Abstract
Background:
Recently, essential oils and extracts derived from plants have received much interest as potential bio-active agents against mosquito vectors.
Methods:
The essential oils extract from fresh peel of ripe fruit of Citrus aurantium and Citrus paradisi were tested against mosquito vector Anopheles stephensi (Diptera: Culicidae) under laboratory condition. Then chemical composition of the essential oil of C. aurantium was analyzed using gas chromatography-mass spectrometry (GC–MS).
Results:
The essential oils obtained from C. aurantium, and C. paradisi showed good larviciding effect against An. stephensi with LC50 values 31.20 ppm and 35.71 ppm respectively. Clear dose response relationships were established with the highest dose of 80 ppm plant extract evoking almost 100% mortality. Twenty-one (98.62%) constituents in the leaf oil were identified. The main constituent of the leaf oil was Dl-limonene (94.81).
Conclusion:
The results obtained from this study suggest that the limonene of peel essential oil of C. aurantium is promising as larvicide against An. stephensi larvae and could be useful in the search for new natural larvicidal compounds.
Keywords: Citrus aurantium, Citrus paradisi, Essential oil, Larvicidal activity, Anopheles stephensi
Introduction
Mosquitoes are very significant vectors from the medical entomology's point of view. They are responsible for the transmission of many diseases to man and animals such as malaria, dengue, yellow fever, encephalitis or filariasis (Eldridge 2000). Human malaria, as a mosquito-borne disease, caused by parasitic protozoa Plasmodium consider as the most important vector-borne disease, which is transmitted only by females of Anopheles mosquitoes (Lehane 1991). Malaria is still an endemic disease in certain foci located in south and southeast of Iran (Manouchehri et al. 1992, Sedaghat et al. 2003 a,b, Vatandoost et al. 2012). In this part of the country six anopheline mosquitoes including An. stephensi, are known as the main malaria vectors (Manouchehri et al. 1976, Sedaghat and Harbach 2005). Anopheles (Cellia) stephensi Liston 1901 is an important malaria vector with wide distribution in the Arabian Peninsula and the Indian subcontinent. It has also distributed in in Khuzestan, Fars, Kerman, Hormozgan, Sistan va Baluchestan and southern Kermanshah provinces in Iran (Manouchehri et al. 1976, Vatandoost et al. 2006, Hanafi et al. 2011).
Several methods have been applied for control of the vectors of the disease including using synthetic pesticides as larvicides or imagicides in the malaria control program. Organophosphate compounds are the most common chemical larvicides which are used in control of Anopheles. However, their toxicity to fish and other non-target organisms and the environment are increased (Mittal et al. 1991, Pinkney et al. 1999). Also resistance of anopheles mosquitoes to these compounds has appeared in many areas (Vatandoost and Borhani 2004, Vatandoost et al. 2004). A lot of attention is being paid to the plant extracts or their essential oils as an alternative source of mosquito larval control agents (Isman 2000, Sedaghat et al 2011a, b, Vatandoost et al. 2012).
Citrus aurantium Linnaeus (Rutaceae) which called as bitter orange or marmalade orange is too sour, but the juice of ripe fruit is used as a condiment in Iran. The peel of C. aurantium is often used in marmalade and dried peel is used in different food and drinks. The flowers are used in tea and its essential oil is used in perfumes and orange-flower water, which is used to flavor sweets (Kiple and Ornelas 2000). The dried whole fruit or peel of the fruit is used in Asian and Western herbal medicine to treat digestive problems (Wichtl 1994). The hydrolate of the flowers has been used for treatment of mild depression, sedation and as a heart tonic for many years in Iran (Zargary 1986, Ayenechi 1991). The studies on biologic effects of C. aurantium indicated potential mosquito repellent, larvicidal and insecticidal activities of this plant (Cetin et al. 2006, Sumroiphon et al. 2006, Yoon et al. 2009).
Citrus paradisi Macfadyen (Rutaceae) or grapefruit like other citrus fruits contains many phytochemicals which contribute to a healthy diet (Fellers et al. 1990). It is used in Persian traditional medicine to treat infected injuries, some digestive problems, cold and helps to lower cholesterol. Recent studies have shown the potential of C. paradise to favorably affect metabolic syndrome, lipid and sugar metabolism (Fujioka et al. 2006, Goldwasser et al. 2010, Ogura et al. 2011). Its seed extract has shown the antimicrobial properties against bacteria and fungi (von et al. 1999).
The objective of this work was to study the effect of the peel essential oils of ripe fruits of C. aurantium and C. paradisi against fourth instar larvae of An. stephensi under laboratory conditions and determine the chemical composition of C. aurantium essential oil.
Materials and Methods
Collection of plant materials
Fresh plants samples of ripe fruit of C. aurantium and C. paradisi were collected in October 2012 from Babol, Iran (52º 41’E, º36 32’N, elevation: −5 m above sea level) and Jiroft (57º 45’E, 28º 37’N, elevation: 650 m above sea level) respectively. The plants were identified and authenticated and the voucher specimen was deposited at Vector Biology Laboratory, Department of Medical Entomology, Tehran University of Medical Sciences, Iran.
Extraction of essential oils
The peel essential oils of fresh (50 g) of C. aurantium and C. paradisi were hydrodis-tilled using a Clevenger-like apparatus (Model: British Pharmacopoeia, Manufactured by Pyrexfan Company, Iran and mantle model EM manufactured by Bibby Scientific Company, United Kingdom) for 3 hours at 70 °C. The yields were averaged over four experiments and calculated according to fresh weight of the plant materials. The obtained oil was dried over anhydrous Na2SO4 and transferred into an airtight amber-colored vial at 4 °C for further experimentation by gas chromatography–mass spectrometry (GC and GC-MS).
GC and GC/MS analysis of essential oil
GC analysis was carried out using an HP6890 gas chromatograph equipped with flame ionization detector and an Hp-1 capillary column (30 m× 0.25 mm I.D., film thickness 0.25 μm) and split ratio, 1:25. The GC settings were as follows: initial oven temperature was held at 40 °C for 1 min, rising to 250 °C at 5 °C/min. The injector temperature was maintained at 250 °C. The detector temperature was at 230 °C. The carrier gas used was Helium at a flow rate of 1 ml/min.
GC-MS was performed on Agilent Technology 5973 mass selective detector connected with an HP 6890 gas chromatograph. The oil of C. aurantium was analyzed using an HP-1MS (Fused silica) with the same column and temperature programmed as above. The MS operated at 70 eV ionization energy. Quantitative data were obtained from the electronic integration of the Flame Ionization Detector (FID) peak areas.
Determination of oil composition
Identification of the oil components were assigned based on retention indices which were calculated by using retention times of n-alkanes that were injected after the oil at the same chromatographic conditions. The compounds were identified by comparison of their relative retention indices and with those in the literature. In addition, computer searching followed by matching of the mass spectra data with those stored in the computer library. The percentage of each component is presented in Table 1.
Table 1.
Probit regression line parameters of Anopheles stephensi to peel essential oil extraction of Citrus aurantium and C. aurantium at different interval concentrations
| Species | A | B±SE | LC50, 95% C.I. | LC90, 95% C.I. | X2 (df) | Heterogeneity P-value |
|---|---|---|---|---|---|---|
| C. aurantium | −5.12 | 3.43±0.28 | 28.17 | 63.30 | 3.15 (2) | > 0.05 |
| 31.20 | 73.83 | |||||
| 34.62 | 90.08 | |||||
| C. paradisi | −5.51 | 3.55±0.29 | 32.28 | 82.03 | 2.54 (2) | > 0.05 |
| 35.71 | 70.23 | |||||
| 39.62 | 100.47 |
A= y-intercept, B= the slope of the line, SE= Standard error, LC50, 95% CI= lethal concentration causing 50% mortality and its 95% confidence interval, LC90, 95% CI= lethal concentration causing 90% mortality and its 95% confidence interval, X2= heterogeneity about the regression line.
Mosquito culture
Fourth instar larvae Anopheles stephensi was used in this study. All larvae of An. stephensi were obtained from laboratory culture of Department of Medical Entomology, Tehran University Medical Sciences (TUMS). The Anopheles colony was maintained at 27 °C with 12: 12 light and dark photoperiod in 60±10% relative humidity.
Larvicidal bioassay
Tests of mosquito larval activity were conducted with reference to the standard method recommended by the World Health Organization (WHO 2005). Since the essential oils do not dissolve in water ethanol 99.0% was used as co-solvent. Different concentrations of the essential oils in distillated water and the co-solvent were prepared. The oil-ethanol-water solution was gently stirred for 30 seconds with a glass rod to ensure a homogeneous test solution and each glass beaker was left at room temperature. After 15 minutes 20 larvae were taken with a fine mesh strainer and transferred gently to a 400 ml glass beaker. Control group included batches of mosquitoes from the colony exposed to water and the solvent alone. The larvae were exposed to the concentrations of 10, 20, 40, 80 and 160 ppm of essential oil in distilled water for 24 hours at room temperature. In the control beakers only 1 ml of solvent was added to each beaker. Mortality was recorded after 24 h of exposure while during the test no food was given to the larvae. Each treatment was done with five replicates.
Statistical analysis
Toxicity and activity were reported as LC 50 and LC90, representing the concentrations in ppm that killed 50% and 90%, respectively of larvae in 24 h. The LC50 and LC90 values and their 95% confidence intervals of each essential oil were calculated by log concentration-probit equation (Finney 1971) using the SPSS 16.0 probit procedure. Controls with mortality between 5–20% were corrected using Abbott's formula (Abbott 1925). When mortality in controls exceeded 20%, test results were rejected or repeated. Comparison of the LC50 and LC90 values were analyzed using ANOVA Test with SPSS version 17.0. Differences between means were considered significant at P≤ 0.05.
Result
Yields and chemical constituents of essential oil
The yields of peel essential oils of C. aurantium and C. paradisi were 0.7±0.12% and 0.85±0.14% (w/w) based on fresh weight respectively. The chemical composition of C. aurantium peel essential oil is presented in Table 2. A total of 21 compounds were identified representing about 98.62% of the oil. The results revealed that terpenoids in the oil were predominant. The main constituents in the C. aurantium peel essential oil were Dl-limonene (94.81%), β-myrcene (1%) and α-pinene (0.65%) respectively.
Table 2.
Chemical constituents of peel essential oil from Citrus aurantium
| n | Compound | KIa | Concentration (%) |
|---|---|---|---|
| 1 | α-Pinene | 909 | 0.30 |
| 2 | β-Pinene | 947 | 0.65 |
| 3 | β-Myrcene | 958 | 1.00 |
| 4 | Dl-Limonene | 997 | 94.81 |
| 5 | Trans-Ocimene | 1016 | 0.19 |
| 6 | Gamma-Terpinene | 1029 | 0.01 |
| 7 | Linalool Oxide | 1039 | 0.04 |
| 8 | 1-Octanol | 1043 | 0.13 |
| 9 | Trans-Linalool Oxide | 1044 | 0.02 |
| 10 | Isoterpinolene | 1045 | 0.01 |
| 11 | Nonanal | 1054 | 0.02 |
| 12 | α-Terpinolene | 1057 | 0.44 |
| 13 | Linalyl acetate | 1234 | 0.32 |
| 14 | Sabinene Hydrate Acetate | 1235 | 0.03 |
| 15 | Neryl Acetate | 1338 | 0.03 |
| 16 | geranyl acetate | 1358 | 0.13 |
| 17 | trans-Caryophyllene | 1386 | 0.03 |
| 18 | Germacrene-D | 1453 | 0.08 |
| 19 | Nerolidol | 1536 | 0.06 |
| 20 | Palmitic Acid | 1929 | 0.06 |
| 21 | Trans-Oleic Acid | 1930 | 0.26 |
| Total | 98.62 | ||
Larvicidal activity of essential oils
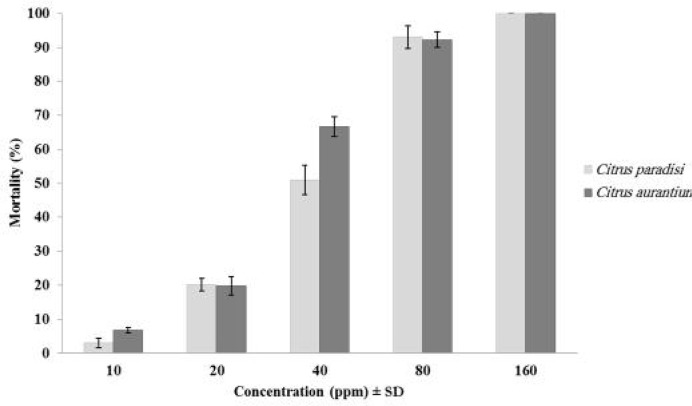
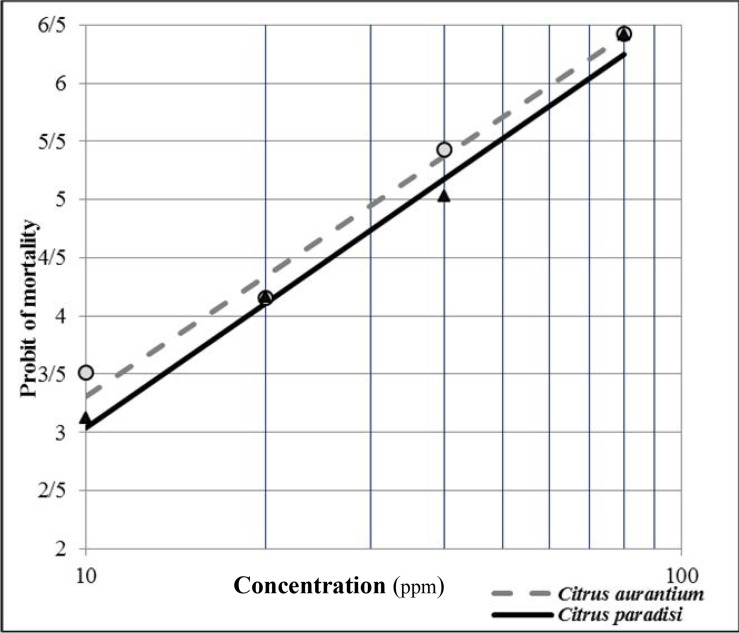
The LC50 and LC90 values of C. aurantium and C. paradisi oils against An. stephensi larvae were 31.20 ppm and 73.83 ppm, 35.71 ppm and 70.23 respectively (Table 1). Both peels essential oils at the 80 ppm concentrations killed more than 90% of the fourth instars larvae (Fig. 1). The mortality rates in the control groups were lower than 5% in all concentrations, no correction were applied. The probit regression lines of An. stephensi exposed to different interval concentrations of a C. aurantium and C. paradisi extractions are shown in Fig 2. The statistical test (ANOVA) showed that there was no significant statistical difference in mortality rate among two essential oils (P≤ 0.628).
Fig. 1.
Percentage of larval mortality of Anopheles. stephensi after treatment with Citrus aurantium and C. paradise
Fig. 2.
Probit regression line of Anopheles stephensi exposed to different interval concentrations of a Citrus aurantium in comparison with C. paradisi essential oils
Discussion
During past five decades using of chemical insecticides against vector mosquitoes have been developed the resistance in vectors to the insecticides and also hazards to the environment (Mittal 1991, Gunasekaran et al. 2004, Vatandoost et al. 2012). Although chemical larval control is considered as a major component in malaria prevention strategies, this part of control has side-effects on human and animal health and also the environment (Sedaghat et al. 2010, Vatandoost et al. 2012). In order to reduce the dependency on chemical insecticides, alternative methods including botanical insecticides for the control of vectors are considered. Certain plant's essential oils or extracts have been found effective for mosquito larval control. It is important to identify chemical constituents of the indigenous plans and their efficacies of essential oils as natural larvicides.
The yield of oil obtained of C. aurantium was 0.7%. It was nearly as same as reported from Pakistan (Siddique et al. 2011) but less than as reported in Egypt (Hifnaway et al. 2004). Gas chromatography–mass spectrometry of peel essential oil of C. aurantium revealed the presence of 21 components. In this study, major constituent of peel essential oil of C. aurantium was Dl-limonene, 94.81 % of the oil. In previous studies, various constituents of the oil of C. aurantium were reported. Although limonene is the most abundant constituent in the oils obtained from all similar studies, its percentage were varied, based on the origins of the plant (Caccioni et al. 1998, Boussaada and Chemli 2007, Moraes et al. 2009, Hosni et al. 2010). The result is in agreement with the results obtained from other study in Iran (Hosni et al. 2010). The other constituents of the peel oil were α-pinene (0.30%) and β-pinene (0.65%), while the results obtain from a study in Pakistan α-pinene and β-pinene were reported 0.476% and 0.176% respectively (Siddique et al. 2011). The result of the larval bioassay tests showed that essential oils of C. aurantium and C. paradise have a same level of bioactivity against An. stephensi larvae. Previous studies have demonstrated the same level of larvicidal activity against mosquito larvae. The LC50 of the essential oil of C. paradise were 47.3 ppm and 85.1 ppm for Ae. aegypti and Ae. albopictus, respectively (Morales-Saldañaet al. 2007). In another study reported by Boussaada and Chemli, the content of limonene in Tunisian C. aurantium from 87 % to 92.2% on fresh weight basis (Boussaada and Chemli 2007). The results nearly similar from the study of Caccioni et al. (1998) which reported limonene (94.3%) myrcene (1.88%) as the main constituents of C.s aurantium. In another study on the peel oil composition of Brazilian C. aurantium, limonene (97.5–98%), myrcene (1.2–1.45%) and octanol (0.34–0.54%) were found as the main constituents (Moraes et al. 2009). The results of constituents of the peel oil of C. aurantium obtained from this study are similar with all prior studies.
Our previous studies on the same strain of An. stephensi larvae revealed that the efficacy of Eucalyptus camaldulensis, Cupressus arizonica, Coriandrum sativum and Heracleum persicum oils were less than the efficacy of C. aurantium and C. paradise oils, while the efficacy of Foeniculum vulgare and Kelussia odoratissima are more than the two Citrus (Sedaghat et al. 2010, 2011a,b, Vatandoost et al. 2012).
According to the classification of Vatandoost et al. (2012) the level of larvicidal activity of C. aurantium and C. paradise demonstrated them as active plants. If we accept Cheng et al. (2003) suggestion, these plants should be considered as very active. Based on both above classifications these two plants need more attention as they lied in active or very active categories.
Results on the larval mortality of C. aurantium and C. paradise oils against An. stephensi confirm their potential to control of the mosquito populations. It seems that the presence of a high amount of Dl-limonene in C. aurantium oil, could demonstrate its efficacy against An. stephensi larvae. In brief, this study clearly illustrated the potential use of C. aurantium and C. paradise as natural mosquito larvicides.
Conclusion
Essential oils from aromatic plants are the complex mixture of constituents with several usages in health and medical sciences. The essential oils of C. aurantium and C. paradise oils show larvicideal activity against An. stephensi the main vector of malaria in Indo-Persian areas including southern areas of Iran. The results obtained from this study suggest that the limonene of peel essential oil of C. aurantium is promising as larvicide and could be useful in the search for new natural larvicidal compounds.
Acknowledgements
The authors would like to appreciate Dr Khanavi for her comments. Special thanks to Department of Medical Entomology and Vector Control, School of Public Health, TUMS for providing the test mosquito, An. stephensi.
References
- Abbott WS. (1987) A method of computing the effectiveness of an insecticide. J Am Mosq Control Assoc. 3: 302–303. [PubMed] [Google Scholar]
- Aynehchi Y. (1991) Pharmacognosy and Medicinal Plant of Iran. Tehran University Publication, Tehran. [Google Scholar]
- Wichtl M. (2004) Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis CRC Press, New York. [Google Scholar]
- Boussaada O, Chemli R. (2007) Seasonal variation of essential oil composition of Citrus aurantium L. Var amara. J. Essent. Oil Bear. Pl. 10: 109–120. [Google Scholar]
- Caccioni DRL, Guizzardi M, Biondi DM, Agatino R, Ruberto G. (1998) Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int J of Food Microbiol. 43: 73–79. [DOI] [PubMed] [Google Scholar]
- Cheng SS, Chang HT, Chang ST, Tsai KH, Chen WJ. (2003) Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour Technol. 89: 99–102. [DOI] [PubMed] [Google Scholar]
- Cetin H, Erler F, Yanikoglu A. (2006) Toxicity of essential oils extracted from Origanum onites L. and Citrus aurentium L. against the pine processionary moth, Thaumetopoea wilkinsoni Tams. Folia Biol (Krakow). 54: 153–157. [DOI] [PubMed] [Google Scholar]
- Eldridge BF. (2000) Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods. Kluwer Academic Publications, Boston, MA. [Google Scholar]
- Fellers PJ, Nikdel S, Lee HS. (1990) Nutrient content and nutrition labeling of several processed Florida citrus juice products. J Am Diet Assoc. 90: 1079–1084. [PubMed] [Google Scholar]
- Finney DJ. (1971) Probit analysis. Cambridge University Press, London. [Google Scholar]
- Fujioka K, Greenway F, Sheard J, Ying Y. (2006) The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. J Med Food. 9: 49–54. [DOI] [PubMed] [Google Scholar]
- Goldwasser J, Cohen PY, Yang E, Balaguer P, Yarmush ML, Nahmias Y. (2010) Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: role of PPARalpha, PPARgamma and LXRalpha. PLoS One. 5 (8): e12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekaran K, Boopathi Doss PS, Vaidyanathan K. (2004) Laboratory and field evaluation of Teknar HP-D, a biolarvicidal formulation of Bacillus thuringiensis ssp. israelensis, against mosquito vectors. Acta Tropica 92: 109–118. [DOI] [PubMed] [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, Haghdoost AA, Shahi M, Sedaghat MM, Abedi F, Yeryan M, Pakari A. (2012) Entomological and Epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 121: 85–92. [DOI] [PubMed] [Google Scholar]
- Hifnawy MS, Azzam SM, Sabry OMM. (2004) Essential oils, pectins and lipids of certain Citrus species growing in Egypt. Bull Fac pharm. 42(2): 177–192. [Google Scholar]
- Hosni K, Zahed N, Chrif Rf, Abid I, Medfei W, Kallel M, Brahim NB, Sebei H. (2010) Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 123: 1098–1104. [Google Scholar]
- Isman MB. (2000) Plant essential oils for pest and disease management. Crop Prot. 19: 603–608. [Google Scholar]
- Kiple KF. (2000) The Cambridge world history of food. Vol. 2. Cambridge University Press, London. [Google Scholar]
- Lehane MJ. (1991) Biology of Blood-Sucking Insects. Harper Collins Academic, London, UK. [Google Scholar]
- Manouchehri AV, Javadian E, Eshighy N, Motabar M. (1976) Ecology of Anopheles stephensi Liston in southern Iran. Trop Geogr Med. 28: 228–232. [PubMed] [Google Scholar]
- Manouchehri AV, Zaim M, Emadi AM. (1992) A review of malaria in Iran, 1975–90. J Am Mosq Control Assoc. 8: 381–385. [PubMed] [Google Scholar]
- Ogura M, Yagi M, Nomoto K, Ryo Miyazaki Ryo, Kongoji M, Watanabe S, Hamada U, Yonei Y. (2011) Effect of Grapefruit Intake on Postprandial Plasma Glucose. Anti-Aging Med. 8: 60–68 [Google Scholar]
- Mittal PK, Adak T, Sharma VP. (1991) Acute toxicity of certain organochlorine, organophosphorus, synthetic pyrethroid and microbial insecticides to the mosquito fish Gambusia affinis (Baird and Girard). Indian J Malariol. 28: 167–170. [PubMed] [Google Scholar]
- Morales-Saldana J, Gomez N, Rovira J, Abrahams M. (2007) Larvicidal activity of the grapefruit Citrus paradisi (Rutaceae) on two vectors of dengue. Rev Peru Biol. 14: 297–299. [Google Scholar]
- Moraes TM, Kushima H, Moleiro FC, Santos RC, Rocha LR, Marques MO, Vilegas W, Hiruma-Lima CA. (2009) Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem Biol Interact. 180: 499–505. [DOI] [PubMed] [Google Scholar]
- Pinkney AE, McGowan PC, Murphy DR, Lowe TP, Sparling DW, Meredith WH. (1999) Effects of temephos (Abate 4E) on fiddler crabs (Uca pugnax and Uca minax) on a Delaware salt marsh. J Am Mosq Control Assoc. 15: 321–329. [PubMed] [Google Scholar]
- Sedaghat MM, Linton YM, Nicolescu G, Smith L, Koliopoulos G, Zounos AK, Oshaghi MA, Vatandoost H, Harbach RE. (2003a) Morphological and molecular characterization of Anopheles (Anopheles) sacharovi Favre, a primary vector of malaria in the Middle East. Syst Entomol. 28: 241–256. [Google Scholar]
- Sedaghat MM, Linton YM, Oshaghi MA, Vatandoost H, Harbach RE. (2003b) The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterization and recognition of a new species. Bull Entomol Res. 93: 527–535. [DOI] [PubMed] [Google Scholar]
- Sedaghat MM, Harbach RE. (2005) An annotated checklist of the Anopheles mosquitoes (Diptera: Culicidae) in Iran. J Vector Ecol. 30: 272–276. [PubMed] [Google Scholar]
- Sedaghat MM, Dehkordi AS, Khanavi M, Abai MR, Hadjiakhoondi A, Mohtarami F, Vatandoost H. (2010) Phyto-chemistry and larvicidal activity of Eucalyptus camaldulensis against malaria vector, Anopheles stephensi. Asian Pac J Trop Med. 3: 841–845. [Google Scholar]
- Sedaghat M, Dehkordi AS, Abai M, Khanavi M, Mohtarami F, Abadi YS, Rafi F, Vatandoost H. (2011a) Larvicidal Activity of Essential Oils of Apiaceae Plants against Malaria Vector, Anopheles stephensi. Iran J Arthropod Borne Dis. 5: 51–59. [PMC free article] [PubMed] [Google Scholar]
- Sedaghat MM, Dehkordi AS, Khanavi M, Abai MR, Mohtarami F, Vatandoost H. (2011b) Chemical composition and larvicidal activity of essential oil of Cupressus arizonica E.L. Greene against malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Pharmacognosy Res. 3: 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique S, Shafique M, Parveen Z, Khan SJ, Khanum R. (2011) Volatile components antioxidant and antimicrobial activity of Citrus auratium var bitter orange peel oil. PhOL. 2: 499–507. [Google Scholar]
- Sumroiphon S, Yuwaree C, Arunlertaree C, Komalamisra N, Rongsriyam Y. (2006) Bioactivity of citrus seed for mosquito-borne diseases larval control. Southeast Asian J Trop Med Public Health. 37: 123–127. [PubMed] [Google Scholar]
- Vatandoost H, Oshaghi MA, Abaie MR, Shahi M, Yaaghoobi F, Baghaii M, Hanafi-Bojd AA, Zamani G, Townson H. (2006) Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran, 2002. Acta Trop. 97: 196–203. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Borhani N. (2004) Susceptibility level and irritability of synthetic pyrethroids against main malaria vectors in the endemic areas of Iran. Acta Med Iran. 42: 247–255. [Google Scholar]
- Vatandoost H, Shahi H, Abai MR, Hanafi-Bojd AA, Oshaghi MA, Zamani G. (2004) Larval habitats of main malaria vectors in Hormozgan Province and their susceptibility to different larvicides. Southeast Asian J Trop Med Public Health. 35: 22–25. [PubMed] [Google Scholar]
- Vatandoost H, Sanei Dehkordi A, Sadeghi SM, Davari B, Karimian F, Abai MR, Sedaghat MM. (2012) Identification of 1 chemical constituents and larvicidal activity of Kelussia odoratissima Mozaffarian essential oil against two mosquito vectors Anopheles stephensi and Culex pipiens (Diptera: Culicidae). Exp Parasitol. 132: 470–474. [DOI] [PubMed] [Google Scholar]
- von WT, Schluter B, Pflegel P, Lindequist U, Julich WD. (1999) Aspects of the antimicrobial efficacy of grapefruit seed extract and its relation to preservative substances contained. Pharmazie. 54: 452–456. [PubMed] [Google Scholar]
- WHO (2005) Guidelines for laboratory and field testing of mosquito larvicides, WHO/CDS/WHOPES/GCDPP/2005.13. Geneva, Switzerland. [Google Scholar]
- Yoon C, Kang SH, Yang JO, Noh DJ, Indiragandhi P, Kim GH. (2009) Repellent activity of citrus oils against the cockroaches Blattella germanica, Periplaneta americana and P. fuliginosa. J Pestic Sci. 34: 77–88. [Google Scholar]
- Zargari A. (1986) Medicinal Plants. Tehran University Press, Tehran. [Google Scholar]