Abstract
Background:
Malaria is still a health problem in Iran. There are several vector control activities, including Indoor Residual spraying, using insecticide treated nets and larviciding including Temephos. In addition nuisance mosquitos are prevalent in the urban areas. So that evaluation of this species to larvicide will provide a clue for management of vector control activities.
Methods:
Two mosquito species were used in this study: Anopheles stephensi were collected from Kazeroun and Culex pipiens from Tehran, capital of Iran. All the tests were carried out according to the WHO method. All the test kis was provided by WHO.
Results:
Results showed a LC50= 0.0523 and LC90=0.3822 mg/l for An. stephensi. The figure for Cx. pipiens was 0.1838 and 0.8505 mg/l respectively.
Conclusion:
monitoring of insecticide resistance to Temephos should be evaluated regularly for management of vector control.
Keywords: Temephos, Anopheles stephensi, Culex pipiens, Evaluation, Efficacy
Introduction
Mosquito-borne diseases are from the main public health problems around the world, especially in tropical and semi-tropical areas. Among them malaria, dirofilariasis, dengue, west Nile, yellow fever, and some other viral diseases have the main morbidity. Malaria occurs in tropical and some temperate regions of the world with an annual morbidity of 300–500 million cases and 574000 deaths in 2013 (WHO 2014). Iran is going to eliminate the disease by 2025 although local transmission happens in some areas in south and southeast. Anopheles stephensi is one of the main malaria vectors is coastal lowlands of southern Iran (Hanafi-Bojd et al. 2011). West Nile and dirofilariasis are diseases which can be transmitted by Cx. pipiens, a worldwide distributed mosquito species. Both diseases are potentially transmittable in Iran (Azari-Hamidian et al. 2009, Ahmadnejad et al. 2011). The first line in malaria control program in Iran is indoor residual spraying to interrupt the transmission cycle, followed by distributing long lasting insecticide impregnated nets (LLINs) and larviciding. The latter is doing by biological larvicides in current years, although Temephos, an organophosphate compound, has been used for years in mosquito larvae control. There are reports of resistance to some insecticides such as DDT, diledrin and malathion in An. stephensi, as well as some indications of resistance to pyrethroids in current years (Davari et al. 2006, Hanafi-Bojd et al. 2012, Vatandoost and Hanafi-Bojd 2012), although current studies have confirmed full susceptibility of this species to malathion (Iranpour et al. 1993, Vatandoost et al. 2005, Hanafi-Bojd et al. 2012). By the way, larvicides tests showed resistance/tolerance to fenthion and fenitrothion in this species, but susceptibility to temephos (Vatandoost et al. 2004a, 2005, Vatandoost and Hanafi-Bojd 2005, Hanafi-Bojd et al. 2012, Soltani et al. 2013).
In insecticide resistance management it is necessary to evaluate used insecticides/larvicides periodically to monitor susceptibility status of the vectors. Considering the extensive use of insecticides in public health sector as well as agriculture and urban pests, physiological and behavioral resistance is a big issue that must be determined in mosquito vector species. Organophosphates are using widely in the agriculture sector, while they are using for larviciding against mosquitoes, it is possible to develop resistance in some vectors. A study on different geographical strains of An. stephensi in Iran showed considerable variations in temephos resistance ratios collected from different localities (Soltani et al. 2013).
Culex pipiens is known as urban pest nuisance around the world and has a wide distribution in the northern and central parts of Iran (Azari-Hamidian 2007). It can transmit dirofilariasis and has high resistance levels to different insecticides (Maraghi et al. 2006, Azari-Hamidian et al. 2009). This species usually breeds in waste waters which are contaminated with different detergents, insecticides, industrial pollutants, oil compounds etc. Therefore it is possible to develop resistance to insecticides and/or larvicides in this mosquito, as it has been reported in recent years (Vatandoost et al. 2004b).
Temephos, a most widely used organophosphate insecticide, has been included in the list of World Health Organization (WHO) as a suitable and safe mosquito larvicide that can be used even in drinking water for controlling of the most mosquito vectors. The toxicity of this insecticide is low and unlikely to present acute hazard for human (WHO 2006). In 2006 for the first time in the Middle East, resistance to temephos was confirmed in An. stephensi breeding in water storage tanks in the Al-Dhahira region of Oman (Anderasen 2006).
During last three decades temephos has considered as a safe larvicide (LC50= 8600 mg/l) in vector control programs (Pierce et al. 1989). World health organization suggested 1 ppm of this compound for larviciding in drinking water bodies. It is effective for 3 months in this concentration (Bang et al. 1972), and this is confirmed by several studies against different mosquito species (Mulla et al. 2004, Thavara et al. 2004, 2005). Also larval control in natural breeding places such as riversides has achieved by temephos EC 1% (Shililu 2001, Parvez and Al Wahaibi 2003). Laboratory tests on this formulation resulted to 15 weeks residual and larvicidal effect against Aedes aegypti (Chen et al. 2006).
With due attention to the long history of insecticide/larvicides resistance in Anopheles stephensi and Culex pipiens in Iran and other countries it is necessary to screen the susceptibility status of these species at regular intervals using WHO standard bioassay tests and to map the spatial and temporal changes in their level of susceptibility/resistance. Therefore this study was aimed to determine the susceptibility of these species to temephos in two sentinel sites in Iran.
Materials and Methods
Mosquito collection and rearing
Two mosquito species were used in this study: An. stephensi mysorensis collected from Kazeroun County, south of Iran and Cx. pipiens collected from Tehran, the capital city of Iran. Collected larvae were transferred to the insectary (Kazeroun Station, School of Public Health, Tehran University of Medical Sciences for An. stephensi and Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences for Cx. pipiens) and fed on fish flakes (Tetramine®). The specimens were reared under 28±1 °C temperature and 14:12 L: D photo-period to late 3rd early 4th instars, the suitable physiological ages for performing tests.
Susceptibility tests
Standard WHO method (1981, 2005) was followed for the tests using WHO standard kit and 1.25, 6.25, 31.25 and 156.25 mg/l concentration were used to provide the final concentration of 0.005, 0.025, 0.125 and 0.0625 when added 1 cc of each concentration to 249 cc of tape water. The obtained results were corrected by Abbotts’ formula when mortality rate in control group was 5– 20 percent (Abbott 1925) and analyzed by Probit (Finney 1971) to obtain LC50, LC90 and regression line. For each larvicides concentration 4 replicates of 25 larvae were used and 2 replicated for control. After 24 hrs all larvae without movement on the water surface were considered as dead.
Results
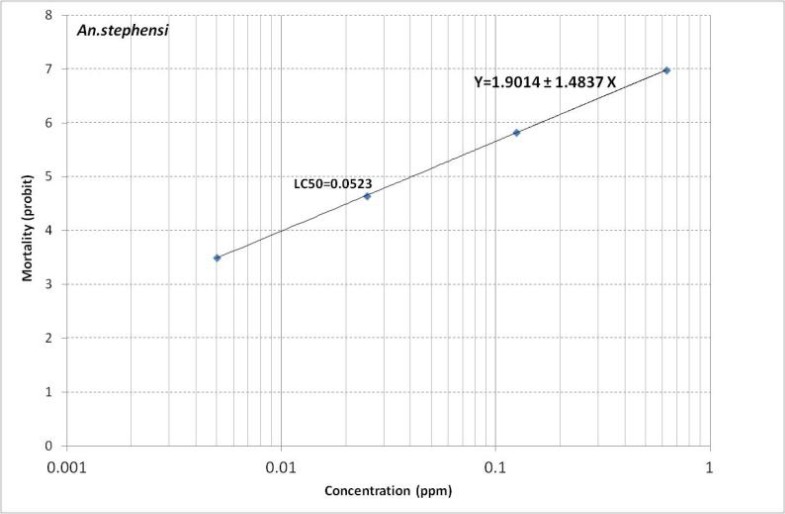
Table 1 shows the results of mortality of An. stephensi to temephos. We obtained LC50= 0.0523 and LC90=0.3822 for this species (Tables 1 and 2). Regression line is presented in Fig. 1. It shows Y= 1.9014+1.4837X.
Table 1.
Mortality rate of Anopheles stephensi larvae (Kazeroun Strain) against WHO standard concentrations of Temephos, 2014
| Concentration (PPM) | Replicates | No. of tested | No. of dead | Mortality rate (%) | Observed probit mortality | Expected probit mortality |
|---|---|---|---|---|---|---|
| 0.005 | 4 | 100 | 8 | 8 | 3.595 | 3.487 |
| 0.025 | 4 | 100 | 26 | 26 | 4.357 | 4.524 |
| 0.125 | 4 | 100 | 77 | 77 | 6.555 | 5.561 |
| 0.625 | 4 | 100 | 93 | 93 | 6.476 | 6.599 |
| Control | 2 | 50 | 0 | 0 | - | - |
Table 2.
Lethal concentration values obtained from regression analysis of Temephos against Anopheles stephensi larvae of Kazeroun Strain, 2014
| A | b ± SE | LC50 (ppm) ± 95%C.L. | LC90 (ppm) ± 95%C.L. | λ2 (heterogeneity) | λ2 table (df) | p-Value |
|---|---|---|---|---|---|---|
| 1.9014 | 1. 4837 ± 0.128 | 0.0411 | 0.2695 | 3.906 * | 5.991 (2) | 0.001 |
| 0.0523 | 0.3822 | |||||
| 0.0664 | 0.6001 |
No Heterogeneity
Fig. 1.
Mortality regression line of Temephos against Anopheles stephensi of Kazeroun Strain, 2014
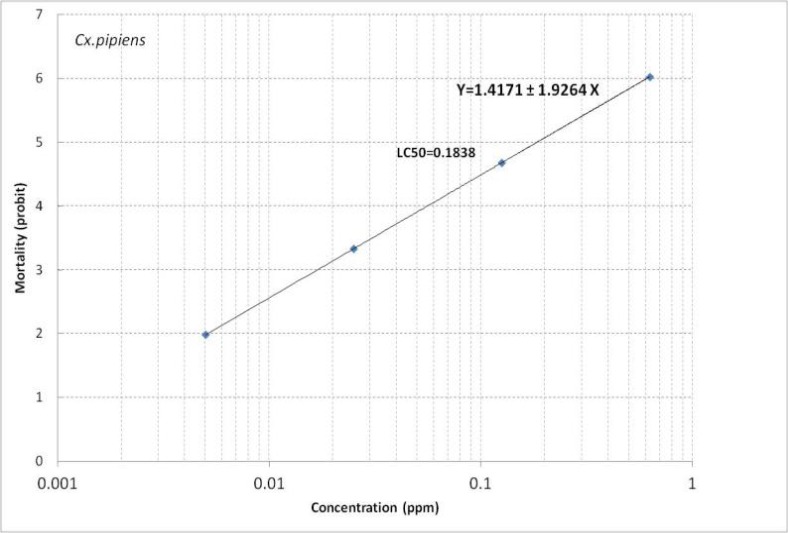
For Cx. pipiens a mortality rate of 3–99% was obtained in different concentrations (Table 3). LC50 and LC90 for this species were calculated as 0.1838 and 0.8505 ppm (Table 4). Regression line is presented in Fig. 2. It shows Y=1.4171+1.9264 X.
Table 3.
Mortality rate of Culex pipiens larvae (Tehran Strain) against WHO standard concentrations of Temephos, 2014
| Concentration (PPM) | Replicates | No. of Tested larvae | No. of mortality | Mortality rate (%) | Observed mortality probit | Expected mortality probit |
|---|---|---|---|---|---|---|
| 0.005 | 4 | 100 | 3 | 3 | 3.119 | 1.984 |
| 0.025 | 4 | 100 | 8 | 8 | 3.595 | 3.331 |
| 0.125 | 4 | 100 | 11 | 11 | 3.773 | 3.677 |
| 0.625 | 4 | 100 | 99 | 99 | 7.327 | 6.024 |
| Control | 2 | 50 | 0 | 0 | - | - |
Table 4.
Lethal concentration values obtained from regression analysis of Temephos against Culex pipiens larvae of Tehran Strain, 2014
| A | b ± SE | LC50 (ppm) ± 95%C.L. | LC90 (ppm) ± 95%C.L. | λ2 (heterogeneity) | λ2 table (df) | p-Value |
|---|---|---|---|---|---|---|
| 1.4171 | 1.9264 ± 0.174 | 0.1493 | 0.6186 | 11.113 * | 5.991 (2) | 0.001 |
| 0.1838 | 0.8505 | |||||
| 0.2285 | 1.3039 |
Heterogeneity
Fig. 2.
Mortality regression line of Temephos against Culex pipiens of Tehran Strain, 2014
Discussion
Our study showed LC50 value of 0.0523 for An. stephensi in Kazeroun area, southern Iran. Previous studies on this species from different localities of southern regions of the country have reported 0.001613 mg/l (Vatandoost and Hanafi-Bojd 2005) and 0.0022–0.0141 mg/l (Soltani et al. 2013). Other studies on diagnostic dose of temephos (0.25 mg/l) found An. stephensi was susceptible to this larvicide in Iran (Vatandoost et al. 2004a, Hanafi-Bojd et al. 2012). By the way as it shown in table 1, in concentration of 0.625 mg/l only 93% mortality has occurred. Considering diagnostic dose for this species, Kazeroun strain is resistant to temephos. By the way these findings should be confirmed by more studies with different batches of WHO standard kits for temephos on more larval population collected from the same area.
Resistance of An. stephensi to this larvicide has previously reported from Oman Country (Parvez and Al Wahaibi 2003). Anopheles stephensi has been reported susceptible to temephos in India with LC50 range of 0.008–0.015 (Tikar et al. 2011, Singh et al. 2014). Study on An. labranchiae in Morocco found low resistance to temephos in a sentinel site. They revised the diagnostic dose of temephos for this species (Faraj et al. 2010). A more recent study in Iran showed that the altered enzymes are responsible for temephos resistance in An. stephensi (Soltani et al. 2015). Although temephos is not used in national malaria program in last years but the study area has different rice fields and citrus gardens, and farmers use organophosphate insecticides to control pests. This may affect susceptibility of An. stephensi as well. Integrated pest management (IPM) in agriculture sector should be considered as an important priority parallel to integrated vector management (IVM) in health sector to overcome vector/pests resistance to pesticides.
Our results on Cx. pipiens also showed LC50 (0.1838 mg/l) was more than diagnostic dose (0.02 mg/l) recommended by WHO for this species. It means resistance to temepho. Other studies on temephos in Cyprus and Tunisia against Cx. pipiens reported LC50 ranges of 0.00076–0.310 and 0.0021–0.015, respectively (Ben Cheikh et al. 1993, Vasques et al. 2009). Dose response tests of the technical temephos against Cx. pipiens in India showed LC50 of 0.01 mg/l in this species (Badawy et al. 2015). This value for Culex quinquefasciatus was 0.0000473 mg/l in another study in that country (Dorta et al. 1993). In Tunisia esterases were found to be contributed to temephos resistance by their increased activity (Ben Cheikh et al. 2008).
Although there is no national program to control this species, but because wastewaters are the main breeding places for Cx. pipiens and they are contaminated with different pollutants, insecticides, detergents, etc this species has developed resistance to these compounds to save itself. A longitudinal on Cx. pipiens in Martinique showed that resistance ratio for temephos was 8.6 to 42-folds during 1991–1999 comparing 2.9 to 4.6-folds in 1990. They found organophosphate resistance was associated with decreasing of susceptible genotypes at Ester and ace-1 foci, as well as to an allele replacement at the Easter locus (Yebakima et al. 2004).
Our results on LC50 of Cx. pipiens were higher than the above mentioned studies in different countries. Therefore, it is necessary to do biochemical and molecular studies to find the reason for resistance to temephos.
In conclusion, it is recommended to do temephos susceptibility tests against both species from the same localities with more larvae and different batches of WHO standard kits of temephos to confirm their resistance status. The present study shows indications of resistance to this larvicides in An. stephensi and Cx. pipiens for the first time in Iran.
Conclusions
The results indicated that the monitoring and evaluation of insecticide resistance should be carried out regularly against mosquito vectors.
Acknowledgements
Authors would like to thanks Eng. AR Ghane, Dr Y Rassi, Mr N Vade, Mr K Hemati, Mr H Salmanpour, Mr Gh R Afroozandeh from Kazerounand Eng. F Rafi, Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciecnes. This project has financially supported by Deputy of Research, Tehran University of Medical Sciecnes, Grant No. 18404.
References
- Abbott WS. (1925) A method of comparing the effectiveness of an insecticide. J Econ Entomol. 18: 265–267. [Google Scholar]
- Ahmadnejad F, Otarod V, Fallah MH, Lowenski S, Sedighi-Moghaddam R, Zavareh A, Durand B, Lecollinet S, Sabatier P. (2011) Spread of West Nile virus in Iran: a cross-sectional serosurvey in equines, 2008–2009. Epidemiol Infect. 139: 1587–1593. [DOI] [PubMed] [Google Scholar]
- Anderasen MH. (2006) Emerging resistance to temephos in Anopheles stephensi in the Al-Dhahira region of oman. Mission report of World Health Organization, pp. 1–13. [Google Scholar]
- Azari-Hamidian S. (2007) Checklist of Iranian mosquitoes (Diptera: Culicidae). J Vector Ecol. 32(2): 235–242. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, Harbach RE. (2009) Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 23(2): 111–121. [DOI] [PubMed] [Google Scholar]
- Badawy MEI, Taktak NEM, Awad OM, Elfiki SA, Abou El-Ela NE. (2015) Larvicidal activity of temephos released from new chitosan/alginate/gelatin capsules against Culex pipiens. Int J Mosq Res. 2(3): 45–55. [Google Scholar]
- Bang YH, Tonn RJ, Jatanasen S. (1972) Pilot studies of Abate as a larvicide for control of Aedes aegypti in Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 3: 106–115. [PubMed] [Google Scholar]
- Ben Cheikh II, Ben Ali-Haouas Z, Marquine M, Pasteur N. (1998) Resistance to Organophosphorus and Pyrethroid Insecticides in Culex pipiens (Diptera: Culicidae) from Tunisia. J Med Entomol. 35(3): 251–260. [DOI] [PubMed] [Google Scholar]
- Ben Cheikh R, Berticat C, Ben Cheikh H, Berthomieu A, Weille M. (2008) Characterization of a novel high-activity esterase in Tunisian populations of the mosquito Culex pipiens. J Econ Entomol. 101(2): 484–491. [DOI] [PubMed] [Google Scholar]
- Chen CD, Lee HL. (2006) Laboratory bioefficacy of CREEK 1.0G (temephos) against Aedes (Stegomyia) aegypti (Linnaeus) larvae. Trop Biomed. 23(2): 220–223. [PubMed] [Google Scholar]
- Davari B, Vatandoost H, Ladonni H, Shaeghi M, Oshaghi MA, Basseri HR, Enayati AA, Rassi Y, Abai MR, Hanafi-Bojd AA, Akbarzadeh K. (2006) Comparative efficacy of different imagicides against different strains of Anopheles stephensi in the malaroius areas of Iran, 2004–2005. Pakistan J Biol Sci. 9(5): 885–892. [Google Scholar]
- Dorta DM, Vasuki V, Rajavel A. (1993) Evaluation of organophosphorus and synthetic pyrethroid insecticides against six vector mosquito species. Rev Saude Publica. 27(6): 391–397. [DOI] [PubMed] [Google Scholar]
- Faraj C, Adlaoui E, Elkohli M, Her rak T, Ameur B, Chandre F. (2010) Review of temephos discriminating concentration for monitoring the susceptibility of Anopheles labranchiae (Falleroni, 1926), malaria vector in Morocco. Malar Res Treat. 2010: 126085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney JD. (1971) Probit analysis, 3rd ed., Cambridge University Press, Cambridge, p. 333. [Google Scholar]
- Hanafi-Bojd AA, Azari-Hamidian S, Vatandoost H, Charrahy Z. (2011) Spatio-temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 4(6): 498–504. [DOI] [PubMed] [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Haghdoost AA, Shahi M, Abedi F, Yeryan M, Pakari A, Sedaghat MM. (2012) Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 121: 85–92. [DOI] [PubMed] [Google Scholar]
- Iranpour M, Yaghoobi-Ershadi MR, Motabar M. (1993) Susceptibility tests of Anopheles stephensi with some chlorine, phosphorus, carbamate and pyrethriod insecticides in south of Iran. Iranian J Publ Health. 22 (1–4): 74–85. [Google Scholar]
- Maraghi Sh, Rahdar M, Akbari H, Radmanesh M, Saberi AA. (2006) Human dirofilariasis due to Dirofilaria repens in Ahvaz, Iran: a report of three cases. Pakistan J Med Sci. 22: 211–213. [Google Scholar]
- Mulla MS, Thavara U, Tawatsin A, Chompoosri J. (2004) Procedures for evaluation of field efficacy of slow release formulations of larvicides against Aedes aegypti in water storagecontainers. J Am Mosq Control Assoc. 20: 64–73. [PubMed] [Google Scholar]
- Parvez SD, Al-Wahaibi SS. (2003) Comparison of three larviciding options for malaria vector control. East Mediterr Health J. 9: 627–636. [PubMed] [Google Scholar]
- Shililu J. (2001) Eritrea field studies on efficacy of bacterial larvicides for use in malaria control. Environment Health Project. 112: 1–15. [Google Scholar]
- Singh PK, Mittal PK, Kumar G, Dhiman RC. (2014) Insecticide susceptibility status of Aedes aegypti and Anopheles stephensi larvae against temephos in Delhi, India. Int J Mosq Res. 1(3): 69–73. [Google Scholar]
- Soltani A, Vatandoost H, Oshaghi MA, Enayati AA, Raeisi A, Eshraghian MR, Soltan-Dallal MM, Hanafi-Bojd AA, Abai MR, Rafi F. (2013) Baseline susceptibility of different geographical strains of Anopheles stephensi (Diptera: Culicidae) to Temephos in malarious areas of Iran. J Arthropod-Borne Dis. 7(1): 56–65. [PMC free article] [PubMed] [Google Scholar]
- Soltani A, Vatandoost H, Oshaghi MA, Maleki Ravasan N, Enayati AA, Asgarian F. (2015) Resistance mechanisms of Anopheles stephensi (Diptera: Culicidae) to Temephos. J Arthropod-Borne Dis. 9(1): 71–83. [PMC free article] [PubMed] [Google Scholar]
- Thavara U, Tawatsin A, Kong-ngamsuk W, Mulla MS. (2004) Efficacy and longevity of a new formulation of temephos larvicide tested in village-scale trials against Aedes aegypti larvae in water storage containers. J Am Mosq Control Assoc. 20: 176–182. [PubMed] [Google Scholar]
- Thavara U, Tawatsin A, Srithommarat R, Zaim M, Mulla MS. (2005) Sequentialrelease and residual activity of temephos applied as sand granules to waterstoragejars for the control of Aedes aegypti larvae (Diptera: Culicidae). J Vector Ecol. 30: 62–72. [PubMed] [Google Scholar]
- Tikar SN, Mendki MJ, Sharma AK, Sukumaran D, Veer V, Parashar BD. (2011) Resistance status of the malaria vector mosquitoes, Anopheles stephensi and Anopheles subpictus towards adulticides and larvicides in arid and semi-arid areas of India. J Insect Sci. 11: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasques MI, Violaris M, Wirth MC, Hadjivassilis A. (2009) Susceptibility of Culex pipiens (Diptera: Culicidae) field populations in Cyprus to conventional organic insecticides, Bacillus thuringiensis subsp. israelensis, and Methoprene. J Med Entomol. 46(4): 881–887. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Shahi H, Abai MR, Hanafi-Bojd AA, Oshaghi MA, Zamani G. (2004a) Larval habitats of main malaria vectors in Hormozgan province and their susceptibility to different larvicides. Southeast Asian J Trop Med Public Health. 35: 22–25. [PubMed] [Google Scholar]
- Vatandoost H, Ezeddinloo L, Mahvi A, Abai M, Kia E, Mobedi I. (2004b) Enhanced tolerance of house mosquito to different insecticides due to agricultural and household pesticides in sewage system of Tehran, Iran. Iranian J Environ Health Sci Eng. 1(1): 46–50. [Google Scholar]
- Vatandoost H, Mashayekhi M, Abaie MR, Aflatoonian MR, Hanafi-Bojd AA, Sharifi I. (2005) Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj district Kerman province, southeastern Iran. J Vector-Borne Dis. 42: 100–108. [PubMed] [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. (2005) Current resistant status of Anopheles stephensi Liston to different larvicides in Hormozgan Province, southeastern Iran. Pakistan J Biol Sci. 8(11): 1568–1570. [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. (2012) Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac J Trop Med. 5(9): 722–726. [DOI] [PubMed] [Google Scholar]
- WHO (1981) Instruction for determining the susceptibility or resistance of mosquito larvae to insecticides. WHO/VBC/81.807.
- WHO (2005) Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/2005.13.
- WHO (2014) World Malaria Report 2013. World Health Organization, Geneva Switzerland, p. 227. [Google Scholar]
- Yebakima A, Marquine MT, Rosine J, Pasteur N. (2004) Evolution of resistance under insecticide selection pressure in Culex pipiens quinquefasciatus (Diptera: Culicidae) from Martinique. J Med Entomol. 41(4): 718–725. [DOI] [PubMed] [Google Scholar]