;67:3505–3511. doi: 10.1158/0008-5472.CAN-06-3714. [DOI] [PubMed] [Google Scholar]
- 74.Wang W, et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer research. 2004;64:8585–8594. doi: 10.1158/0008-5472.CAN-04-1136. [DOI] [PubMed] [Google Scholar]
- 75.Goswami S. Breast Cancer Cells Isolated by Chemotaxis from Primary Tumors Show Increased Survival and Resistance to Chemotherapy. Cancer research. 2004;64:7664–7667. doi: 10.1158/0008-5472.CAN-04-2027. [DOI] [PubMed] [Google Scholar]
- 76.Qian X, et al. p21CIP1 mediates reciprocal switching between proliferation and invasion during metastasis. Oncogene. 2013;32:2292–2303. 2303, e2291–2297. doi: 10.1038/onc.2012.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehn S, et al. Down-regulation of the oncogene cyclin D1 increases migratory capacity in breast cancer and is linked to unfavorable prognostic features. Am J Pathol. 2010;177:2886–2897. doi: 10.2353/ajpath.2010.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berglund P, et al. Cyclin E overexpression obstructs infiltrative behavior in breast cancer: a novel role reflected in the growth pattern of medullary breast cancers. Cancer research. 2005;65:9727–9734. doi: 10.1158/0008-5472.CAN-04-3984. [DOI] [PubMed] [Google Scholar]
- 79.Gil-Henn H, et al. Arg/Abl2 promotes invasion and attenuates proliferation of breast cancer in vivo. Oncogene. 2013;32:2622–2630. doi: 10.1038/onc.2012.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patsialou A, et al. Autocrine CSF1R signaling mediates switching between invasion and proliferation downstream of TGFbeta in claudin-low breast tumor cells. Oncogene. 2015;34:2721–2731. doi: 10.1038/onc.2014.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubio CA. Arrest of cell proliferation in budding tumor cells ahead of the invading edge of colonic carcinomas. A preliminary report. Anticancer Res. 2008;28:2417–2420. [PubMed] [Google Scholar]
- 82.Rubio CA. Difference in cell proliferation between two structurally different lesions in colorectal adenomas: high-grade dysplasia and carcinoma in situ. Anticancer Res. 2007;27:4321–4324. [PubMed] [Google Scholar]
- 83.Rubio CA. Further studies on the arrest of cell proliferation in tumor cells at the invading front of colonic adenocarcinoma. J Gastroenterol Hepatol. 2007;22:1877–1881. doi: 10.1111/j.1440-1746.2007.04839.x. [DOI] [PubMed] [Google Scholar]
- 84.Rubio CA. Cell proliferation at the leading invasive front of colonic carcinomas. Preliminary observations. Anticancer Res. 2006;26:2275–2278. [PubMed] [Google Scholar]
- 85.Wassermann S, et al. p16INK4a is a beta-catenin target gene and indicates low survival in human colorectal tumors. Gastroenterology. 2009;136:196–205. e192. doi: 10.1053/j.gastro.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 86.Jung A, et al. The invasion front of human colorectal adenocarcinomas shows co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A and is a region of low proliferation. Am J Pathol. 2001;159:1613–1617. doi: 10.1016/s0002-9440(10)63007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oshimori N, et al. TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell. 2015;160:963–976. doi: 10.1016/j.cell.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fero ML, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 90.Romagosa C, et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30:2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 91.Tumuluri V, et al. The relationship of proliferating cell density at the invasive tumour front with prognostic and risk factors in human oral squamous cell carcinoma. J Oral Pathol Med. 2004;33:204–208. doi: 10.1111/j.0904-2512.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 92.Dissanayake U, et al. Comparison of cell proliferation in the centre and advancing fronts of oral squamous cell carcinomas using Ki-67 index. Cell Prolif. 2003;36:255–264. doi: 10.1046/j.1365-2184.2003.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kagawa Y, et al. Cell cycle-dependent Rho GTPase activity dynamically regulates cancer cell motility and invasion in vivo. PLoS One. 2013;8:e83629. doi: 10.1371/journal.pone.0083629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaggioli C. Collective invasion of carcinoma cells: when the fibroblasts take the lead. Cell adhesion & migration. 2008;2:45–47. doi: 10.4161/cam.2.1.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harney AS, et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015;5:932–943. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 97.Hawinkels LJ, et al. Interaction with colon cancer cells hyperactivates TGF-beta signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107. doi: 10.1038/onc.2012.536. [DOI] [PubMed] [Google Scholar]
- 98.Qian LW, et al. Radiation-induced increase in invasive potential of human pancreatic cancer cells and its blockade by a matrix metalloproteinase inhibitor, CGS27023. Clin Cancer Res. 2002;8:1223–1227. [PubMed] [Google Scholar]
- 99.Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 100.O'Leary B, et al. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 101.Toogood PL, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48:2388–2406. doi: 10.1021/jm049354h. [DOI] [PubMed] [Google Scholar]
- 102.Hu H, et al. Antibiotic drug tigecycline inhibits melanoma progression and metastasis in a p21CIP1/Waf1-dependent manner. Oncotarget. 2016;7:3171–3185. doi: 10.18632/oncotarget.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dai M, et al. A novel function for p21Cip1 and acetyltransferase p/CAF as critical transcriptional regulators of TGFbeta-mediated breast cancer cell migration and invasion. Breast Cancer Res. 2012;14:R127. doi: 10.1186/bcr3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu F, Korc M. Cdk4/6 inhibition induces epithelial-mesenchymal transition and enhances invasiveness in pancreatic cancer cells. Mol Cancer Ther. 2012;11:2138–2148. doi: 10.1158/1535-7163.MCT-12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Powell AA, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One. 2012;7:e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao L, et al. 3D live fluorescence imaging of cellular dynamics using Bessel beam plane illumination microscopy. Nat Protoc. 2014;9:1083–1101. doi: 10.1038/nprot.2014.087. [DOI] [PubMed] [Google Scholar]
- 107.Sakaue-Sawano A, et al. Visualizing Spatiotemporal Dynamics of Multicellular Cell-Cycle Progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 108.Spencer SL, et al. The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell. 2013;155:369–383. doi: 10.1016/j.cell.2013.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu Q, et al. Imaging multicellular specimens with real-time optimized tiling light-sheet selective plane illumination microscopy. Nature communications. 2016;7:11088. doi: 10.1038/ncomms11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weigert R, et al. Imaging cell biology in live animals: ready for prime time. The Journal of cell biology. 2013;201:969–979. doi: 10.1083/jcb.201212130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Entenberg D, et al. subcellular resolution optical imaging in the lung reveals early metastatic proliferation and motility. Intravital. 2015:4. doi: 10.1080/21659087.2015.1086613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manning CS, et al. Intravital imaging of SRF and Notch signalling identifies a key role for EZH2 in invasive melanoma cells. Oncogene. 2015;34:4320–4332. doi: 10.1038/onc.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zomer A, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161:1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]