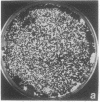
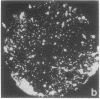
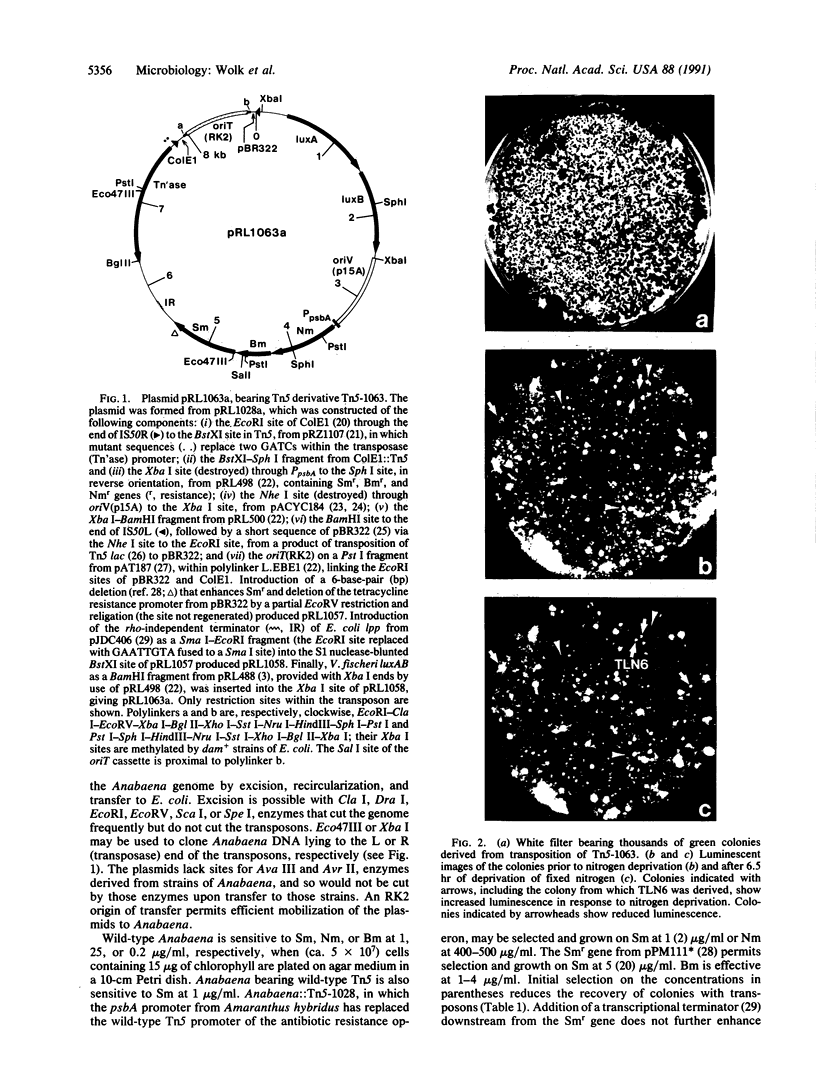
Abstract
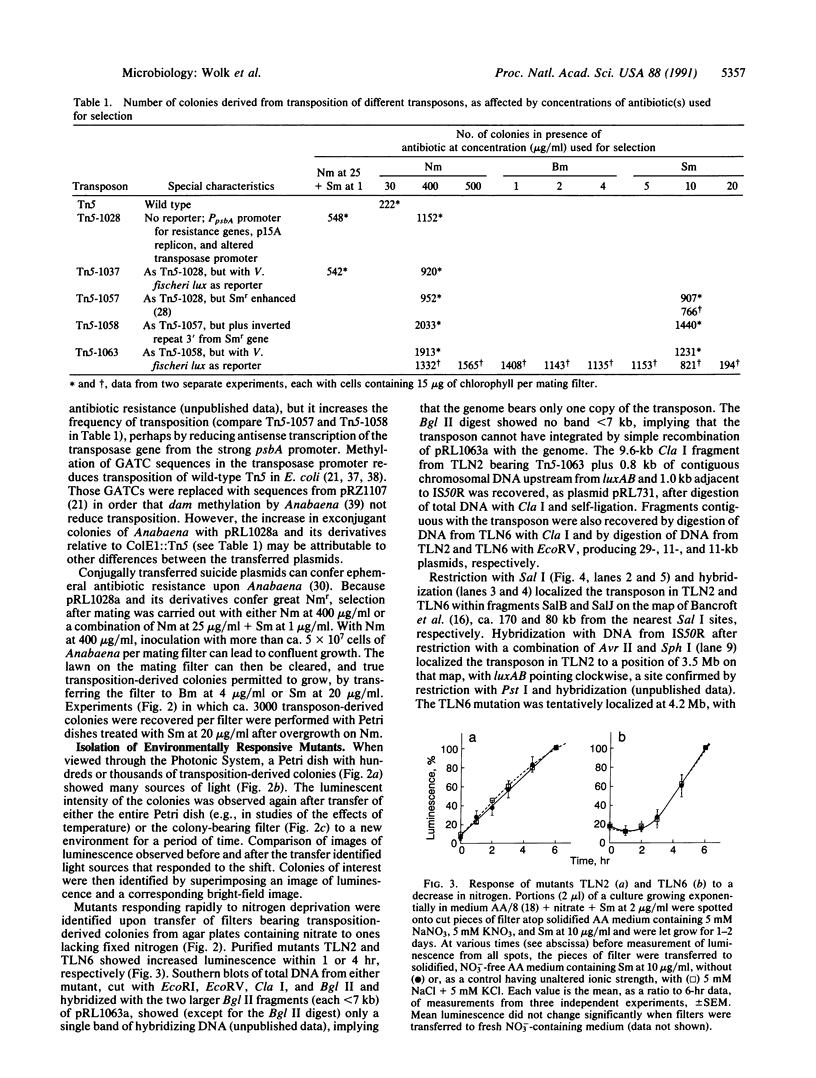
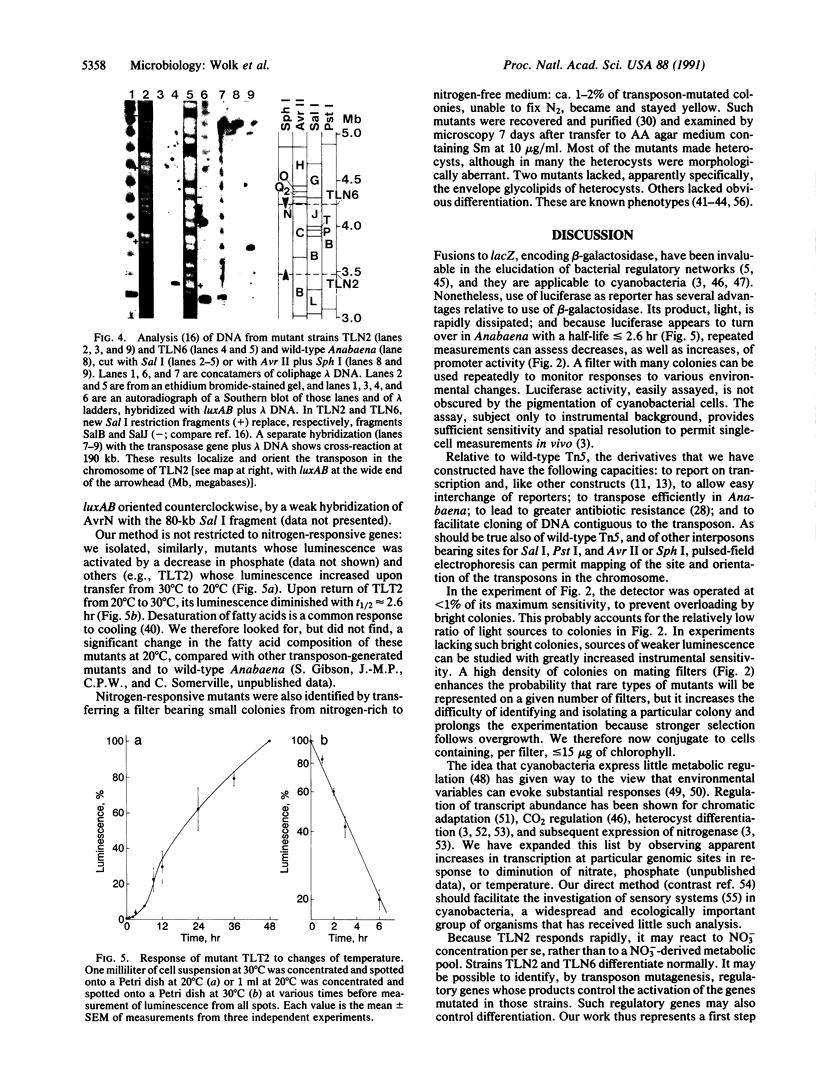
Anabaena, a filamentous cyanobacterium, is of developmental interest because, when deprived of fixed nitrogen, it shows patterned differentiation of N2-fixing cells called heterocysts. To help elucidate its early responses to a decrease in nitrogen, we used a derivative of transposon Tn5 to generate transcriptional fusions of promoterless bacterial luciferase genes, luxAB, to the Anabaena genome. Genes that responded to removal of fixed nitrogen or to other environmental shifts by increased or decreased transcription were identified by monitoring the luminescence of colonies from transposon-generated libraries. The Tn5 derivative transposed in Anabaena at ca. 1-4 x 10(-5) per cell and permitted high-resolution mapping of its position and orientation in the genome and facile cloning of contiguous genomic DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balbás P., Soberón X., Merino E., Zurita M., Lomeli H., Valle F., Flores N., Bolivar F. Plasmid vector pBR322 and its special-purpose derivatives--a review. Gene. 1986;50(1-3):3–40. doi: 10.1016/0378-1119(86)90307-0. [DOI] [PubMed] [Google Scholar]
- Bancroft I., Wolk C. P., Oren E. V. Physical and genetic maps of the genome of the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1989 Nov;171(11):5940–5948. doi: 10.1128/jb.171.11.5940-5948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin R., Chalifour F. P., Dion P. Construction of a Tn5 derivative encoding bioluminescence and its introduction in Pseudomonas, Agrobacterium and Rhizobium. Mol Gen Genet. 1988 Jul;213(1):50–55. doi: 10.1007/BF00333397. [DOI] [PubMed] [Google Scholar]
- Borthakur D., Haselkorn R. Tn5 mutagenesis of Anabaena sp. strain PCC 7120: isolation of a new mutant unable to grow without combined nitrogen. J Bacteriol. 1989 Oct;171(10):5759–5761. doi: 10.1128/jb.171.10.5759-5761.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Haselkorn R. Isolation and complementation of nitrogen fixation mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 1991 Mar;173(6):1879–1885. doi: 10.1128/jb.173.6.1879-1885.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Cai Y. P., Wolk C. P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990 Jun;172(6):3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. T., Ohmori H., Tomizawa J., Lebowitz J. Nucleotide sequence and gene organization of ColE1 DNA. J Biol Chem. 1985 Jul 25;260(15):8925–8935. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Hirashima A., Inokuchi Y., Green P. J., Inouye M. A novel immune system against bacteriophage infection using complementary RNA (micRNA). Nature. 1985 Jun 13;315(6020):601–603. doi: 10.1038/315601a0. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Dodson K. W., Berg D. E. Factors affecting transposition activity of IS50 and Tn5 ends. Gene. 1989;76(2):207–213. doi: 10.1016/0378-1119(89)90161-3. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988 Aug 15;68(1):119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- Elhai J., Wolk C. P. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990 Oct;9(10):3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Foran D. R., Brown W. M. Nucleotide sequence of the LuxA and LuxB genes of the bioluminescent marine bacterium Vibrio fischeri. Nucleic Acids Res. 1988 Jan 25;16(2):777–777. doi: 10.1093/nar/16.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J., Pollitt N. S., Inouye M. Major cold shock protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jan;87(1):283–287. doi: 10.1073/pnas.87.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–441. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- Guijarro J., Santamaria R., Schauer A., Losick R. Promoter determining the timing and spatial localization of transcription of a cloned Streptomyces coelicolor gene encoding a spore-associated polypeptide. J Bacteriol. 1988 Apr;170(4):1895–1901. doi: 10.1128/jb.170.4.1895-1901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn R., Rice D., Curtis S. E., Robinson S. J. Organization and transcription of genes important in Anabaena heterocyst differentiation. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):181–193. doi: 10.1016/s0769-2609(83)80104-5. [DOI] [PubMed] [Google Scholar]
- Haury J. F., Wolk C. P. Classes of Anabaena variabilis mutants with oxygen-sensitive nitrogenase activity. J Bacteriol. 1978 Nov;136(2):688–692. doi: 10.1128/jb.136.2.688-692.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Wolk C. P. Identification and characterization of hetA, a gene that acts early in the process of morphological differentiation of heterocysts. J Bacteriol. 1990 Jun;172(6):3131–3137. doi: 10.1128/jb.172.6.3131-3137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N. T., Thiel T., Giddings T. H., Jr, Wolk C. P. New Anabaena and Nostoc cyanophages from sewage settling ponds. Virology. 1981 Oct 15;114(1):236–246. doi: 10.1016/0042-6822(81)90269-5. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kuspa A., Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986 Sep;117(1):252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- Maly F. E., Urwyler A., Rolli H. P., Dahinden C. A., De Weck A. L. A single-photon imaging system for the simultaneous quantitation of luminescent emissions from multiple samples. Anal Biochem. 1988 Feb 1;168(2):462–469. doi: 10.1016/0003-2697(88)90344-2. [DOI] [PubMed] [Google Scholar]
- Mazodier P., Genilloud O., Giraud E., Gasser F. Expression of Tn5-encoded streptomycin resistance in E. coli. Mol Gen Genet. 1986 Sep;204(3):404–409. doi: 10.1007/BF00331016. [DOI] [PubMed] [Google Scholar]
- Murata N. Low-temperature effects on cyanobacterial membranes. J Bioenerg Biomembr. 1989 Feb;21(1):61–75. doi: 10.1007/BF00762212. [DOI] [PubMed] [Google Scholar]
- Padhy R. N., Hottat F. G., Coene M. M., Hoet P. P. Restriction analysis and quantitative estimation of methylated bases of filamentous and unicellular cyanobacterial DNAs. J Bacteriol. 1988 Apr;170(4):1934–1939. doi: 10.1128/jb.170.4.1934-1939.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D., Hoopes B. C., McClure W. R., Kleckner N. IS10 transposition is regulated by DNA adenine methylation. Cell. 1985 Nov;43(1):117–130. doi: 10.1016/0092-8674(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Rose R. E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988 Jan 11;16(1):355–355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan D. J., Bloye S. A., Mann N. H., Hodgson D. A., Carr N. G. Construction of lacZ promoter probe vectors for use in Synechococcus: application to the identification of CO2-regulated promoters. Gene. 1990 May 31;90(1):43–49. doi: 10.1016/0378-1119(90)90437-v. [DOI] [PubMed] [Google Scholar]
- Schauer A., Ranes M., Santamaria R., Guijarro J., Lawlor E., Mendez C., Chater K., Losick R. Visualizing gene expression in time and space in the filamentous bacterium Streptomyces coelicolor. Science. 1988 May 6;240(4853):768–772. doi: 10.1126/science.3363358. [DOI] [PubMed] [Google Scholar]
- Scherer S., Potts M. Novel water stress protein from a desiccation-tolerant cyanobacterium. Purification and partial characterization. J Biol Chem. 1989 Jul 25;264(21):12546–12553. [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985 Dec;49(4):398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., Quandt J., Klipp W. New derivatives of transposon Tn5 suitable for mobilization of replicons, generation of operon fusions and induction of genes in gram-negative bacteria. Gene. 1989 Aug 1;80(1):161–169. doi: 10.1016/0378-1119(89)90262-x. [DOI] [PubMed] [Google Scholar]
- Singer R. A., Doolittle W. F. Control of gene expression in blue-green algae. Nature. 1975 Feb 20;253(5493):650–651. doi: 10.1038/253650a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Mitchison G. J., Smith R. J. Mutants of Anabaena cylindrica altered in heterocyst spacing. Arch Microbiol. 1975 May 5;103(3):219–223. doi: 10.1007/BF00436353. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Cai Y., Cardemil L., Flores E., Hohn B., Murry M., Schmetterer G., Schrautemeier B., Wilson R. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J Bacteriol. 1988 Mar;170(3):1239–1244. doi: 10.1128/jb.170.3.1239-1244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J. C., Krebs M. P., Reznikoff W. S. Effect of dam methylation on Tn5 transposition. J Mol Biol. 1988 Jan 5;199(1):35–45. doi: 10.1016/0022-2836(88)90377-4. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Herrero M., Jakubzik U., Timmis K. N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990 Nov;172(11):6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]