Abstract
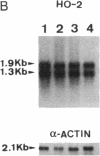
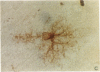
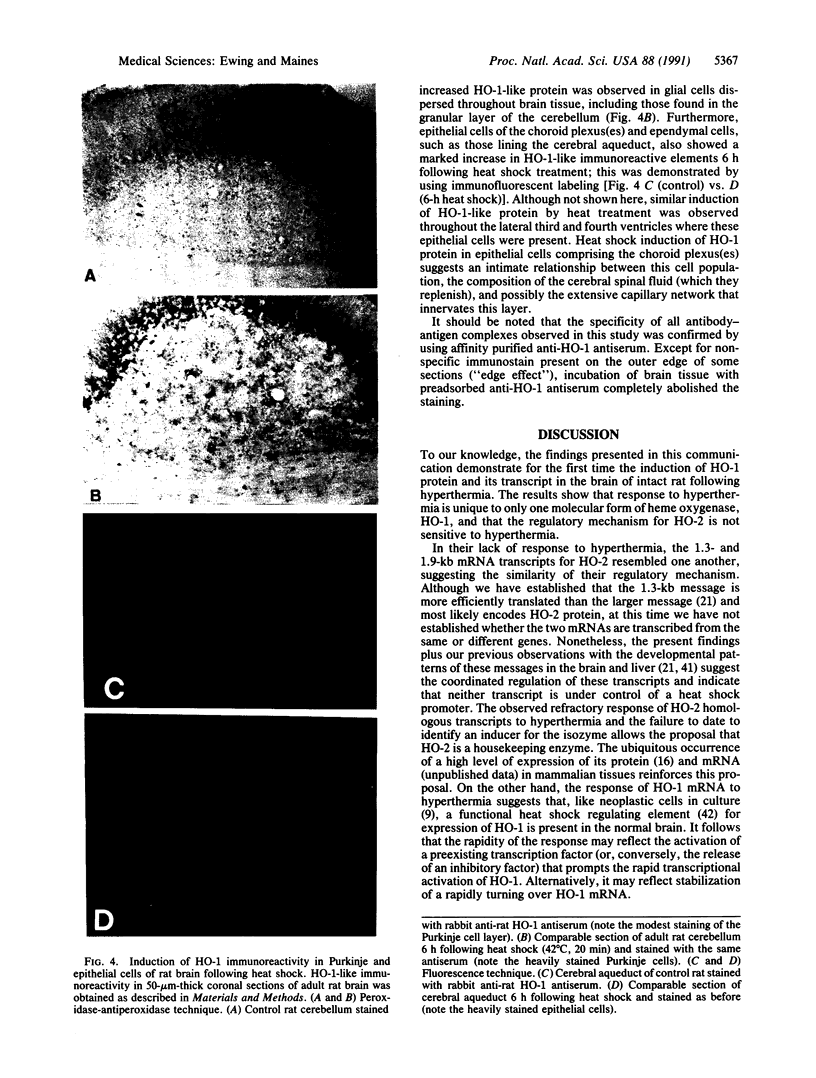
Catalytic activity of heme oxygenase (heme, hydrogen-donor:oxygen oxidoreductase, EC 1.14.99.3) isozymes, HO-1 and HO-2, permits production of physiologic isomers of bile pigments. In turn, bile pigments biliverdin and bilirubin are effective antioxidants in biological systems. In the rat brain we have identified only the HO-1 isozyme of heme oxygenase as a heat shock protein and defined hyperthermia as a stimulus that causes an increase in brain HO-1 protein. Exposure of male rats to 42 degrees C for 20 min caused a rapid and marked increase in brain 1.8-kilobase HO-1 mRNA. Specifically, a 33-fold increase in brain HO-1 mRNA was observed within 1 h and sustained for at least 6 h posttreatment. In contrast, the two HO-2 homologous transcripts (1.3 and 1.9 kilobases) did not respond to heat shock; neither the ratio nor the level of the two messages differed from that of the control when measured either at 1, 6, or 24 h after hyperthermia. The induction of a 1.8-kilobase HO-1 mRNA resulted in a pronounced increase in HO-1 protein 6 h after hyperthermia, as detected by both Western immunoblot and RIA. Immunocytochemistry of rat brain showed discrete localization of HO-1-like protein only in neurons of select brain regions. Six hours after heat shock, an intense increase in HO-1-like protein was observed in both Purkinje cells of the cerebellum and epithelial cells lining the cerebral aqueduct of the brain. We suggest that the increase in HO-1 protein, hence increased capacity to form bile pigments, represents a neuronal defense mechanism against heat shock stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienz M., Pelham H. R. Mechanisms of heat-shock gene activation in higher eukaryotes. Adv Genet. 1987;24:31–72. doi: 10.1016/s0065-2660(08)60006-1. [DOI] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Heat-shock proteins and development. Adv Genet. 1987;24:1–29. doi: 10.1016/s0065-2660(08)60005-x. [DOI] [PubMed] [Google Scholar]
- Brown I. R. Induction of heat shock (stress) genes in the mammalian brain by hyperthermia and other traumatic events: a current perspective. J Neurosci Res. 1990 Nov;27(3):247–255. doi: 10.1002/jnr.490270302. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cruse I., Maines M. D. Evidence suggesting that the two forms of heme oxygenase are products of different genes. J Biol Chem. 1988 Mar 5;263(7):3348–3353. [PubMed] [Google Scholar]
- Haber S. N., Watson S. J. The comparison between enkephalin-like and dynorphin-like immunoreactivity in both monkey and human globus pallidus and substantia nigra. Life Sci. 1983;33 (Suppl 1):33–36. doi: 10.1016/0024-3205(83)90437-x. [DOI] [PubMed] [Google Scholar]
- Hershko A. Ubiquitin-mediated protein degradation. J Biol Chem. 1988 Oct 25;263(30):15237–15240. [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981 May;75(5):734–738. doi: 10.1093/ajcp/75.5.734. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G., Yoshida T. Function and induction of the microsomal heme oxygenase. Mol Cell Biochem. 1983;53-54(1-2):163–183. doi: 10.1007/BF00225252. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Daniel R. F., Ryan D. E., Levin W., Maines M. D. Rat liver cytochrome P-450b, P-420b, and P-420c are degraded to biliverdin by heme oxygenase. Arch Biochem Biophys. 1988 Feb 1;260(2):638–644. doi: 10.1016/0003-9861(88)90492-4. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Maines M. D. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981 Apr 25;256(8):3956–3962. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Maines M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988 Jul;2(10):2557–2568. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Trakshel G. M., Kutty R. K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986 Jan 5;261(1):411–419. [PubMed] [Google Scholar]
- Marini A. M., Kozuka M., Lipsky R. H., Nowak T. S., Jr 70-kilodalton heat shock protein induction in cerebellar astrocytes and cerebellar granule cells in vitro: comparison with immunocytochemical localization after hyperthermia in vivo. J Neurochem. 1990 May;54(5):1509–1516. doi: 10.1111/j.1471-4159.1990.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Nowak T. S., Jr, Bond U., Schlesinger M. J. Heat shock RNA levels in brain and other tissues after hyperthermia and transient ischemia. J Neurochem. 1990 Feb;54(2):451–458. doi: 10.1111/j.1471-4159.1990.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Raps S. P., Lai J. C., Hertz L., Cooper A. J. Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res. 1989 Jul 31;493(2):398–401. doi: 10.1016/0006-8993(89)91178-5. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Chow L. T., Broker T. R. Characterization of rare human papillomavirus type 11 mRNAs coding for regulatory and structural proteins, using the polymerase chain reaction. Virology. 1989 Oct;172(2):489–497. doi: 10.1016/0042-6822(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Maines M. D. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J Biol Chem. 1990 May 5;265(13):7501–7506. [PubMed] [Google Scholar]
- Schacter B. A. Heme catabolism by heme oxygenase: physiology, regulation, and mechanism of action. Semin Hematol. 1988 Oct;25(4):349–369. [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Shibahara S., Müller R. M., Taguchi H. Transcriptional control of rat heme oxygenase by heat shock. J Biol Chem. 1987 Sep 25;262(27):12889–12892. [PubMed] [Google Scholar]
- Shibahara S., Müller R., Taguchi H., Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka A., Mytilineou C., Cohen G. Histochemical evaluation of glutathione in brain. Brain Res. 1987 Apr 21;409(2):275–284. doi: 10.1016/0006-8993(87)90712-8. [DOI] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Sun Y., Maines M. D. Heme oxygenase-2 mRNA: developmental expression in the rat liver and response to cobalt chloride. Arch Biochem Biophys. 1990 Nov 1;282(2):340–345. doi: 10.1016/0003-9861(90)90126-j. [DOI] [PubMed] [Google Scholar]
- Sun Y., Rotenberg M. O., Maines M. D. Developmental expression of heme oxygenase isozymes in rat brain. Two HO-2 mRNAs are detected. J Biol Chem. 1990 May 15;265(14):8212–8217. [PubMed] [Google Scholar]
- Taketani S., Kohno H., Yoshinaga T., Tokunaga R. The human 32-kDa stress protein induced by exposure to arsenite and cadmium ions is heme oxygenase. FEBS Lett. 1989 Mar 13;245(1-2):173–176. doi: 10.1016/0014-5793(89)80215-7. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Trakshel G. M., Kutty R. K., Maines M. D. Purification and characterization of the major constitutive form of testicular heme oxygenase. The noninducible isoform. J Biol Chem. 1986 Aug 25;261(24):11131–11137. [PubMed] [Google Scholar]
- Trakshel G. M., Kutty R. K., Maines M. D. Resolution of the rat brain heme oxygenase activity: absence of a detectable amount of the inducible form (HO-1). Arch Biochem Biophys. 1988 Feb 1;260(2):732–739. doi: 10.1016/0003-9861(88)90503-6. [DOI] [PubMed] [Google Scholar]
- Trakshel G. M., Maines M. D. Multiplicity of heme oxygenase isozymes. HO-1 and HO-2 are different molecular species in rat and rabbit. J Biol Chem. 1989 Jan 15;264(2):1323–1328. [PubMed] [Google Scholar]
- Vierling E., Nagao R. T., DeRocher A. E., Harris L. M. A heat shock protein localized to chloroplasts is a member of a eukaryotic superfamily of heat shock proteins. EMBO J. 1988 Mar;7(3):575–581. doi: 10.1002/j.1460-2075.1988.tb02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]