Abstract
Background:
Dirofilaria immitis and Dirofilaria repens are the most common species of filarial nematodes described in the dogs. A single-step multiplex PCR was applied to detect and differentiate simultaneously and unequivocally D. immitis and D. repens on DNA extracted from canine peripheral blood and besides to detect the seroprevalance of D. immitis by ELISA in Elazig Province, Turkey. A PCR detection of the Wolbachia, which plays an important role in D. immitis biology and contributes to the inflammatory pathology of the heartworm, was also applied for the first time in Turkey.
Methods:
A total of 161 whole blood and sera samples were collected from stray dogs and stored at –20 °C until used. After DNA extraction, all samples were processed with Dirofilaria primers by multiplex-PCR and Wolbachia primers by conventional PCR besides ELISA for serology. The amplification was performed using a set of primers designed on a portion of the small subunit ribosomal RNA gene of the mitochondrion (12S rDNA).
Results:
Three of the examined dogs (1.8%) were found to be infected with only D. immitis, one (0.6%) with D. repens and three (1.8%) with both parasites. Besides, 10 out of 161 dogs (6.2%) were found infected with Wolbachia sp. Finaly, the seroprevalence of dirofilariosis in the examined dogs was found to be 3.7% (6/161).
Conclusion:
Although dirofilariosis is not a serious problem in the region, the stray dogs still continue to be a source of infection.
Keywords: Dirofilaria immitis, Dirofilaria repens, Wolbachia, Multiplex-PCR, ELISA
Introduction
Dirofilariosis, caused by Dirofilaria immitis, is found world-wide, but the most endemic areas are those with high temperatures and appropriate mosquito vector populations. Dirofilaria immitis typically inhabits the right ventricle and pulmonary arteries of dogs. “This vector-borne parasite can cause patent infections in dogs, cats and wild canidae” (Dillon 2000). It is one of the most pathogenic nematode parasite of dogs. Adult heartworms may cause clinical signs ranging from mild cough to congestive heart failure, intravascular hemolysis and pulmonary thromboembolism which are often fatal if untreated (Soulsby 1986). Dirofilaria immitis in dogs can be diagnosed through careful morphological examination of circulating microfilariae, detection of circulating antigens, histochemical or immuno-histochemical staining of circulating microfilariae or, more recently, through molecular approaches. Morphological identification of circulating microfilariae, however, is not always easy and is potentially misleading (Rishniw et al. 2006). Dirofilaria repens, a filarial parasite of canids, is transmitted by mosquitoes. The adult worms are observed mainly in the subcutaneous tissue of dogs, and produce microfilariae that circulate in the blood stream of infected dogs. Dignosis of it can be done by blood smear evaluation for the presence of microfilariae, serologic detection antigen or antibodies and detection of microfilarial DNA by PCR (Lee et al. 2004).
Dirofilaria immitis is transmitted by several culicid mosquito species belonging to a wide range of genera, including Culex, Aedes, Ochlerotatus, Anopheles, Armigeres and Mansonia (Cancrini et al. 1995). Aedes vexans and Culex pipiens were detected as the potential vectors of D. immitis in Turkey (Yildirim et al. 2011). For the first time, cytochrome c oxidase I (COI) sequences were obtained from Iranian specimens of An. hyrcanus, An. pseudopictus, Cx. theileri and Oc. caspius s.l. Only Culex theileri were found naturally infected with third-stage (infective) larvae of D. immitis (Azari-Hamidian et al. 2009).
DNA-based diagnostic tests for D. immitis and D. repens infections have been shown to overcome some deficiencies of parasitological and serological diagnosis, and specific and sensitive polymerase chain reaction (PCR)-based assays have been reported (Mar et al. 2002, Rishniw et al. 2006). The usefulness of different PCR methods for the identification of Dirofilaria spp microfilaria in dog blood (the definitive host) has been reported in recent publications (Gioia et al. 2010, Simsek et al. 2011, Giangaspero et al. 2012, Latrofa et al. 2012).
Dirofilaria immitis is one of the several species of parasitic nematodes that hold the obligate symbiont bacteria Wolbachia spp. large colonies of Wolbachia live in the subdermal lateral cords of both female and male nematodes, as well as in the reproductive structures of females (McHaffie 2012).
The aim of the current study was to performe a single-step multiplex PCR to detect and differentiate D. immitis and D. repens on genomic DNA isolated from dog blood and also detect the seroprevalance of D. immitis by ELISA. The amplification was performed using a set of primers designed on a portion of the small subunit ribosomal RNA gene of the mitochondrion (12S rDNA). The other aim of this work was to PCR detection of the Wolbachia which is play an important role in D. immitis biology and contributes to the inflammatory pathology of the heart-worm.
Materials and Methods
Samples collection
A total of 161 whole blood and sera samples were obtained from stray dogs in Elazig Province of eastern Turkey within 2010. These dogs had been captured from suburbs by the local authorized for the aim of spaying and during this procedure the blood samples were acquired under anesthesia. The blood and sera samples were stored in –20 °C untill use and age, breed and genders were recorded.
DNA (gDNA) Isolation, PCR amplification and sequencing
The blood samples were removed from freezer and waited at room temperature untill thawed. Then 1 ml blood sample was putted into an eppendorf tube and centrifuged during 5 min by 5000 rpm for sink to the bottom of possible microfilaria. Supernatant was removed and prior to gDNA isolation pellet was digested overnight at 56 °C with 600 μl lysis buffer of the kit to which 20 μl Proteinase-K (20 mg/ml) (Sigma, USA) were added. The tubes were incubated at 56 °C for overnight and the kit procedure was followed and at the last step the pellet was resuspended in 80 μl sterile distilled water, and the gDNA samples were stored at –20 °C until use.
The multiplex-PCR reactions for D. immitis and D. repens were performed using two sets of primer in the same reaction. General primer pairs 12SF (5’-GTTCCAGAATAATCGGCTA-3’) and 12SRdeg (5’-ATTGACGGATG(AG)TTTGTACC-3’) were used previously designed on the 12S rDNA region (Casiraghi et al. 2004). Besides we used a specific forward primer for D. immitis (12SF2B 5’-TTTTTACTTTTTTGGTAATG-3’) and a specific reverse primer for D. repens (12SR2 5’-AAAAGCAACACAAATAA (CA)A-3’) previously designed by Gioia et al. (2010). The PCR reactions were carried out in a total volume of 50 μl containing 5 μl of genomic DNA for each sample amplification, 5 μl of MgCl2, 1.25 mM of each dNTP, 5 μl 10 X PCR buffer, 0.5 IU Taq DNA polymerase and 20 pmol of each primers. The thermal profile used was 92 °C for 1 min; 40 cycles of 92 °C for 30 s, 52 °C for 45 s, 72 °C for 1 min and final elongation step at 72 °C for 10 min. The amplified products were separated by electrophoresis in 2% agarose gel with a Tris-boric acid–EDTA (TBE, pH 8.3) buffer at 90 V for 45 min. Following electrophoresis, the amplified products were visualized with ethidium bromide (0,5 μg/ml) staining for 45 min at room temperature. Dirofilaria immitis genomic DNA positive control sample was extracted from microfilariae present in the blood of infected dogs (gifted from another research group) (Yildirim et al. 2007). Another gDNA control sample was extracted from an adult D. repens parasite (this worm was gifted by Luigi Venco (Veterinary Hospital “Citt`a di Pavia”, Viale Cremona Pavia, Italy).
Extracted DNA was also tested for the presence of Wolbachia using a PCR-based assay and the gene primer wsp. A specific primer sets (Forward 5′-TGGTCCAATAA GTGATGAAG AAACTAGCTA-3′, reverse 5′-AAAATTAAACGCTACTCCAGCTTCT GCAC-3′) previously described by Zhou et al. (1998) were used for the amplification of gDNA. The PCR mixtures were composed of 5 μl of 10X PCR buffer, 5 μl of MgCl2, 125 μM of each dNTPs, 20 pmol of each primers, 0.2 μl (5 IU) Taq-DNA Polymerase and 5 μl of genomic DNA was used for each PCR reaction. The reactions were performed on a PCR thermal cycler (Thermo Electron Corporation, Waltham, MA, USA) under the following conditions: 94 °C for 3 min, 40 cycles of 94 °C for 1 min, 52 °C for 1 min and 72 °C for 1 min with a final extension at 72 °C for 5 min. PCR products were analyzed on 1.4% agarose gels stained by ethidium bromide and visualized under ultra-violet light.
Randomly selected six Dirofilaria and two Wolbachia samples were sequenced for confirmation of the PCR results.
Serological Analysis
Filarcheck (Agrolab, Italy) kit was used for working the dog sera for serological analysis. The test is based on a sandwich ELISA technique. Microplate wells were coated with a monoclonal antibody against the circulating antigen of D. immitis. Canine serum was added into the wells. If the serum contained the antigen, wells gave blue colour otherwise colorless.
Statistical Analysis
The data were evaluated by SPSS 15.0 programme using of 2X2 Fischer's Exact test and Pearson's Chi square test.
Results
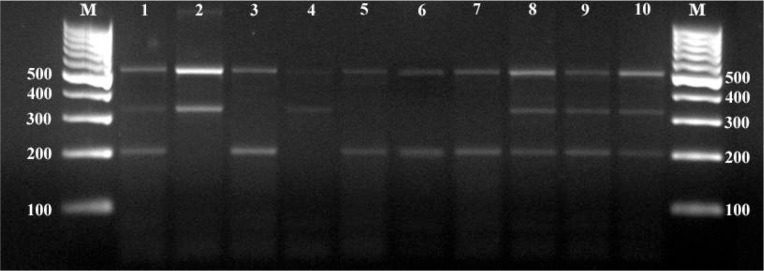
Multiplex-PCR reaction showed the expected amplification products of approximately 500 bp for the genus Dirofilaria, 327 bp for D. repens and of 204 bp for D. immitis. (Fig. 1). Wolbachia surface protein PCR amplified 630 bp band as shown in Fig. 2.
Fig. 1.
Multiplex-PCR bands of samples. M: Molecular weight marker (100 bp), 1: Positive control of mix infection (500 bp, 327 bp and 204 bp), 2: Positive control of Dirofilaria repens (500 bp and 327 bp), 3: Positive control of Dirofilaria immitis (500 bp and 204 bp), 4: Only D. repens detected sample, 5, 6, 7: Only D. immitis detected samples, 8, 9, 10: Mix infected samples.
Fig. 2.
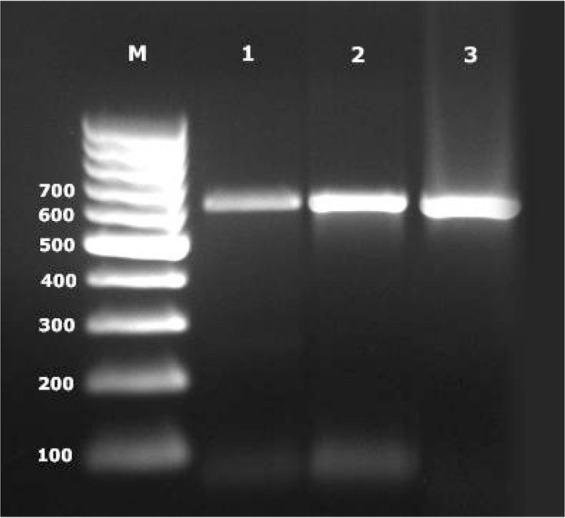
PCR bands of Wolbachia surface protein. M: Molecular weight marker (100 bp), 1, 2: Positive samples (630 bp), 3: Positive control
The results of the PCR assay according to the ages and gender of filarial agents and Wolbachia are shown in Table 1. Thirty five male dogs were examined by multiplex-PCR and the prevalance values were 5.7% for D. immitis, 2.8% for D. repens and 2.8% for mixed infection (both D. immitis and D. repens). On the other hand, 126 female dogs were examined by PCR and only one case was D. immitis (0.8%) and two cases were D. repens (1.5%). There was no any mix infection in female dogs.
Table 1.
Positivity of filarial agents and Wolbachia according to ages and gender
| Inspected Dog (n) | Only D. immitis | Only D. repens | Mix | Wolbachia | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | P | n | % | P | n | % | P | n | % | P | |||||||
| Gender | Male | 35 | 2 | 5.7 | 0.119 | 1 | 2.8 | 0.217 | 1 | 2.8 | 0.523 | 4 | 11.4 | 0.226 | ||||
| Female | 126 | 1 | 0.8 | - | - | 2 | 1.5 | 6 | 4.7 | |||||||||
| χ2 | P | χ2 | P | χ2 | P | χ2 | P | |||||||||||
| Ages | 0–1 | 69 | - | - | 3.875 | 0.144 | - | - | 1.276 | 0.528 | - | - | 2.628 | 0.269 | 3 | 4.3 | 1.098 | 0.577 |
| 2–4 | 71 | 3 | 4.2 | 1 | 1.4 | 2 | 2.8 | 6 | 8.4 | |||||||||
| 4> | 21 | - | - | - | - | 1 | 4.7 | 1 | 4.7 | |||||||||
| Total | 161 | 3 | 1.8 | 1 | 0.6 | 3 | 1.8 | 10 | 6.2 | |||||||||
Among the 161 samples screened by the ELISA, 6 samples (3.7%) tested positive for the D. immitis. There was no significant difference in the number of positive D. immitis infection among female dogs (4 out of 126, 3.2%) and male dogs (2 out of 35, 5.7%). Only 2 out of the 69 dogs belonging to the 0–1 yrs old group were positive (2.9%), while 4 out of 71 dogs belonging to the 2–4 yrs old group were positive (5.6%). A total of 21 dogs belonging to the >4 yrs old group showed no seropositivity of D. immitis infection.
Discussion
Adult D. immitis, inhabit the right ventricle of the heart the pulmonary arteries where they cause canine heartworm disease while the adults D. repens usually inhabit the subcutaneous tissue. In addition, it is well-known that D. immitis and D. repens produce microfilariae that circulate in the blood of dogs (Soulsby 1986). Dirofilaria immitis occurs worldwide in tropical, sub-tropical and temperate climates however D. repens occurs in the oldworld, in particular, throughout the Mediterranian sub-region, South Asia and sub-Saharan Africa (Cringoli et al. 2001).
Vector borne pathogens are sensitive to climatic condition, and there are some evidence that climate change may increase the incidence and dentensity of the diseases transmission (Purse et al. 2005). By altering the global environment, climate change has significant potential to intensify the vector borne diseases (Khasnis and Nettleman 2005). Dirofilaria immitis vectors are mosquitoes of Culicidae family with nearly 70 species susceptible for developing of parasite and thus considered potential vectors (Vezzani and Carbajo 2006). Aedes albopictus is reported as the primary potential vector of D. immitis in Italy (Cancrini et al. 2003). Whereas, Cx. theileri was detected as a vector of D. immitis in Portugal (Santa-Ana et al. 2006). There are limited study about vectors of Dirofilaria species in Turkey. Yildirim et al. (2011) determined that Ae. vexans and Cx. pipiens are the main potential vectors for D. immitis in Central Turkey. In the current study we could not investigate the potential vectors of Dirofilaria species.
Several studies have been published regarding the distribution and prevalence of D. immitis in dogs in Turkey. It was first reported in a dog the year of 1951 in Turkey (Guralp 1981). Tasan (1983) detected micro-filaraemia in 53/283 (18.7%) stray dogs in Elazig. The prevalence was recorded as 1.52% in Istanbul (Oncel and Vural 2005), 9.6% in Kayseri, (Yildirim et al. 2007), 8.1% in Erzurum (Simsek et al. 2011) besides 12.3%, 18.3%, 10.5% and 14.8% in Sakarya, Kocaeli, Mersin and Ankara, respectively (Simsek et al. 2008). These different prevalence rates may reflect different testing methodologies or true regional differences. The prevalence of D. immitis in dogs has been determined traditionally by postmortem inspection, detection of micro-filariae and serological testing. However, dogs with occult heartworm infections are amicrofilaraemic. In addition, some antiparasitic treatments such as macrolides may render an infected dog amicrofilaraemic for 6–9 months (Hoover et al. 1996). Thus, serological and microfilarial examinations should be applied together for screening D. immitis in dogs. DNA based diagnostic tests for D. immitis infections have been shown to overcome some deficiencies of parasitological and serological diagnosis, and specific and sensitive polymerase chain reaction (PCR)-based assays have been reported (Rishniw et al. 2006). The current study describes a quick and accurate molecular method for the simultaneous detection of the D. immitis and D. repens for the first time in Turkey.
Although there have been some records about variability between age and dirofilariosis prevalence (Montoya et al. 1998, Song et al. 2003, Fan et al. 2003), some authors (Rowley 1981, Martin and Collins 1985) have reported that age has no effect on dirofilariosis and it can be occur in all ages dog. While the others (Song et al. 2003, Fan et al. 2001) have stressed that ages and positivity, have positive relation and especially 3–7 ages group have higher risk than the others. Fan et al. (2001) found the lowest prevalence in 1–3 ages (6.3%) and following 3–6 ages (14.1%) while the highest rates has been found in up to 6 ages (23.7%). Simsek et al. (2008) determined the highest percent 3–5 ages dog (17.5%) while there was no positivity in up to 6 ages. In the current study, all dogs that were defined only D. immitis by PCR were in 2–4 ages group (4.2%). Similarly, the ELISA seropositivity was 2.9% in 0–1 ages and 5.6% in 2–4 ages dog. A possible explanation for higher seroprevalence of D. immitis infection in older dogs might be due to their longer exposure to the risk factor like mosquito (Fan et al. 2001). Selby et al. (1980) also indicated that the age of dogs was an important risk factor and determined by time of exposure in the endemic area.
In the present study, ELISA and PCR positivity were higher in male than female dogs. Similarly, Montoya et al. (1998) indicated that heartworm infection was more common in male dogs than female dogs, and the generally higher infection rate in male dogs had been postulated to be due to their stronger attraction to mosquitoes. However, Simsek et al. (2008), reported 10.7% for males and 14.4% for females. More male dogs live in the outdoor, due to their use in defence of property. They are, therefore, more likely to be bitten by mosquitoes (Montoya et al. 1998). However, all studied stray dogs in this work were living in out-door. Thus, living conditions are not unique on the prevalence. We believe that, some individual parameters like hormonal changes and immune deficiencies in female dogs may more tend to dirofilariosis.
Canine heartworm disease is generally diagnosed by antigen testing for D. immitis, and/or identification of microfilariae in the blood of infected dogs. However, some other filariae, including Dipetalonema reconditum, D. repens and approximately 1% of D. immitis infestations, can produce persistent microfilaremias with negative heartworm antigen tests (Rishniw et al. 2006). Thus, serological and molecular techniques should be use as combined. In this study, the sero-prevalence with ELISA was 3.7% while the positivity was 1.8% by PCR. This differences might be related with some possible cross reactions with the other nematodes, single-sex adults and/or possible treatment of microfilaria by macrolids.
Dirofilaria repens is transmitted by mosquitoes. The adult parasites are observed in the subcutaneous tissues of dogs and produce microfilariae that circulate in the perifer blood of infected dogs. It is less remarkable than D. immitis due to the lower pathogenicity (Soulsby 1982). Dirofilaria repens was detected first time in Turkey in 1962 (Merdivenci 1970). Tasan (1984), necropsied 120 dogs and found the occurence of D. repens as 2.5% in Elazig province of Turkey. Whereas, Yildirim (2004) examined a total of 300 dog blood by modified knott and membrane filtration tests and no detected any D. repens micofilaria in Ankara. In the current study, one of the examined dogs (0.6%) was infected with only D. repens by PCR and three of them (1.8%) were infected with both D. immitis and D. repens. These rates are close to reported by Tasan (1984). Microfilariae of D. repens are difficult to discriminate from D. immitis, since they have similar morphology. Staining of microfilaria was widely used for discriminate the both species. In the last years, PCR analysis was reported to be quite sensitive and specific for the differentiate the species (Lee et al. 2004). Gioia et al. (2010) designed a single-step multiplex PCR was based on the amplification of a partial 12S rRNA gene of the mitochondrion with a mix of general and species-specific filarial primers in a single reaction. We also used the same primers for the amplification of D. immitis and D. repens 12S rRNA genes by PCR in a single tube. Thus, the simultaneous detection of both D. immitis and D. repens in naturally infected dogs has been achived for the first time in Turkey.
Wolbachia is an intracellular alphaproteo-bacteria endosymbionts sheltered in a broad range of insects and nematodes (Pfarr and Hoerauf 2007). According to reports based on DNA amplification, one in five of the arthropods are infected with Wolbachia, rendering this bacterium the most ubiquitous intracellular symbiont yet described (Bourtzis 2008). Dirofilaria immitis and D. repens harboured the Wolbachia endosymbiont (Kozek 2005). We amplified the wsp (Wolbachia surface protein) gene by PCR and detected 6.2% (10/161) positivity in the current study. There are very limited study about this subject in Turkey. Sarali et al. (2009) collected 150 dogs blood from Izmir and Aydın provinces and detected the prevalences as 12.3% for both D. immitis and Wolbachia sp. In the current work, we determined the Wolbachia in 6 samples together with D. immitis and D. repens and in 4 samples without Dirofilaria spp as well. In this instance, either those 4 samples were infected with any Dirofilaria species and the PCR could not detect or those dogs had another residence for Wolbachia in the dogs. It is widely accepted that Wolbachia is released into the tissues of the infected host following worm death and that bacteria derived molecules provoke innate inflammatory responses (Saint Andre et al. 2002). Thus, doxycycline treatment may reduce Wolbachia levels in adultworms and less severe pathology as well.
Conclusion
This is the first study on the detection of Dirofilaria species using of multiplex-PCR in Turkey. Besides, it was attentioned to neglected filarial nematod which is D. repens in Turkey and obtained actual prevalence data about D. repens, D. immitis and Wolbachia, as well. Besides, the seroprevalence of D. immitis was determined by ELISA. Those results have been shown that canine dirofilariosis still prevalent and there is no effective reduction yet.
Acknowledgements
This study was supported by a grant from The Scientific and Technological Research Council of Turkey (TUBİTAK Project no: 110O934). The authors declerate that there is no conflict of interest.
References
- Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, Harbach RE. (2009) Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 23: 111–121. [DOI] [PubMed] [Google Scholar]
- Bourtzis K. (2008) Wolbachia-based technologies for insect pest population control. Adv Exp Med Biol. 627: 104–113. [DOI] [PubMed] [Google Scholar]
- Cancrini G, Pietrobelli M, Frangipane di Regalbono A, Tampieri MP, Della Torre A. (1995) Development of Dirofilaria and Setaria nematodes in Aedes albopictus. Parassitologia 37: 141–145. [PubMed] [Google Scholar]
- Cancrini G, Frangipane di Regalbono A, Ricci I, Tessarin C, Gabrielli S, Pietrobelli M. 2003. Aedes albopictusis a natural vector of Dirofilaria immitis in Italy. Vet Parasitol. 118: 195–202. [DOI] [PubMed] [Google Scholar]
- Casiraghi M, Bain O, Guerriero R, Martin C, Pocacqua V, Gardner SL, Franceschi A, Bandi C. (2004) Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int J Parasitol. 34: 191–203. [DOI] [PubMed] [Google Scholar]
- Cringoli G, Rinaldi L, Veneziano V, Capelli G. (2001) A prevalence survey and risk analysis of filariosis in dogs from the Mt. Vesuvius area of southern Italy. Vet Parasitol. 102: 243–252. [DOI] [PubMed] [Google Scholar]
- Dillon R. (2000) Dirofilariasis in Dogs, Cats. In: Ettinger JE, Feldman EC. (Eds) Textbook of Veterinary Internal Medicine, Vol 1 5th ed WB Saunders Company, Philadelphia, pp. 937–961. [Google Scholar]
- Giangaspero A, Marangi M, Latrofa MS, Martinelli D, Traversa D, Otranto D, Genchi C. (2013) Evidences of increasing risk of dirofilarioses in southern Italy. Parasitol Res. 112: 1357–1361. [DOI] [PubMed] [Google Scholar]
- Gioia G, Lecova L, Genchi M, Ferri E, Genchi C, Mortarino M. (2010) Highly sensitive multiplex PCR for simultaneous detection and discrimination of Dirofilaria immitis and Dirofilaria repens in canine peripheral blood. Vet Parasitol. 172: 160–163. [DOI] [PubMed] [Google Scholar]
- Guralp N. (1981) Helmintoloji. Ankara University Basımevi, Ankara, (in Turkish). [Google Scholar]
- Hoover JP, Campbell GA, Fox JC, Claypool PL, Mullins SB. (1996) Comparison of eight diagnostic blood tests for heart-worm infection in dogs. Canine Pract. 21: 11–19. [Google Scholar]
- Khasnis AA, Nettleman MD. (2005) Global warming and infectious disease. Arch Med Res. 36: 689–696. [DOI] [PubMed] [Google Scholar]
- Kozek WJ. (2005) What is new in the Wolbachia/Dirofilaria interaction? Vet Parasitol. 133: 127–132. [DOI] [PubMed] [Google Scholar]
- Latrofa MS, Weigl S, Dantas-Torres F, Annoscia G, Traversa D, Brianti E, Otranto D. (2012) A multiplex PCR for the simultaneous detection of species of filarioids infesting dogs. Acta Trop. 122: 150–154. [DOI] [PubMed] [Google Scholar]
- Lee SE, Song KH, Liu J, Kim MC, Park BK, Cho KW, Hasegawa A, Kim DH. (2004) Comparison of the acid-phosphatase staining and polymerase chain reaction for detection of Dirofilaria repens infection in dogs in Korea. J Vet Med Sci. 66: 1087–1089. [DOI] [PubMed] [Google Scholar]
- Mar PH, Yong IC, Chang GN, Fei AC. (2002) Specific polymerase chain reaction for differantial diagnosis of Dirofilaria immitis and Dirofilaria reconditum using primers derived from internal transcribed spacer region 2 (ITS2). Vet Parasitol. 106: 243–252. [DOI] [PubMed] [Google Scholar]
- Martin TE, Collins GH. (1985) Prevalence of Dirofilaria immitis and Dipetalonema reconditum in Greyhounds. Aust Vet J. 62: 159–163. [DOI] [PubMed] [Google Scholar]
- McHaffie J. (2012) Dirofilaria immitis and Wolbachia pipientis: a thorough investigation of the symbiosis responsible for canine heartworm disease. Parasitol Res. 110: 499–502. [DOI] [PubMed] [Google Scholar]
- Merdivenci A. (1970) Bir köpekte Dirofilaria repens (Railliet et Henry, 1911) olgusu ve insan dirofilariyozuna toplu bir bakış. Pendik Vet Araş Enst Derg. 1: 121–129 (in Turkish). [Google Scholar]
- Montoya JA, Morales M, Ferrer O, Molina JM, Corbera JA. (1998) The prevalence of Dirofilaria immitis in Gran Canaria, Canary islands, Spain. Vet Parasitol. 75: 221–226. [DOI] [PubMed] [Google Scholar]
- Oncel V, Vural G. (2005) Seroprevalence of Dirofilaria immitis in stray dogs in Istanbul and Izmir. Tr J Vet Anim Sci. 29: 785–789. [Google Scholar]
- Pfarr KM, Hoerauf A. (2007) A niche for Wolbachia. Trends Parasitol. 23: 5–7. [DOI] [PubMed] [Google Scholar]
- Purse BV, Mellor PS, Rogers DJ, Samuel AR, Mertens PP, Baylis M. (2005) Climate change and the recent emergence of bluetongue in Europe. Nat Rev Microbiol. 3: 171–181. [DOI] [PubMed] [Google Scholar]
- Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Alpizar JLD. (2006) Discrimination between six species of canine microfilariae by single polymerase chain reaction. Vet Parasitol. 135: 303–314. [DOI] [PubMed] [Google Scholar]
- Rowley J. (1981) The prevalence of hearth-worm infection in three countries in North Carolina. Canine Pract. 8: 46–48. [Google Scholar]
- Saint Andre A, Blackwell NM, May LR, Hoerauf A, Brattig NW, Volkmann L, Taylor MJ, Ford L, Hise AG, Lass JH, Diaconu E, Pearlman E. (2002) The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science. 295: 1892–1895. [DOI] [PubMed] [Google Scholar]
- Santa-Ana M, Khadem M, Capela R. (2006) Natural infection of Culex theileri (Diptera: Culicidae) with Dirofilaria immitis (nematoda: filarioidea) on Maderia Island, Portugal. J Med Entomol. 43: 104–106. [DOI] [PubMed] [Google Scholar]
- Sarali H, Aypak S, Karagenc T. (2009) Detection of Wolbachia in Dirofilaria species in dog. The 16th National Congress of Parasitology, 2009 December 1–7, University of Cukurova, Adana, Turkey, p. 248. [Google Scholar]
- Selby LA, Corwin RM, Hayes HM. (1980) Risk factors associated with canine heartworm infection. J Am Vet Med Assoc. 176: 33–35. [PubMed] [Google Scholar]
- Simsek S, Ozkanlar YE, Balkaya I, Aktas MS. (2011) Microscopic, serologic and molecular surveys on Dirofilaria immitis in stray dogs, Turkey. Vet Parasitol. 183: 109–113. [DOI] [PubMed] [Google Scholar]
- Simsek S, Utuk AE, Koroglu E, Rishniw M. (2008) Serological and molecular studies on Dirofilaria immitis in dogs from Turkey. J Helminthol. 82: 181–186. [DOI] [PubMed] [Google Scholar]
- Soulsby EJL. (1986) Helminths, Arthropods and Protozoa of Domesticated Animals. Seventh ed Bailliere and Tindall, London. [Google Scholar]
- Tasan E. (1983) Prevalence of canine filaria in Elazig province of Turkey. Doğa Bilim Dergisi 7: 63–70 (in Turkish). [Google Scholar]
- Tasan E. (1984) Elazığ kırsal yöre köpeklerinde helmintlerin yayılışı ve insan sağlığı yönünden önemi. Doğa Bilim Dergisi 8: 160–167. [Google Scholar]
- Vezzani D, Carbajo AE. (2006). Spatial and temporal transmission risk of Dirofilaria immitis in Argentina. Int J Parasitol. 36: 1463–1472. [DOI] [PubMed] [Google Scholar]
- Yildirim A. (2004) Prevalance of canine filaria in Ankara province of Turkey. Vet J Ankara Univ. 51: 35–40. [Google Scholar]
- Yildirim A, Ica A, Atalay O, Duzlu O, Inci A. (2007) Prevalence and epidemiological aspects of Dirofilaria immitis in dogs from Kayseri province, Turkey. Res Vet Sci. 82: 358–363. [DOI] [PubMed] [Google Scholar]
- Yildirim A, Inci A, Duzlu O, Biskin Z, Ica A, Sahin I. (2011) Aedes vexans and Culex pipiens as the potential vectors of Dirofilaria immitis in Central Turkey. Vet Parasitol. 178: 143–147. [DOI] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O'neill S. (1998) Phylogeny and PCR-based classification of Wolbachia strains using Wsp gene sequences. Proc Biol Sci. 265: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]