Abstract
Background:
Antimicrobial peptides play a role as effectors substances in the immunity of vertebrate and invertebrate hosts. In the current study, antimicrobial peptide was isolated from the haemolymph of the American cockroach, Periplaneta americana.
Methods:
Micrococcus luteus as Gram-positive bacteria and Escherichia coli as Gram-negative bacteria were candidate for injection. Induction was done by injecting both bacteria into the abdominal cavity of two groups of cockroaches separately. The haemolymphs were collected 24 hours after post injection and initially tested against both bacteria. Subsequently, the immune induced haemolymph was purified by high performance liquid chromatography (HPLC) to separate the proteins responsible for the antibacterial activity.
Results:
The non-induced haemolymph did not show any activity against both bacteria whereas induced haemolymph exhibited high activity against M. luteus but did less against E. coli. Two fractions showed antibacterial activity against M. luteus. Finally the molecular weight of the isolated antibacterial proteins were determined as 72 kDa and 62 kDa using SDS-PAGE.
Conclusion:
Induced haemolymph of American cockroaches has the ability to produce peptides to combat against Gram-positive bacteria when an immune challenge is mounted. Further work has to be done to sequence of the protein, which it would be advantageous.
Keywords: American cockroach, Antibacterial protein, Isolation, Micrococcus luteus, Escherichia coli
Introduction
Insects exhibit an amazing evolutionary success that can be explained by a variety of reasons (Jarosz 1996, Lazzaro 2008, Gao and Zhu 2012), among which the fact that their potent immunity play a major role in defense against bacteria (Hoffmann et al. 1996, Wilson et al. 1999, Lamberty et al. 2001, Lazzaro 2008). Based on habitat of cockroaches, they are always exposed to potentially pathogenic microorganisms and parasites, but only a few encounters result in infection (Gillespie and Kanost 1997).
Antimicrobial peptides play an essential role in fighting against invading pathogens in insects, especially those that lack an adaptive immunity (Toke 2005). Normally due to microbial infection, antimicrobial peptides are synthesized in fat body or certain haemolymph cells of insects or body injury, and then rapidly released into haemolymph to kill microorganisms (Brivio et al. 2006, Yu et al. 2010, Yakovlev 2011). However, insects count on cellular and humoral mechanisms to fight against pathogens and subsequently, innate immunity, which is being dominant in the final category.
Insects are remarkably resistant to bacterial infections by detecting of bacteria, a complex genetic cascade is activated, which eventuates in the production of a series of antibacterial peptides and is released into the haemolymph (Eleftherianos et al. 2006, Eleftherianos et al. 2007). These antimicrobial peptides are mostly small, amphipathic, cationic molecules (Gao and Zhu 2013). They have an effect on membrane of microbial cell changing permeability or by breakdown bacteria membrane (Toke 2005, Dai et al. 2008, Huang et al. 2008). In addition, insect peptides may affect the synthesizing of DNA or protein as well as the protein folding of the bacteria (Otvos 2000, Huang et al. 2008, Shen et al. 2010, Bang et al. 2012). Insects can synthesize some antimicrobial peptides such as cecropins, which exhibit anti-cancer activity (Ye et al. 2004). Some insects can synthesize inducible antibacterial peptides such as lysozyme which is also constitutive like lipopolysaccharide (LPS)-binding protein which was isolated from the haemolymph of the American cockroach (Periplaneta americana) (Ha Lee et al. 2007, Fiolka 2008). This protein acts as an opsonin (Jomori and Natori 1992, Hashimoto et al. 2009, Kim et al. 2010). Generally, five major groups of antibacterial peptides have been introduced (Hultmark 1993) including cecropins, insect defensins, attacin-like (glycine-rich) proteins, proline rich peptides and lysozymes. The mechanisms of some these peptides have been studied extensively (Sawa and Kurahashi 1999, Imler and Bulet 2005, Wang et al. 2009).
American cockroach spends most of its time in sewage, sewer pipe. These environments usually contain high density of bacteria. Therefore, it is likely to defend itself against invading pathogens by means of antimicrobial compounds. The purpose of the current study was to isolate and purify an antimicrobial protein from the haemolymph of American cockroach.
In this study, we isolated and purified an antimicrobial protein in immune induced haemolymph of P. americana which may open up new way of research to detect new antimicrobial pathogens.
Materials and Methods
Insect rearing and haemolymph collection
American cockroaches, P. americana, were maintained in an insectary at 25±2 °C with a 12h light/dark ratio, and fed on dried bred, date and water. To collect non-induced haemolymph, two groups each included 30 adults and final instars cockroaches were anaesthetized with CO2. The ventral surface of sternum of each insect was sterilized with 70% ethanol, and the coxal membranes of legs were punched with still needle. The exuded haemolymph from the wounds was immediately collected, centrifuged at 1800×g for 10 minutes. For collecting of induced haemolymph, 100 μl of M. luteus or E. coli (106 cells/ml) was injected into the abdominal cavity of each cockroach. Based on preliminary time optimization, the insects were anesthetized 20 hours after injection and the haemolymph was collected using sterile syringe, and then centrifuged. All collected samples were transferred into clean and chilled eppendorf tubes containing few crystals of phenyl thiourea in order to prevent melanization. Finally, the supernatants and pallets were kept in −20 °C until used. The protein concentration of all samples was analyzed by Bradford method before being used.
Bacterial strains
In order to screen the antibacterial activity of the heamolymph compounds on and based on evidences mentioned in previous studies (Jomori and Natori 1990, Serja et al. 2003), two strains of bacteria, one Gram positive and one Gram negative bacteria were chosen. The bacteria were collected from Persian type culture collection, Science and Industrial Research Organization of Iran. The bacteria strains used for screening antimicrobial peptides were nonpathogenic bacteria, ATCC 9341recently named as Kocuria rhizophila and E. coli ATCC25922 which is susceptible to all of the antibiotic, and Microccus leteus ATCC 9341 is resistance to all antibiotic except chloramphenicol, doxy cycline, hydramycinand tetracline.
Antibacterial assay
The antibacterial assays were done by diffusion disc method. Sterile Petri dishes received 20 ml of melted Luria Burtenii medium, pH 7.0. After solidification of the medium, the agar surface was inoculated with 0.1 ml (106 cells/ml) of the test bacterial strain and spread with the help of a glass rod. Sterile paper discs soaked with 20 μl of the haemolymph (at concentration of 1.21mg/ml) was places on the medium. As control, paper discs soaked with 20 μl of non-induced haemolymph (at concentration of 1.34 mg/ml) were used. The plate was incubated overnight at 37 °C, and the diameters of the clear zones were recorded. The assay was carried out three times.
Reverse Phase-High Performance Liquid Chromatography (RP-HPLC)
The antibacterial compounds from haemolymph was purified by semi-preparative high performance liquid chromatography (RPHPLC) (Knauer, Germany) under the following conditions: flow rate =1 ml min−1, stationary phase = Spherisorb C18 column (Waters, USA, ODS2 Column 5 μm, 250 mm×4.6mm), mobile phase = acetonitrile in water containing 0.1% trifluoroacetic acid (TFA), detector wavelength at 230 nm. The fractions were eluted in an elution range of 20% to 80% acetonitrile in water for 40 min. The fractions were concentrated by freeze-dried, redissolved in 50 ml of apyrogenic water and then the antimicrobial activity of all fractions against both bacteria was tested using disc diffusion assay.
Separation of Antibacterial Agents by SDS-PAGE
The eluted protein was applied to SDS-PAGE as discussed by Laemmeli (1970). The protein was mixed with 5μl of 5x SDS-PAGE sample buffer under non-reducing conditions and then heated for 10 minutes at 100 °C. The sample (20 μl) was then centrifuged at 10000g for 5 min to remove debris before loading into the gel. The supernatant was applied to SDS-PAGE electrophoresis in a 10% polyacrylamide gel to analyse the eluted protein. Electrophoresis was performed at a constant voltage of 200v for ca, 45 min using the bio-Rad mini-protean II apparatus (Bio-Rad laboratories LTD, Hemel Hempstead, Hertforshire, UK). After separation, the gel was fixed by 30-min-long gentle shaking in 10% acetic acid, 50% methanol (v/v) and visualized by staining with Coomassie Brillant Blue R-250.
Results
Antibacterial assay
The whole non-induced haemolymph of cockroach did not show any antibacterial activity against M. luteus and E. coli. On contrary, the induced haemolymph showed high antibacterial activity against M. luteus and less activity against E. coli (Fig. 1A, B).
Fig. 1.
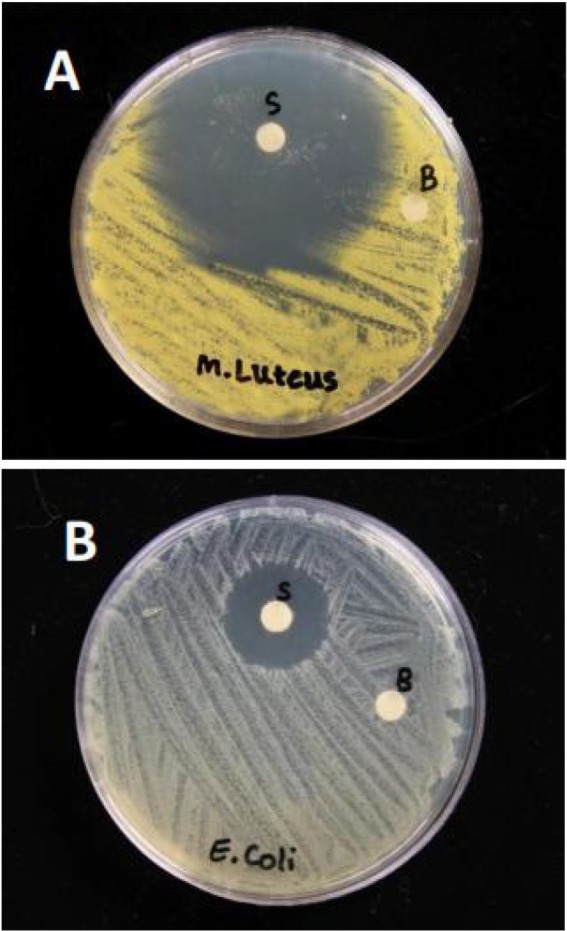
Antibacterial activity of whole haemolymph against Micrococcus luteus (A) and Escherichia coli (B) where ‘S’ indicates treatment and ‘B’ control (non-induced haemolymph). The clear zone around the discs indicates antibacterial activity
Purification of the antibacterial peptides
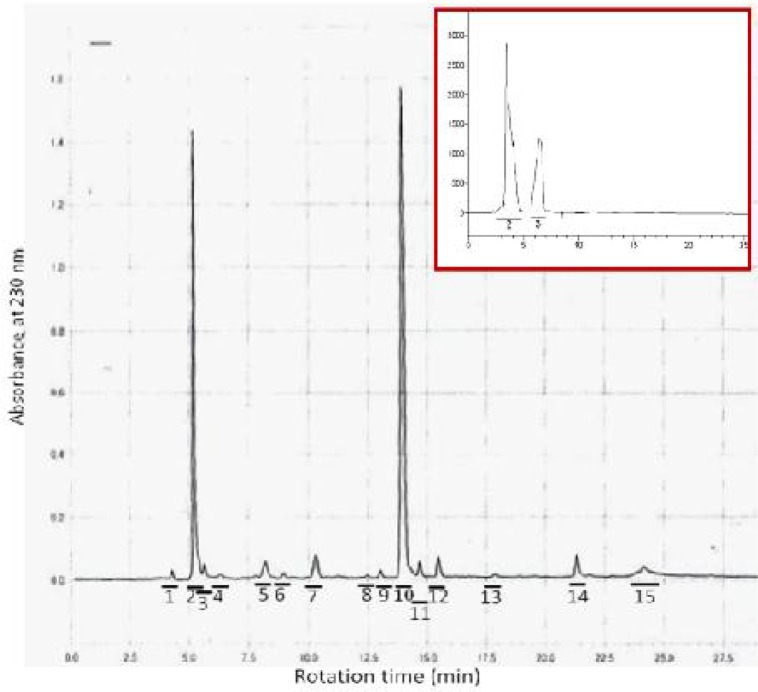
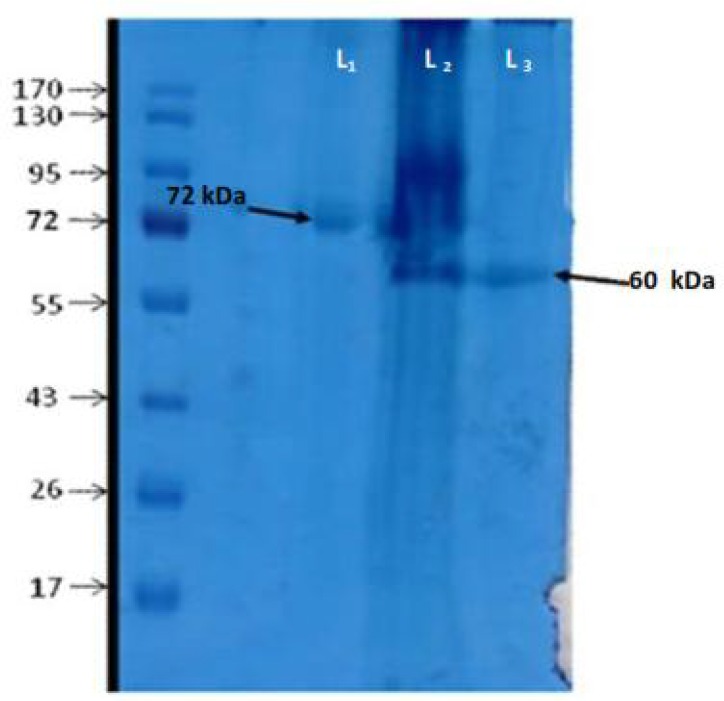
The haemolymph with antibacterial activity subjected to semi-preparative Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) for separation and purification of the peptides. Fifteen different peaks were obtained and the fractions collected (Fig. 2). All fractions were freeze-dried and redissolved in 50 ml of apyrogenic water and then separately subjected to antibacterial susceptibility test against both M. luteus and E. coli. Factions 2 and 3 showed high antibacterial activity against only M. luteus (Fig. 3). No activity was observed against E. coli. To remove any possible impurity, the active fractions were reloaded onto RP-HPLC, tested for antibacterial activity and then subjected to SDS-PAGE. Two protein bands were observed at 60 kDa and 72 kDa on the polyacrylamide gel (Fig. 4).
Fig. 2.
RP-HPLC chromatogram of Periplaneta americana heamolymph induced with Micrococcus luteus. The absorbance was measured at 230 nm. The antibacterial activity of all fractions was separately tested against M. luteus. Only fractions 2 and 3 showed antibacterial activity. Fractions 2 and 3 were concentrated and reloaded onto RP-HPLC to remove any possible impurity
Fig. 3.
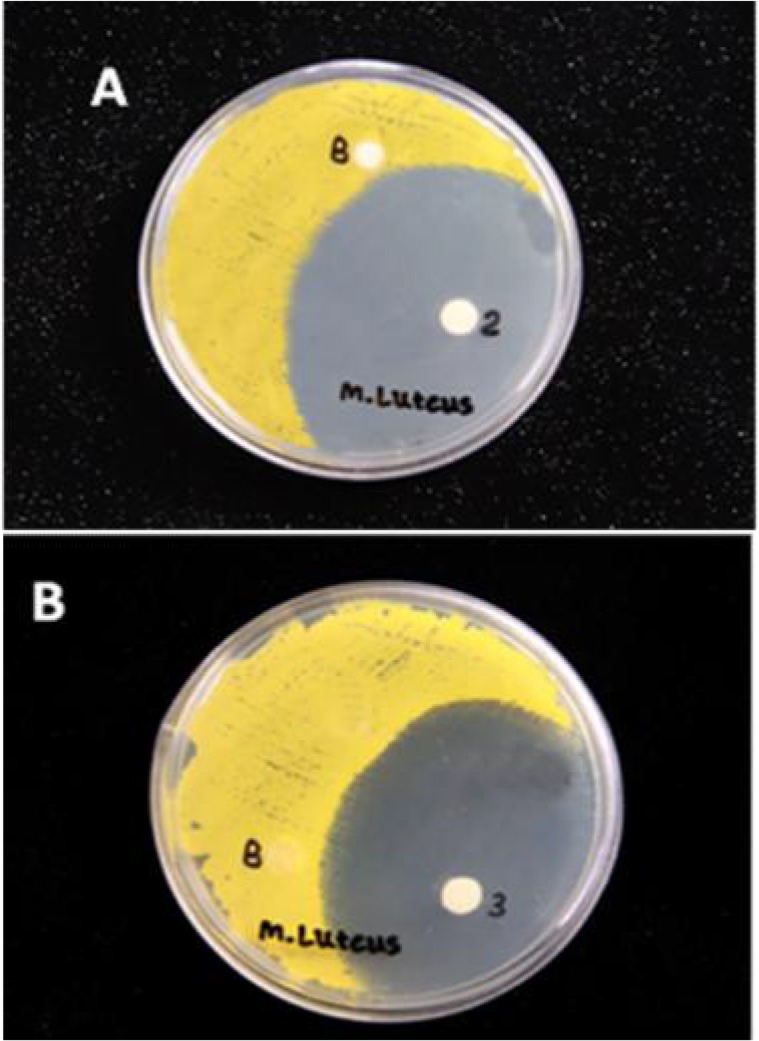
Antibacterial activity of fraction 2 (A) and 3 (B) against Micrococcus luteus (A). The fractions were concentrated by freeze-dried, redissolved in 50 ml of apyrogenic water
Fig. 4.
SDS-PAGE of fractions 2 and 3 L2 shows molecular mass of whole heamolymph proteins and L1 and L3 show molecular weight of fraction 2 and 3 respectively
Discussion
Insects have many systems that work together to limit the spread of bacteria and other pathogens. They can synthesis antimicrobial peptides within their body. They have pattern recognition proteins that can bind to the external surface of bacteria or other pathogens (Vilcinskas 2013). In this study the non-induced haemolymph and the induced haemolymph of American cockroach were screened for the antimicrobial activity against two Gram negative and Gram-positive bacterial strains. The non-induced haemolymph did not show inhibitory activity against any of the tested bacterial strains. It does not indicate that peptides are absent but they may be present in lesser quantity so that no detectible action in vitro studies is seen. It has been stated that the adult American cockroach can generate an adaptive humoral immune response to pathogens (Karp 1985, George et al. 1987, Faulhaber and Karp 1992). In this scenario, defensive peptides or proteins play a main and crucial in insect humoral immune response against invading microorganisms (Leclerc and Reichhart 2004, Levy et al. 2004, Mak et al. 2010).
Generally, each insect species may possess an individual set of antimicrobial peptides synthesized in response to non-self recognition (Engstrom 1999). For example, Drosophila melanogaster metchnikowins peptides have no activity against Gram-negative bacteria but they inhibit growth of M. luteus (Imler and Bulet 2005, Rahnamaeian et al. 2009, Rahnamaeian and Vilcinskas 2012). We found similar result by injecting Gram-negative bacteria, E. coli and Gram-positive bacteria, M. luteus. Although, whole haemolymph of immune cockroaches showed antimicrobial activity against E. coli (Fig. 1) but we could not find these activities in any fraction. It seems that they are not directly involved in killing E. coli, and some molecules may be involved in signaling mechanism to remove the bacteria. Another possibility is that the quantity of antimicrobial activity against E. coli may too less to see visible action.
Various insect species, which bacteria injected into the haemocoel, elicit the synthesis of a number of peptides and proteins, which are individually or cooperatively active against the foreign microorganisms (Cociancich et al. 1994, Vilcinskas 2013). Induction is a common process in many insect species (Cociancich et al. 1994). In the present study, induction of such peptide(s) was done by injecting E. coli or M. luteus into the abdominal cavity of American cockroaches. The immune induced peptides were active against tested bacterial strains and this result suggests that peptides are produced to combat bacterial infection. However, constitutive and inducible proteins may be present in haemolymph of some insects and may act as signaling molecules such as lysozyme (Royet and Dziarski 2007, Royet et al. 2011, Bosco-Drayon et al. 2012).
We found two proteins with antibacterial activity and then they were subjected to non-reducing SDS-PAGE to determine the size of proteins. The molecular weight of proteins was 60 and 72 kDa (Fig. 4) and these separated proteins allow us to observe antibacterial activity of them separately.
The purified proteins are predominantly active against the Gram-positive bacteria; it suggests that the antibacterial activity of the peptides is related to the cell wall of the bacteria. It may be assumed that the proteins identified in this study might play an important role in their self-defense against bacterial infection in American cockroaches individually or cooperatively.
However, further studies are needed to work out the combined effect of peptides. At this point, it is also important to remember the development of resistance to ordinary antibiotic like Gentamicin, penicillin and so on, by variety of infectious bacteria. It is also believed that antimicrobial peptides will be assumed in the near future as an alternative for the nowadays-classical antibiotics (Małgorzata et al. 2007). The advantages of antimicrobial peptides are many viz, selectivity, fast killing, broad antimicrobial spectra and lack of resistance development (Matsuzaki 1999, Papo and Shai 2005). However, the present result is preliminary and future study will be done in other methods to confirm the antimicrobial property of peptides.
Conclusion
The main idea of this research is to isolate new antimicrobial peptides from haemolymph of American cockroaches, Periplaneta Americana and to study its activity against these two a Gram-positive and Gram-negative bacteria. We found that induced haemolymph of American cockroaches has the ability to produce peptides to combat against Gram-positive bacteria when an immune challenge is mounted. Before immune challenge, antimicrobial peptides were indistinguishable, whereas concentration increased tremendously in the haemolymph after induction peptide. Further work has to be done to improve purification steps of antibacterial proteins without damaging the proteins so that the peptides can act equally or higher than conventional antibiotics, and sequencing of the proteins would be advantageous.
Acknowledgements
We thank Mr Hossaini, for the kind cooperation in insectary of department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences. The authors declare that there is no conflict of interest.
References
- Bang K, Park S, Yoo JY, Cho S. (2012) Characterization and expression of attacin, an antibacterial protein-encoding gene, from the beet armyworm, Spodoptera exigua (Hubner) (Insecta: Lepidoptera: Noctuidae). Mol Biol Rep. 39: 5151–5159. [DOI] [PubMed] [Google Scholar]
- Bosco-Drayon V, Poidevin M, Boneca IG, Narbonne-Reveau K, Royet J, Char-roux B. (2012) Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe. 12: 153–165. [DOI] [PubMed] [Google Scholar]
- Brivio MF., Moro M, Mastore M. (2006) Down-regulation of antibacterial peptide synthesis in an insect model induced by the body-surface of an entomoparasite (Steinernema feltiae). Dev Comp Immunol. 30: 627–638. [DOI] [PubMed] [Google Scholar]
- Cociancich S, Bulet P, Hetru C, Hoffmann JA. (1994) The inducible antibacterial peptides of insects. Parasitol Today. 10: 132–139. [DOI] [PubMed] [Google Scholar]
- Dai H, Rayaprolu S, Gong Y, Huang R, Prakash O, Jiang H. (2008) Solution structure, antibacterial activity, and expression profile of Manduca sexta moricin. J Pept Sci 14: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftherianos I, Marokhazi J, Millichap PJ, Hodgkinson AJ, Sriboonlert A, ffrench-Constant RH, Reynolds SE. (2006) Prior infection of Manduca sexta with non-pathogenic Escherichia coli elicits immunity to pathogenic Photorhabdus luminescens: roles of immune-related proteins shown by RNA interference. Insect Biochem Mol Biol. 36: 517–525. [DOI] [PubMed] [Google Scholar]
- Eleftherianos I, Gokcen F, Felfoldi G, Millichap PJ, Trenczek TE, ffrench-Constant RH, Reynolds SE. (2007) The immunoglobulin family protein Hemolin mediates cellular immune responses to bacteria in the insect Manduca sexta. Cell Microbiol. 9: 1137–1147. [DOI] [PubMed] [Google Scholar]
- Engstrom Y. (1999) Induction and regulation of antimicrobial peptides in Drosophila. Dev Comp Immunol. 23: 345–358. [DOI] [PubMed] [Google Scholar]
- Faulhaber LM, Karp RD. (1992) A diphasic immune response against bacteria in the American cockroach. Immunology. 75: 378–381. [PMC free article] [PubMed] [Google Scholar]
- Fiolka MJ. (2008) Immunosuppressive effect of cyclosporin A on insect humoral immune response. J Invertebr Pathol. 98: 287–292. [DOI] [PubMed] [Google Scholar]
- Gao B, Zhu S. (2012) Alteration of the mode of antibacterial action of a defensin by the amino-terminal loop substitution. Biochem Biophys Res Commun. 426: 630–635. [DOI] [PubMed] [Google Scholar]
- Gao B, Zhu S. (2014) An insect defensin-derived beta-hairpin peptide with enhanced antibacterial activity. ACS Chem Biol. 9: 405–413. [DOI] [PubMed] [Google Scholar]
- George JF, Karp RD, Rellahan BL, Lessard JL. (1987) Alteration of the protein composition in the haemolymph of American cockroaches immunized with soluble proteins. Immunology. 62: 505–509. [PMC free article] [PubMed] [Google Scholar]
- Gillespie JP, Kanost MR, Trenczek t. (1997) Biology mediators of insect immunity. Annu Rev Entomol. 42: 611–643. [DOI] [PubMed] [Google Scholar]
- Ha Lee J, Hee Lee I, Noda H, Mita K, Taniai K. (2007) Verification of elicitor efficacy of lipopolysaccharides and peptidoglycans on antibacterial peptide gene expression in Bombyx mori. Insect Biochem Mol Biol. 37: 1338–1347. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Tabuchi Y, Sakurai K, Kutsuna M, Kurokawa K, Awasaki T, Sekimizu K, Nakanishi Y, Shiratsuchi A. (2009) Identification of lipoteichoic acid as a ligand for draper in the phagocytosis of Staphylococcus aureus by Drosophila hemocytes. J Immunol. 183: 7451–7460. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart JM, Hetru C. (1996) Innate immunity in higher insects. Curr Opin Immunol. 8: 8–13. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lou H, Wu X, Chen Y. (2008) Characterization of the BPI-like gene from a subtracted cDNA library of large yellow croaker (Pseudosciaena crocea) and induced expression by formalininactivated Vibrio alginolyticus and Nocardia seriolae vaccine challenges. Fish Shellfish Immunol. 25: 740–750. [DOI] [PubMed] [Google Scholar]
- Imler JL, Bulet P. (2005) Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy. 86: 1–21. [DOI] [PubMed] [Google Scholar]
- Jarosz J. (1996) Anti-infective defence strategies and methods of escape from entomologic pathogens under immunologic control of insects. Wiad Parazytol. 42: 3–27. [PubMed] [Google Scholar]
- Jomori T, Natori S. (1992) Function of the lipopolysaccharide-binding protein of Periplaneta americana as an opsonin. FEBS Lett. 296: 283–286. [DOI] [PubMed] [Google Scholar]
- Karp RD. (1985) Preliminary characterization of the inducible humoral factor in the American cockroach (Periplaneta americana). Dev Comp Immunol. 9: 569–575. [DOI] [PubMed] [Google Scholar]
- Kim CH, Shin YP, Noh MY, Jo YH, Han YS, Seong YS, Lee IH. (2010) An insect multiligand recognition protein functions as an opsonin for the phagocytosis of microorganisms. J Biol Chem. 285: 25243–25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lamberty M, Zachary D, Lanot R, Bordereau C, Robert A, Hoffmann JA, Bulet P. (2001) Insect immunity. Constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J Biol Chem. 276: 4085–4092. [DOI] [PubMed] [Google Scholar]
- Lazzaro BP. (2008) Natural selection on the Drosophila antimicrobial immune system. Curr Opin Microbiol. 11: 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc V, Reichhart JM. (2004) The immune response of Drosophila melanogaster. Immunol Rev. 198: 59–71. [DOI] [PubMed] [Google Scholar]
- Levy F, Rabel D, Charlet M, Bulet P, Hoffmann JA, Ehret-Sabatier L. (2004) Peptidomic and proteomic analyses of the systemic immune response of Drosophila. Biochimie. 86: 607–616. [DOI] [PubMed] [Google Scholar]
- Mak P, Zdybicka-Barabas A, Cytrynska M. (2010) A different repertoire of Galleria mellonella antimicrobial peptides in larvae challenged with bacteria and fungi. Dev Comp Immunol. 34: 1129–1136. [DOI] [PubMed] [Google Scholar]
- Rahnamaeian M, Vilcinskas A. (2012) Defense gene expression is potentiated in transgenic barley expressing antifungal peptide Metchnikowin throughout powdery mildew challenge. J Plant Res. 125: 115–124. [DOI] [PubMed] [Google Scholar]
- Rahnamaeian M, Langen G, Imani J, Khalifa W, Altincicek B, von Wettstein D, Kogel KH, Vilcinskas A. (2009) Insect peptide metchnikowin confers on barley a selective capacity for resistance to fungal ascomycetes pathogens. J Exp Bot. 60: 4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J, Dziarski R. (2007) Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat Rev Microbiol. 5: 264–277. [DOI] [PubMed] [Google Scholar]
- Royet J, Gupta D, Dziarski R. (2011) Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat Rev Immunol. 11: 837–851. [DOI] [PubMed] [Google Scholar]
- Sawa T, Kurahashi K. (1999) Antimicrobial peptides/proteins-application to the therapy of sepsis. Masui. 48: 1186–1193. [PubMed] [Google Scholar]
- Shen X, Ye G, Cheng X, Yu C, Altosaar I, Hu C. (2010) Characterization of an abaecin-like antimicrobial peptide identified from a Pteromalus puparum cDNA clone. J Invertebr Pathol. 105: 24–29. [DOI] [PubMed] [Google Scholar]
- Toke O. (2005) Antimicrobial peptides: new candidates in the fight against bacterial infections. Biopolymers. 80: 717–735. [DOI] [PubMed] [Google Scholar]
- Vilcinskas A. (2013) Evolutionary plasticity of insect immunity. J Insect Physiol. 59: 123–129. [DOI] [PubMed] [Google Scholar]
- Wade JD, Lin F, Condie BA, Hanrieder J, Hoffmann R. (2005) Designer antibacterial peptides kill fluoroquinolone-resistant clinical isolates. J Med Chem. 48: 5349–5359. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin X, Zhu J, Zeng A, Chu F, Yang X, Ma Y. (2009) Expression pattern of antibacterial genes in the Musca domestica. Sci China C Life Sci. 52: 823–830. [DOI] [PubMed] [Google Scholar]
- Wilson R, Chen C, Ratcliffe NA. (1999) Innate immunity in insects: the role of multiple, endogenous serum lectins in the recognition of foreign invaders in the cockroach, Blaberus discoidalis. J Immunol. 162: 1590–1596. [PubMed] [Google Scholar]
- Yakovlev AY. (2011) Induction of antimicrobial peptide synthesis by the fat body cells of maggots of Calliphora vicina R.-D. (Diptera: Calliphoridae). Zh Evol Biokhim Fiziol. 47(6): 461–468. [PubMed] [Google Scholar]
- Ye JS, Zheng XJ, Leung KW, Chen HM, Sheu FS. (2004) Induction of transient ion channel-like pores in a cancer cell by antibiotic peptide. J Biochem. 136: 255–259. [DOI] [PubMed] [Google Scholar]
- Yu Y, Park JW, Kwon HM, Hwang HO, Jang IH, Masuda A, Kurokawa K, Nakayama H, Lee WJ, Dohmae N, Zhang J, Lee BL. (2010) Diversity of innate immune recognition mechanism for bacterial polymeric meso-diaminopimelic acid-type peptidoglycan in insects. J Biol Chem. 285: 32937–32945. [DOI] [PMC free article] [PubMed] [Google Scholar]