Abstract
Objectives
Surgical site infections (SSIs) constitute a threat, especially in complicated appendicitis, and are commonly due to gram-negative organisms. We sought to describe the incidence of SSIs in appendectomies performed during a three-year period (January 2013 to December 2015) in a community hospital in Qatar, and compare this with external benchmarks.
Methods
We conducted a longitudinal study at The Cuban Hospital, Qatar. We used the standardized surveillance criteria to define SSI developed by the Centers for Disease Control. Information about age, sex, smoking habits, diabetes mellitus status, body mass index, and the result of bacteriologic studies were collected.
Results
Of a total 603 patients, 22 (3.6%) cases of SSI were reported, with an infection rate of 13.6%, 4.5%, and 1.0% in 2013, 2014, and 2015, respectively. SSIs were observed more frequently in patients with contaminated/dirty wounds (6.6%). About 65% of isolates from the surgical site were multidrug-resistant organisms (Escherichia coli and Klebsiella spp.).
Conclusions
This study describes the incidence of SSI in appendectomy, which could be used as a benchmark for the facility improvement program. The high frequency of multidrug-resistant organisms in SSIs requires additional studies focused on evaluating the effectiveness of the current preventive practices with a particular reference to antimicrobial prophylaxis.
Keywords: Surgical Site Infection, Appendectomy, Incidence, Etiology, Qatar
Introduction
Acute appendicitis is the most frequent cause of acute abdominal pain with a lifetime risk of 7%, representing an important proportion of emergency surgical procedures. In the US, approximately 300 000 people undergo appendectomies annually in acute care hospitals.1-3 In Qatar, 2 375 cases were reported in public hospitals during 2013.4
Currently, the majority of appendectomies are performed using the laparoscopic technique, which has contributed to reduced surgical site infections (SSIs), the length of hospital stay, and cost.5,6 Nevertheless, SSIs remain a threat, especially for complicated appendicitis (e.g., gangrenous, perforated) and for those performed via open procedures or when disruption of infection control practices was observed. The prevention of SSIs emphasizes the effective use of antimicrobial prophylaxis, and other control practices including non-hair removal at the operative site (unless it interferes with the procedure), maintaining normothermia during the perioperative period, surveillance for SSIs (using postdischarge surveillance methods), and the compliance with bundles elements for surgical procedures.7
Recently published papers reported the incidence of SSIs as 2.8% in laparoscopic and 4.6% in open appendectomies in Korea,8 and 7.2%, 6.2%, and 5.9% in Brazil,9 China,10 and Sweden,11 respectively. Lower infection rates are reported by the US Center for Disease Control and Prevention National Healthcare Safety Network (CDC/NHSN) and the International Nosocomial Infection Control Consortium (INICC) (1.4% and 2.9%, respectively).12,13 There are no published reports about the incidence of SSIs in healthcare facilities in Qatar. The public health system in Qatar has four general hospitals where appendectomies are performed. These facilities provide care to the population regardless of their nationality, the majority of patients being foreign workers, mainly single males aged between 20–50 years old from South East Asian countries (e.g., India, Nepal, Sri Lanka, and Bangladesh).14
This study was conducted with the objective of describing the incidence of SSIs in appendectomies performed during a three-year period in a community hospital in Qatar, and comparing this with external benchmarks from the CDC/NHSN and INICC.
Methods
A longitudinal study was carried out at The Cuban Hospital, Qatar, from January 2013 to December 2015. The Cuban Hospital is a 75-bed community hospital providing healthcare services for patients from neighboring communities or transfers from other facilities. All patients diagnosed with acute appendicitis and who underwent appendectomy were included in the study. For the diagnosis of appendicitis the clinical picture, laboratory and radiological findings suggestive of acute inflammation of the appendix were considered.
The standardized surveillance criteria for defining SSI developed by the CDC were used.15 The American Society of Anesthesiologists (ASA) score was used to measure the patient´s physical status. Operative procedures were classified according to the degree of contamination into three classes (clean-contaminated, contaminated, or dirty/infected). For each patient, the National Nosocomial Infection Surveillance (NNIS) system risk index was computed on the basis of an ASA score higher than two, a wound class of contaminated or dirty/infected, and the duration of procedure > 81 minutes, with each criterion met adding one point to the index.
Additional information was collected including age, sex, smoking habits, diabetes mellitus status, body mass index, and the result of bacteriologic studies in samples collected from the SSIs.
The surveillance procedure included the diagnosis of SSI within 30 days of surgery, whether during hospitalization or after discharge. A combination of postdischarge surveillance methods was used and included a review of the patient’s file or electronic medical record and a telephone interview. The surveillance was conducted by a trained infection control practitioner and data validation performed as per Joint Commission International requirement.16
The study was performed using administrative data collected as a component of the surveillance system at the corporate level. The registration of this type of study is not required in Qatar, nor is informed consent from patients. Patient confidentiality was protected by the infection control staff who coded all data.
Statistical analysis was performed using JMP software version 10 (SAS Institute Inc., Cary, NC, 1989–2007). The incidence of SSI was calculated using the NNIS operative procedure categories (United States), by dividing the number of infections by the number of operations performed and multiplying by 100. Comparisons among patients according to the report of SSI were made using the chi-square test with Yates correction or the Fisher’s exact test, as appropriate. Relative risk ratios (RR), 95% confidence intervals (CI), and p-values were determined.
Results
The study included 603 patients who underwent appendectomies between January 2013 and December 2015. The mean patient age was 30.7±8.4 years, with men accounting for the 95.3% of patients [Table 1]. Twenty patients of 22 had superficial incisional SSIs, and one patient had deep incisional and organ space SSI. Active smokers most frequently had SSIs (RR 2.21; 95% CI 0.92–5.29; p = 0.060). Diabetes mellitus and obesity were found in 6.3% and 2.1% of patients with SSIs (p > 0.050). The RR of SSI in patients with contaminated/dirty wounds was 6.55 (95% CI 2.45–17.50) (p > 0.001) compared to clean-contaminated wounds. For patients who underwent open appendectomies the RR was 12.38 (95% CI 5.51–27.79) compared to laparoscopic appendectomies, and for risk index (RI) 2,3 (RR 7.28; 3.03–17.51) compared to RI 0,1 [Table 1].
Table 1. Patient and procedure characteristics according to surgical site infection.
| Total | Surgical site infection | ||
|---|---|---|---|
| Yes n = 22 |
No n = 581 |
||
| Age, mean±SD, years | 30.7±8.4 | 30.5±9.0 | 30.7±8.3 |
| Sex | |||
| Male | 575 | 22 (3.8) | 553 (96.2) |
| Female | 28 | 0 (0) | 28 (100) |
| Active tobacco use | 98 | 7 (7.1) | 91 (92.9) |
| Diabetes mellitus | 16 | 1 (6.3) | 15 (93.8) |
| Obesity (BMI ≥ 30 kg/m2) |
48 | 1 (2.1) | 47 (97.9) |
| Wound class | |||
| Clean contaminated | 397 | 5 (1.3) | 392 (98.7) |
| Contaminated | 89 | 6 (6.7) | 83 (93.3) |
| Dirty/Infected | 117 | 11 (9.4)* | 106 (90.6) |
| ASA score | |||
| 1,2 | 594 | 21 (3.5) | 573 (96.5) |
| 3,4 | 9 | 1 (11.1) | 8 (88.8) |
| Type of procedure | |||
| Laparoscopic | 540 | 9 (1.7) | 531 (98.3) |
| Open | 63 | 13 (20.6)* | 50 (79.4) |
| Risk index | |||
| 0,1 | 466 | 7 (1.5) | 459 (98.5) |
| 2,3 | 137 | 15 (10.9)* | 122 (89.1) |
Data is given as n (%) unless otherwise stated.*p < 0.001. ASA: American Society of Anesthesiologists; BMI: body mass index.
Positive laboratory cultures were collected in 20 patients, of which 12 isolates (60.0%) were extended spectrum beta-lactamase producers (ESBL) Escherichia coli, four were Pseudomonas aeruginosa, and one isolate each were reported as E. coli, Enterococcus faecalis, Klebsiella pneumoniae (ESBL) and group B Streptococcus [Figure 1]. Sixty-five percent of the isolates were multidrug-resistant organisms (ESBLs) [Figure 1].
Figure 1.
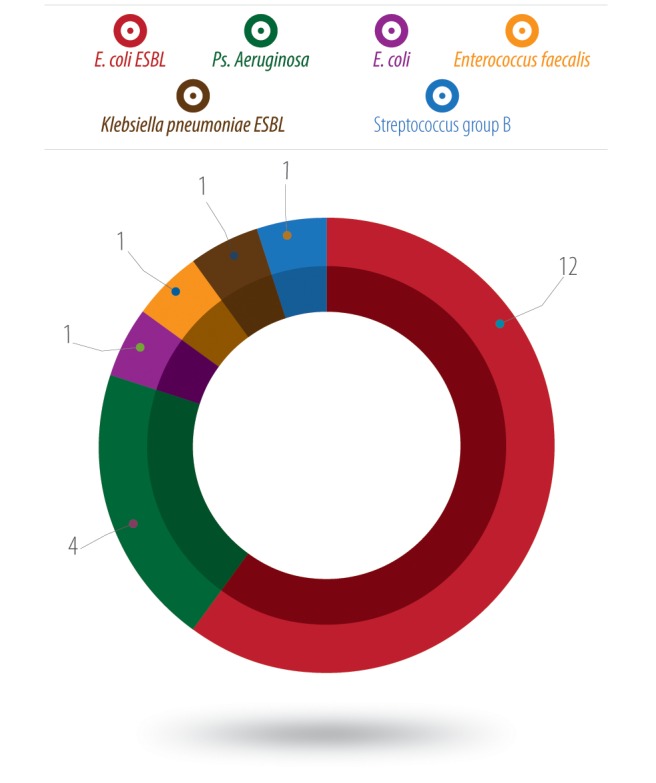
Etiology of surgical site infection in appendectomies (number of isolates).
Table 2 compares SSI rates for The Cuban Hospital with the INICC and CDC/NHSN. The overall SSI rate (3.6%) in our hospital was higher than the INICC and CDC/NHSN reports. Significant differences for RI 2,3, when compared with CDC/NHSN data were observed. For RI 0,1 the infection rate showed no differences. During 2013, 59 appendectomies were performed, and eight patients were reported with SSIs (infection rate 13.6%). There were 244 procedures and 11 cases in 2014 (infection rate 4.5%) and 300 procedures and three cases in 2015 (infection rate 1.0%). The infection rate was not calculated for RI 2,3 in 2013 because of the small number of procedures.
Table 2. Surgical site infection rates (per 100 procedures) in The Cuban Hospital (2013–2015), INICC and CDC-NHSN (2006–2008).
| Location | Procedures, n | SSIs, n |
SSI rate, % | Percentiles | ||||
|---|---|---|---|---|---|---|---|---|
| 10 | 25 | 50 | 75 | 90 | ||||
| TCH | ||||||||
| RI 0,1 | 466 | 7 | 1.5 | 0 | 0 | 2.1 | 6.7 | 6.7 |
| RI 2,3 | 137 | 15 | 10.9 | - | - | - | - | - |
| Total | 603 | 22 | 3.6 | 1 | 1 | 4.5 | 13.6 | 13.6 |
| INICC* | 13 668 | 395 | 2.9 | 0.1 | 1.5 | 2.0 | 5.3 | 8.2 |
| CDC-NHSN | ||||||||
| RI 0,1 | 5 211 | 60 | 1.2 | 0.0 | 0.0 | 0.6 | 1.2 | 2.8 |
| RI 2,3 | 663 | 23 | 3.5 | - | - | - | - | - |
SSI: surgical site infection; TCH: The Cuban Hospital; RI: risk index; INICC: International Nosocomial Infection Control Consortium; CDC-NHSN: Center for Disease Control and Prevention National Healthcare Safety Network.*pooled risk categories.
Discussion
The SSI rate was higher at our hospital than that reported by CDC/NHSN and INICC, which depended mainly on cases with RI 2,3. The infection rate for cases with a RI 0,1 at our hospital were only slightly higher than the CDC/NHSH. Nevertheless, a significant reduction in the infection rate was observed during the study period, which could be explained by the step-by-step implementation of the infection control program in new healthcare facilities (TCH was opened in 2012). Among the infection control practices implemented, it is important to emphasize the surveillance of SSIs and compliance monitoring with the surgical bundles, which included antibiotic prophylaxis, perioperative glucose control, postoperative normothermia, and hair removal practices.7 Additional practices included in the facility program are a preoperative shower with chlorhexidine gluconate solution and standardized antibiotic prophylaxis.
Factors related to SSIs in the patient population included smoking, complicated appendectomies (e.g., gangrenous, perforated), and those performed by open procedures. These factors have been mentioned in other published studies.8-11,17 An analysis of NHSN data (2006–2008) reported male sex (odds ratio 1.70; 95% CI 1.07–2.68) as a risk factor for SSI in appendectomy, which is not consistent with our findings.17 This is probably related to the gender distribution in our patients and the population living in the country. The microbial agents isolated are similar to previous reports about the topic. Nevertheless, it is important to consider the high frequency of ESBL producing organisms, mainly E. coli. The current antimicrobial prophylaxis recommended for appendectomy includes the use of beta-lactam antibiotics with or without metronidazole.18-21 Antimicrobial agents for prophylaxis should be active against the pathogens most likely to contaminate the surgical site, but the patient’s skin and intestinal flora could be colonized by multidrug-resistant organisms before admission, making the current practices ineffective.22 Additional research is needed to clarify this issue.
The language barrier and the efficiency of post-discharge surveillance could be considered the most important limitations of this study. The population studied included nationals from many countries, which is a common characteristic of the population living in Qatar. This makes data collection during physician’s interview more of a challenge (overcome with the use of translators). Despite the methods used for detection of surgical infections after discharge, it is important to consider that patients with SSIs could visit primary health care facilities, which provide free of charge services. In selected cases, we performed telephone interviews using translators according to the patient’s primary language, mainly when there was any suspicion of SSI from the patient’s file or electronic medical record review. In addition, the number of procedures could limit the power of risk factor analysis, but this did not constitute the main objective of the study. Currently, there is no SSIs benchmark at the national or regional level; this is the reason why the national infection control program uses the US CDC/NHSN data for references of program goals and evaluation of quality improvements.
Conclusion
Our study has demonstrated that the incidence of SSIs at our hospital was slightly better than external benchmarks, with a reduced infection rate over the study period. The data collected could be used as a point of reference for the facility improvement program. It also provides additional information about the incidence of ESBL organisms in the etiology of SSIs that require further research.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors would like to thank Mr. Carlos L. Crespo Palacios for reviewing this paper.
References
- 1.Weiss AJ, Elixhauser A, Andrews RM. Characteristics of operating room procedures in U.S. hospitals, 2011: statistical brief #170. Rockville, MD: Agency for Healthcare Research and Quality, 2006 [cited 2016 June]. Available from: https://www. hcup-us. ahrq. gov/ reports/ statbriefs/ sb170-Operating-Room-Procedures-United-States-2011 .jsp. [PubMed]
- 2.Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol 1990. Nov;132(5):910-925. [DOI] [PubMed] [Google Scholar]
- 3.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009. Jun;37(5):387-397. [DOI] [PubMed] [Google Scholar]
- 4.Annual Health Report, 2013. Department of Epidemiology & Medical Statistics. Hamad Medical Corporation, Doha (Qatar), 2014. [Google Scholar]
- 5.Minutolo V, Licciardello A, Di Stefano B, Arena M, Arena G, Antonacci V. Outcomes and cost analysis of laparoscopic versus open appendectomy for treatment of acute appendicitis: 4-years experience in a district hospital. BMC Surg 2014. Mar;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golash V. Laparoscopic assisted two port open appendicectomy. Oman Med J 2008. Jul;23(3):166-169. [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014. Jun;35(6):605-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suh YJ, Jeong SY, Park KJ, Park JG, Kang SB, Kim DW, et al. Comparison of surgical-site infection between open and laparoscopic appendectomy. J Korean Surg Soc 2012. Jan;82(1):35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes CA, Junior CS, Costa EdeF, Alves PdeA, de Faria CV, Cangussu IV, et al. Lessons learned with laparoscopic management of complicated grades of acute appendicitis. J Clin Med Res 2014. Aug;6(4):261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y, Shi G, Zhang J, Cao JG, Liu LJ, Chen TH, et al. Surgical site infection after laparoscopic and open appendectomy: a multicenter large consecutive cohort study. Surg Endosc 2015. Jun;29(6):1384-1393. [DOI] [PubMed] [Google Scholar]
- 11.Andersson RE. Short-term complications and long-term morbidity of laparoscopic and open appendicectomy in a national cohort. Br J Surg 2014. Aug;101(9):1135-1142. [DOI] [PubMed] [Google Scholar]
- 12.Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009. Dec;37(10):783-805. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal VD, Richtmann R, Singh S, Apisarnthanarak A, Kübler A, Viet-Hung N, et al. International Nosocomial Infection Control Consortiuma Surgical site infections, International Nosocomial Infection Control Consortium (INICC) report, data summary of 30 countries, 2005-2010. Infect Control Hosp Epidemiol 2013. Jun;34(6):597-604. [DOI] [PubMed] [Google Scholar]
- 14.Al-Thani MH, Sadoun E, Al-Thani AA, Khalifa SA, Sayegh S, Badawi A. Change in the structures, dynamics and disease-related mortality rates of the population of Qatari nationals: 2007-2011. J Epidemiol Glob Health 2014. Dec;4(4):277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Disease Control and Prevention. National Healthcare Safety Network. Surgical Site Infection (SSI) Event. January 2015 (Modified April 2015) [cited 2016 June]. Available from: http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
- 16.The Joint Commission International Accreditation Standards for Hospitals. 5th Edition, 2014 April [cited 2016 September 10]. Available from: https://www.jointcommissioninternational.org/assets/3/7/Hospital-5E-Standards-Only-Mar2014.pdf.
- 17.Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol 2011. Oct;32(10):970-986. [DOI] [PubMed] [Google Scholar]
- 18.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. American Society of Health-System Pharmacists. Infectious Disease Society of America. Surgical Infection Society. Society for Healthcare Epidemiology of America Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013. Feb;70(3):195-283. [DOI] [PubMed] [Google Scholar]
- 19.Balkhair A, Al-Farsi YM, Al-Muharrmi Z, Al-Rashdi R, Al-Jabri M, Neilson F, et al. Epidemiology of Multi-Drug Resistant Organisms in a Teaching Hospital in Oman: A One-Year Hospital-Based Study. The Scientific World Journal. 2014;2014:157102. [DOI] [PMC free article] [PubMed]
- 20.Ibrahim ME, Bilal NE, Magzoub MA, Hamid ME. Prevalence of Extended-spectrum β-Lactamases-producing Escherichia coli from Hospitals in Khartoum State, Sudan. Oman Med J 2013. Mar;28(2):116-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Yamani A, Khamis F, Al-Zakwani I, Al-Noomani H, Al-Noomani J, Al-Abri S. Patterns of Antimicrobial Prescribing in a Tertiary Care Hospital in Oman. Oman Med J 2016. Jan;31(1):35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kader AA, Kamath KA. Faecal carriage of extended-spectrum beta-lactamase-producing bacteria in the community. East Mediterr Health J 2009. Nov-Dec;15(6):1365-1370. [PubMed] [Google Scholar]


