Abstract
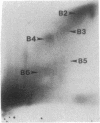
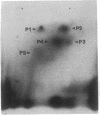
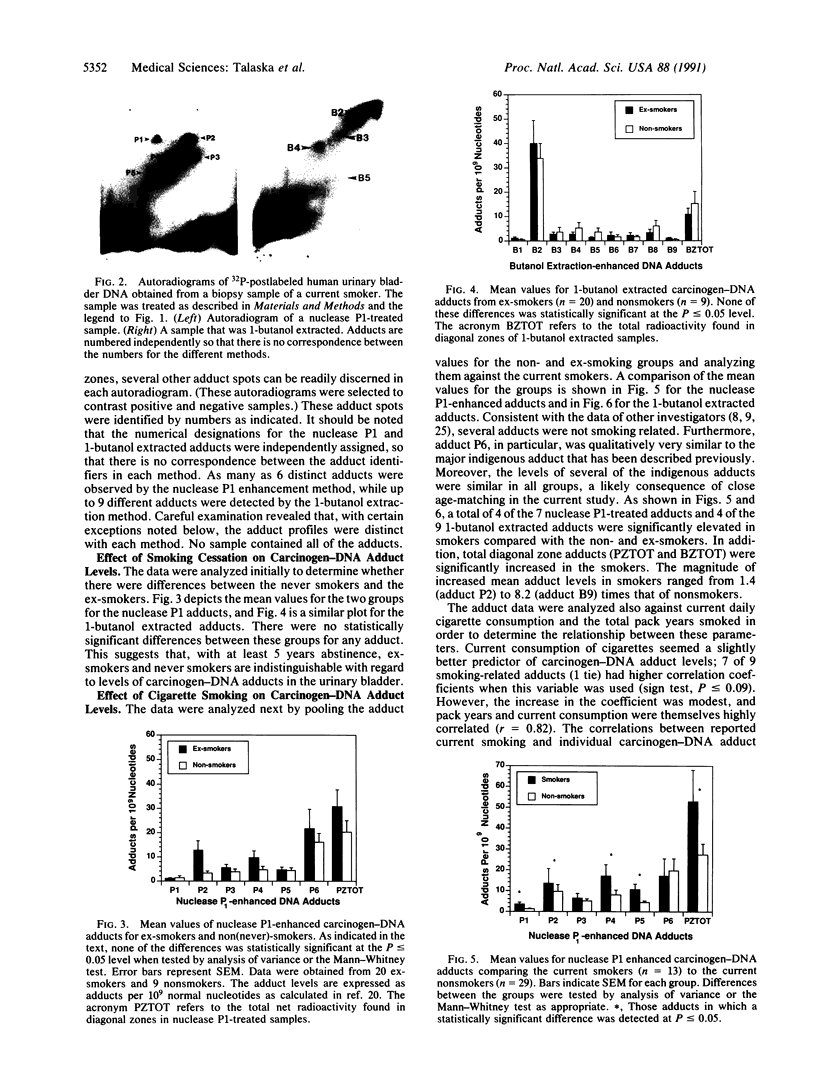
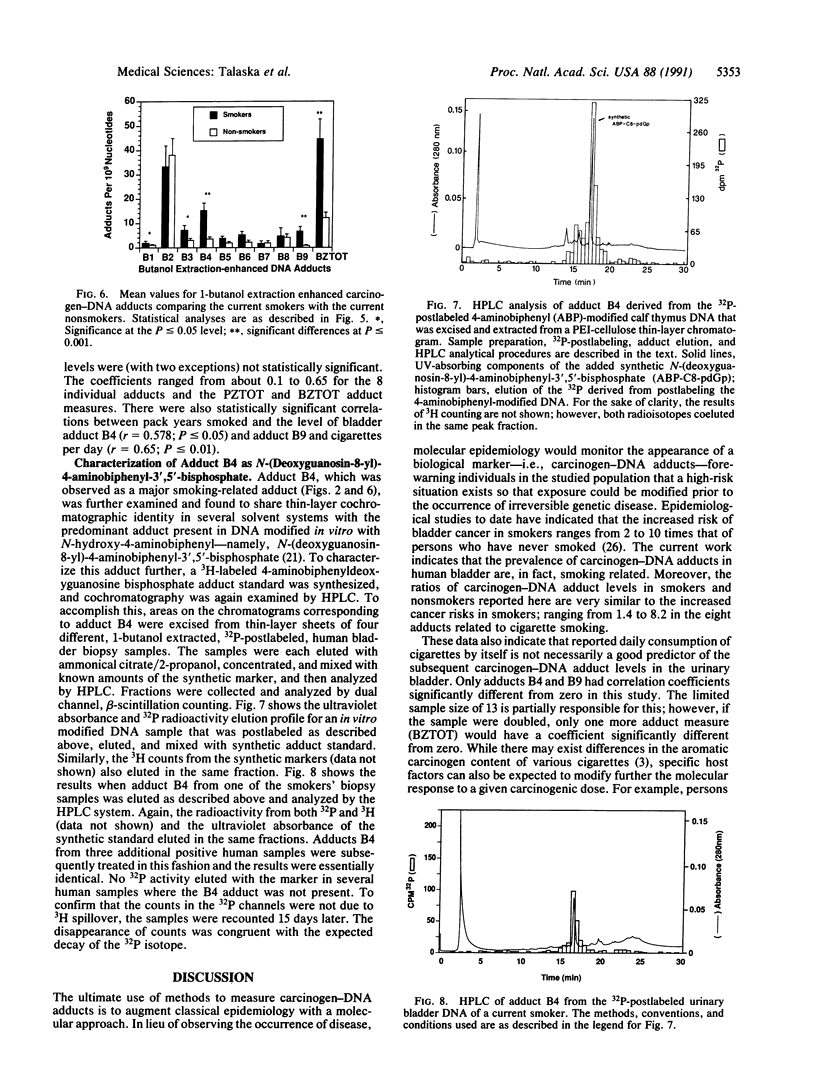
The prevalence of covalent modifications to DNA (carcinogen-DNA adducts) in 42 human urinary bladder biopsy samples was investigated by 32P-postlabeling methods, with enhancement by both nuclease P1 treatment and 1-butanol extraction. Total mean carcinogen-DNA adduct levels and the mean levels of several specific adducts were significantly elevated in DNA samples of 13 current smokers, as opposed to 9 never smokers or 20 ex-smokers (5 years abstinence). There was no significant difference between the latter two groups. Several DNA adducts enhanced by nuclease P1 treatment were chromatographically similar to putative hydrocarbon DNA adducts reported earlier for placenta and lung DNA samples obtained from cigarette smokers. Putative aromatic amine adducts were detected by 1-butanol extraction that were not present when the samples were treated with nuclease P1. One of these displayed chromatographic behavior identical to the predominant adduct induced by the human urinary bladder carcinogen, 4-aminobiphenyl, which is present in cigarette smoke. This adduct comigrated in several thin-layer chromatographic systems with a synthetic N-(deoxyguanosin-8-yl)-4-amino[2,2'-3H]biphenyl-3',5'-bisphosphate marker. Moreover, when this adduct was eluted from the thin-layer chromatograms of several individuals and injected onto an HPLC system, the 32P from the human bladder DNA samples coeluted in the same fraction as the tritiated synthetic N-(deoxyguanosin-8-yl)-4-aminobiphenyl marker. These data reinforce an association between cigarette smoking and DNA damage and suggest a molecular basis for the initiation of human urinary bladder cancer by cigarette smoke.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce G. T., Cline D. T., Jr, Mead J. E. The 32P-post-labeling method in quantitative DNA adduct dosimetry of 2-acetylaminofluorene-induced mutagenicity in Chinese hamster ovary cells and Salmonella typhimurium TA1538. Carcinogenesis. 1987 Apr;8(4):515–520. doi: 10.1093/carcin/8.4.515. [DOI] [PubMed] [Google Scholar]
- Bryant M. S., Skipper P. L., Tannenbaum S. R., Maclure M. Hemoglobin adducts of 4-aminobiphenyl in smokers and nonsmokers. Cancer Res. 1987 Jan 15;47(2):602–608. [PubMed] [Google Scholar]
- Butler M. A., Iwasaki M., Guengerich F. P., Kadlubar F. F. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J., Routledge M. N., Jenkins D., Garner R. C. DNA adducts in different tissues of smokers and non-smokers. Int J Cancer. 1990 Apr 15;45(4):673–678. doi: 10.1002/ijc.2910450417. [DOI] [PubMed] [Google Scholar]
- Everson R. B., Randerath E., Santella R. M., Cefalo R. C., Avitts T. A., Randerath K. Detection of smoking-related covalent DNA adducts in human placenta. Science. 1986 Jan 3;231(4733):54–57. doi: 10.1126/science.3941892. [DOI] [PubMed] [Google Scholar]
- Gallagher J. E., Jackson M. A., George M. H., Lewtas J., Robertson I. G. Differences in detection of DNA adducts in the 32P-postlabelling assay after either 1-butanol extraction or nuclease P1 treatment. Cancer Lett. 1989 Apr;45(1):7–12. doi: 10.1016/0304-3835(89)90029-3. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Earley K. 32P-adduct assay: comparative recoveries of structurally diverse DNA adducts in the various enhancement procedures. Carcinogenesis. 1988 Sep;9(9):1687–1693. doi: 10.1093/carcin/9.9.1687. [DOI] [PubMed] [Google Scholar]
- Gupta R. C. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res. 1985 Nov;45(11 Pt 2):5656–5662. [PubMed] [Google Scholar]
- Gupta R. C. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Reddy M. V., Randerath K. 32P-postlabeling analysis of non-radioactive aromatic carcinogen--DNA adducts. Carcinogenesis. 1982;3(9):1081–1092. doi: 10.1093/carcin/3.9.1081. [DOI] [PubMed] [Google Scholar]
- Jahnke G. D., Thompson C. L., Walker M. P., Gallagher J. E., Lucier G. W., DiAugustine R. P. Multiple DNA adducts in lymphocytes of smokers and nonsmokers determined by 32P-postlabeling analysis. Carcinogenesis. 1990 Feb;11(2):205–211. doi: 10.1093/carcin/11.2.205. [DOI] [PubMed] [Google Scholar]
- Kadlubar F. F., Talaska G., Butler M. A., Teitel C. H., Massengill J. P., Lang N. P. Determination of carcinogenic arylamine N-oxidation phenotype in humans by analysis of caffeine urinary metabolites. Prog Clin Biol Res. 1990;340B:107–114. [PubMed] [Google Scholar]
- Lasko D. D., Basu A. K., Kadlubar F. F., Evans F. E., Lay J. O., Jr, Essigmann J. M. A probe for the mutagenic activity of the carcinogen 4-aminobiphenyl: synthesis and characterization of an M13mp10 genome containing the major carcinogen-DNA adduct at a unique site. Biochemistry. 1987 Jun 2;26(11):3072–3081. doi: 10.1021/bi00385a019. [DOI] [PubMed] [Google Scholar]
- Lu L. J., Disher R. M., Reddy M. V., Randerath K. 32P-postlabeling assay in mice of transplacental DNA damage induced by the environmental carcinogens safrole, 4-aminobiphenyl, and benzo(a)pyrene. Cancer Res. 1986 Jun;46(6):3046–3054. [PubMed] [Google Scholar]
- Maclure M., Katz R. B., Bryant M. S., Skipper P. L., Tannenbaum S. R. Elevated blood levels of carcinogens in passive smokers. Am J Public Health. 1989 Oct;79(10):1381–1384. doi: 10.2105/ajph.79.10.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B. W., Cerniglia C. E., Federle T. W. Metabolism of the benzidine-based azo dye Direct Black 38 by human intestinal microbiota. Appl Environ Microbiol. 1985 Jul;50(1):10–15. doi: 10.1128/aem.50.1.10-15.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommsen S., Aagaard J. Tobacco as a risk factor in bladder cancer. Carcinogenesis. 1983;4(3):335–338. doi: 10.1093/carcin/4.3.335. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Martin C. N., Garner R. C., King M. M. Correlation of DNA adduct levels in human lung with cigarette smoking. Nature. 1988 Dec 22;336(6201):790–792. doi: 10.1038/336790a0. [DOI] [PubMed] [Google Scholar]
- Randerath K., Liehr J. G., Gladek A., Randerath E. Age-dependent covalent DNA alterations (I-compounds) in rodent tissues: species, tissue and sex specificities. Mutat Res. 1989 Mar;219(2):121–133. doi: 10.1016/0921-8734(89)90023-4. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Disher R. M. Age- and tissue-related DNA modifications in untreated rats: detection by 32P-postlabeling assay and possible significance for spontaneous tumor induction and aging. Carcinogenesis. 1986 Sep;7(9):1615–1617. doi: 10.1093/carcin/7.9.1615. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Steineck G., Norell S. E., Feychting M. Diet, tobacco and urothelial cancer. A 14-year follow-up of 16,477 subjects. Acta Oncol. 1988;27(4):323–327. doi: 10.3109/02841868809093549. [DOI] [PubMed] [Google Scholar]
- Talaska G., Au W. W., Ward J. B., Jr, Randerath K., Legator M. S. The correlation between DNA adducts and chromosomal aberrations in the target organ of benzidine exposed, partially-hepatectomized mice. Carcinogenesis. 1987 Dec;8(12):1899–1905. doi: 10.1093/carcin/8.12.1899. [DOI] [PubMed] [Google Scholar]
- Talaska G., Dooley K. L., Kadlubar F. F. Detection and characterization of carcinogen-DNA adducts in exfoliated urothelial cells from 4-aminobiphenyl-treated dogs by 32P-postlabelling and subsequent thin-layer and high-pressure liquid chromatography. Carcinogenesis. 1990 Apr;11(4):639–646. doi: 10.1093/carcin/11.4.639. [DOI] [PubMed] [Google Scholar]
- Vineis P., Caporaso N., Tannenbaum S. R., Skipper P. L., Glogowski J., Bartsch H., Coda M., Talaska G., Kadlubar F. Acetylation phenotype, carcinogen-hemoglobin adducts, and cigarette smoking. Cancer Res. 1990 May 15;50(10):3002–3004. [PubMed] [Google Scholar]