Abstract
The Gram-positive bacterium Staphylococcus carnosus (S. carnosus) TM300 is an apathogenic staphylococcal species commonly used in meat starter cultures. As with all Gram-positive bacteria, its cytoplasmic membrane is surrounded by a thick peptidoglycan (PGN) or murein sacculus consisting of several layers of glycan strands cross-linked by peptides. In contrast to pathogenic staphylococci, mainly Staphylococcus aureus (S. aureus), the chemical composition of S. carnosus PGN is not well studied so far. UPLC/MS analysis of enzymatically digested S. carnosus TM300 PGN revealed substantial differences in its composition compared to the known pattern of S. aureus. While in S. aureus the uncross-linked stem peptide consists of a pentapeptide, in S. carnosus, this part of the PGN is shortened to tripeptides. Furthermore, we found the PGN composition to vary when cells were incubated under certain conditions. The collective overproduction of HlyD, FtsE and FtsX—a putative protein complex interacting with penicillin-binding protein 2 (PBP2)—caused the reappearance of classical penta stem peptides. In addition, under high sugar conditions, tetra stem peptides occur due to overflow metabolism. This indicates that S. carnosus TM300 cells adapt to various conditions by modification of their PGN.
Keywords: S. carnosus, peptidoglycan, muropeptides, UPLC/MS, FtsEX, CcpA, carboxypeptidases
1. Introduction
Staphylococcus carnosus (S. carnosus, S. c.) TM300 is an apathogenic, coagulase-negative, “food grade” staphylococcal species [1] commonly used as meat starter culture for raw sausages [2]. Its 2.56 Mbp genome has the highest GC content of all staphylococcal species sequenced so far. While virulence and toxicity factors are almost completely missing from the genome, most of the metabolic pathways are present. As typical for starter cultures, it harbors various sugar degradation pathways [3,4]. The general features of the peptidoglycan (PGN) of S. carnosus TM300 are already known. Like Staphylococcus aureus (S. aureus, S. a.) it belongs to the A3α-type with a penta glycine interpeptide bridge [1,5]. N-acetylmuramic acid (MurNAc) residues in the glycan backbone are not O-acetylated at position six, therefore rendering S. carnosus sensitive against lysozymes like all apathogenic Staphylococcus species [6,7].
PGN biosynthesis starts in the cytoplasm where the precursor UDP-MurNAc-pentapeptide is synthesized by the sequential addition of the stem peptide to UDP-MurNAc. At the cytoplasmic membrane, this precursor is attached to the lipid carrier undecaprenol leading to lipid I. Addition of the second sugar moiety UDP-N-acetylglucosamine (UDP-GlcNAc) results in lipid II formation, which is flipped across the cytoplasmic membrane by an enzyme, that is still highly debated in its identity, as there are two possible candidates [8]: either FtsW [9,10] or MurJ [11,12]. The S. aureus stem peptide consists of l-Ala-d-iGlu-l-Lys-d-Ala-d-Ala [5] with the d-iGlu being modified at the stage of lipid II to d-iGln [13,14]. The murein synthesizing enzymes—called penicillin-binding proteins (PBPs)—need at least one amidated stem peptide for their transpeptidase activity during cross-linking [15]. In general, PBPs are divided into high molecular weight (HMW) PBPs, that perform PGN synthesis, and low molecular weight (LMW) PBPs, which are PGN hydrolases [16]. S. aureus contains four native synthesizing PBPs and homologues of all of these can be found in the genome of S. carnosus TM300. PBP2 is considered to be the main enzyme for cell wall biosynthesis in staphylococci and is localized to the division septum by binding to its transpeptidation substrate lipid II [17]. PBP2 is the only bifunctional PBP in S. aureus and S. carnosus. It catalyzes the polymerization of the glycan moieties of lipid II by its transglycosylase activity resulting in glycan chains of alternating β-1,4-linked GlcNAc-MurNAc residues. In addition PBP2 is able to cross-link adjacent stem peptides by its transpeptidase activity [18,19]. Transpeptidation occurs between the d-Ala on position four of the donor stem peptide and the N-terminal Gly of the interpeptide bridge of the acceptor stem peptide by the expense of the last d-Ala of the donor stem peptide. The resulting tetra stem peptide can serve as an acceptor for further cross-linking reactions (Supplementary Materials Figure S1). The penta stem peptide of the first acceptor or of free acceptors in S. aureus however has never been reported to be modified in any way.
In S. aureus there are three additional monofunctional PBPs: (1) the essential enzyme PBP1, which is crucial for the cell division mechanism [20]; (2) the non-essential PBP3, which is involved in autolysis [21] and (3) the non-essential PBP4, which belongs to the low molecular weight PBPs and normally is considered to be a murein hydrolase. In S. aureus however, PBP4 is responsible for the high degree of cross-linking [22] and for the resistance against β-lactams in community-acquired methicillin-resistant S. aureus strains (caMRSA) [23]. There are two possible candidates for PBP4 in S. carnosus TM300 found in KEGG [24]: SCA_0291 with 64.7% or SCA_2445 with 37.6% sequence identity to S. aureus COL, respectively. While PBPs are generally considered to contain a non-cleavable signal peptide functioning as a single N-terminal transmembrane anchor [16], these three proteins are all predicted to contain a second transmembrane domain on the C-terminus [25].
The concerted actions of transglycosylase and transpeptidase reactions of the PBPs build a mesh-like macromolecule that surrounds the whole cell. During cell division this PGN sacculus has to be divided into two parts without compromising integrity. S. aureus is a spherical bacterium that divides along three orthogonal planes over the course of three division cycles [26]. While it was long believed that spheres like staphylococci grow by PGN synthesis at the cell division site only, recent investigations by super-resolution microscopy revealed additional peripheral PGN biosynthesis catalyzed by PBP4 [27]. Cell division in all bacteria depends on formation of the FtsZ-ring and the sequential recruitment of various proteins such as FtsE and FtsX to the divisome [28,29]. FtsEX is similar to an ABC transporter on a structural and sequence level [30] with FtsE being the ATP binding subunit and FtsX being the integral membrane part. Rather than being transporters, these two proteins are proposed to regulate the activity of murein hydrolases like PcsB in Streptococcus pneumoniae [31,32,33] and the amidases AmiA and AmiB—both via EnvC—in E. coli [34]. In Bacillus subtilis, FtsX interacts with the endopeptidase CwlO [35]. Genes encoding for FtsE and FtsX can also be found in S. carnosus TM300. However, the resulting proteins do not possess sequence similarity but only structural similarity. The role of FtsE and FtsX during synthesis of the PGN sacculus has not been investigated in staphylococci so far.
The PGN sacculus can be isolated and digested by a muraminidase such as mutanolysin into fragments called muropeptides. Muropeptides are disaccharide units with peptide moieties that can be cross-linked to other stem peptides and disaccharides thereby resulting in oligomeric structures [36]. During the analyses of S. carnosus TM300 muropeptides, we observed degradation of the first acceptor stem peptides from five (pentapeptide) to three (tripeptide) amino acids, which is in contrast to the known muropeptide pattern of S. aureus [37,38]. This gave us reason to perform a detailed Ultra Performance Liquid Chromatography (UPLC) and UPLC mass spectrometry (UPLC/MS) analysis of the S. carnosus TM300 muropeptide pattern. Our results show that almost all stem peptides that were not part of a cross-link contain only three amino acids (l-Ala-d-iGln-l-Lys) under standard conditions. However, overexpression of HlyD-FtsE-FtsX, a putative protein complex interacting with PBP2, partly inhibited this degradation process. A similar effect was observed for high glucose and fructose concentrations.
2. Results
2.1. Peptidoglycan Analysis
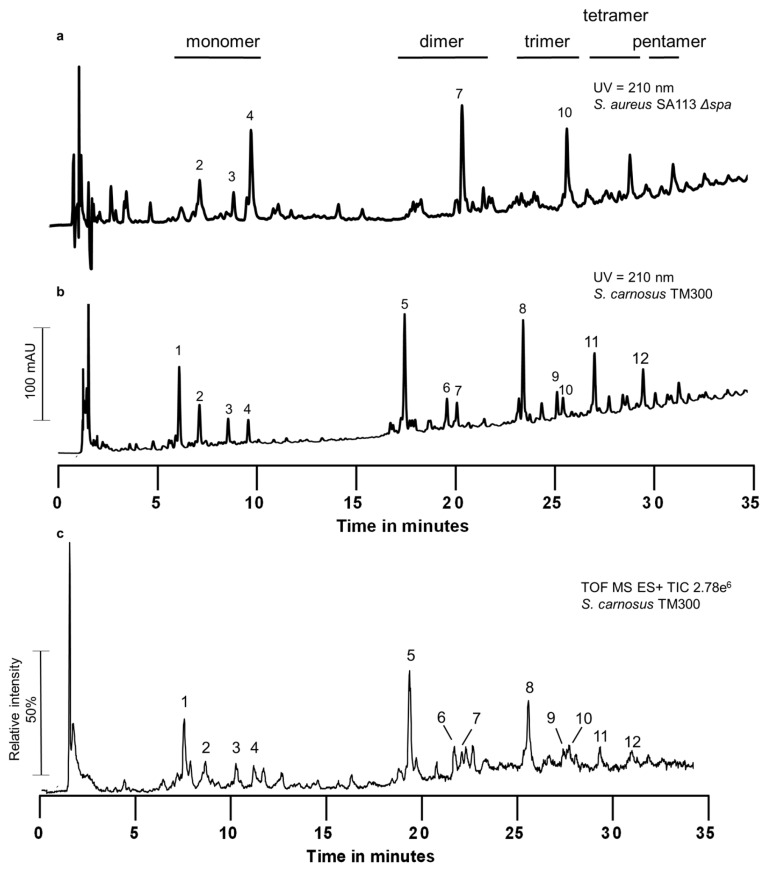
We analyzed the PGN of S. carnosus TM300 by UPLC (Figure 1) and observed that it differed substantially from the known pattern of S. aureus [37,38]. The whole muropeptide pattern of S. carnosus was shifted to shorter retention times compared to S. aureus. We performed UPLC-MS analysis to determine the chemical composition of the muropeptide peaks (Table 1). All main peaks in the monomeric as well as in the cross-linked fractions (dimer to pentamer fraction) contained muropeptides with stem peptides only consisting of three amino acids: l-Ala-d-iGln-l-Lys, explaining the shift in retention time. In S. aureus, the non-cross-linked stem peptides of monomeric muropeptides as well as the acceptor stem peptide of multimeric muropeptides harbor the complete penta stem peptide. These penta muropeptides are also present in S. carnosus, but in small amounts compared to the respective tri muropeptide. The cross-linked stem peptides are still tetra peptides and do not get degraded into tri peptides, leading to Tri-Tetran muropeptides (Table 1). During the course of our experiments, we realized that the amount of different muropeptides varied and was influenced by genetic factors as well as composition of the medium.
Figure 1.
Muropeptide profile of S. carnosus TM300 by UPLC and UPLC/MS. Peptidoglycan was isolated, digested into muropeptides and analyzed by reversed phase UPLC and UPLC/MS. (a) Muropeptide profile of S. aureus SA113 Δspa obtained by UPLC. Muropeptide peaks that are identical with the ones of S. carnosus TM300 are numbered; (b) Muropeptide profile of S. carnosus TM300 as obtained by UPLC; (c) TIC of UPLC/MS analysis of S. carnosus TM300 peptidoglycan. Muropeptides of S. carnosus TM300 exhibit shorter retention times compared to S. aureus SA113 Δspa. UPLC/MS analysis revealed the presence of stem peptides containing only three amino acids instead of five. Masses of indicated peaks are shown in Table 1 including molecule composition and proposed molecular formula. Retention time of peaks in the UPLC (quaternary pumping system) was about 1 min longer than in the binary UPLC/MS system. Therefore, in Table 1 only TIC retention times are given (TIC: Total Ion current, recorded in positive mode).
Table 1.
Muropeptides of S. carnosus TM300 analyzed by UPLC-MS. Each muropeptide contains one to five stem-peptides and each consists of the three to five amino acids l-Ala-d-iGln-l-Lys(-d-Ala)(-d-Ala). The interpeptide bridges are built of Gly molecules. The sum of all Gly residues present in each muropeptide is given. Muropeptide peaks 4, 7 and 10 (highlighted in bold) are strongly elevated by hlyD-ftsE-ftsX overexpression. Muropeptide peaks 3, 6 and 9 (highlighted in italics) are strongly elevated by high glucose and fructose concentrations.
| Peak | Retention Time (min) | M + H+ (Da) | Proposed Molecular Formula | Lengths of the Stem Peptides | Inter-Peptide Bridges |
|---|---|---|---|---|---|
| Gly | |||||
| 1 | 7.5 | 1111.5192 | C43H75N12O22+ | Tri | 5 |
| 2 | 8.6 | 1036.4937 | C40H70N13O19+ | Tetra w/o GlcNAc | 6 |
| 1093.5164 | C42H73N14O20+ | 7 | |||
| 1150.5417 | C44H76N15O21+ | 8 | |||
| 1207.5699 | C46H79N16O22+ | 9 | |||
| 1239.5699 | C48H83N14O24+ | Tetra | 6 | ||
| 1296.5939 | C50H86N15O25+ | 7 | |||
| 1353.6135 | C52H89N16O26+ | 8 | |||
| 1410.6370 | C54H92N17O27+ | 9 | |||
| 3 | 10.2 | 979.4691 | C38H67N12O18+ | Tetra w/o GlcNAc | 5 |
| 1182.5502 | C46H80N13O23+ | Tetra | 5 | ||
| 4 | 11.1 | 1253.5856 | C49H85N14O24+ | Penta | 5 |
| 5 | 19.2 | 2275.0595 | C89H152N25O44+ | Tri-Tetra | 10 |
| 6 | 21.5 | 2346.0815 | C92H157N26O45+ | Tetra2 | 10 |
| 7 | 21.9 | 2417.1143 | C95H162N27O46+ | Penta-Tetra | 10 |
| 8 | 25.4 | 3438.5927 | C135H229N38O66+ | Tri-Tetra2 | 15 |
| 9 | 27.2 | 3509.6465 | C138H234N39O67+ | Tetra3 | 15 |
| 10 | 27.5 | 3580.6553 | C141H239N40O68+ | Penta-Tetra2 | 15 |
| 11 | 29.1 | 4602.0920 | C181H306N51O88+ | Tri-Tetra3 | 20 |
| 12 | 31.6 | 5765.6142 | C227H383N64O110+ | Tri-Tetra4 | 25 |
Note: w/o—without.
2.2. Search for New Proteins Involved in PGN Biosynthesis
We performed a Bacterial-Two-Hybrid (BTH) screen using a genomic library of S. carnosus TM300 to search for new interaction partners of PBP2. This bifunctional transglycosylase/transpeptidase is considered to be one of the main players of PGN biosynthesis in S. aureus [16,17,18,19,39,40]. Among others, we found the so far uncharacterized protein SCA_1997, a conserved hypothetical protein that belongs to the HlyD family of secretion proteins (K02005). Therefore, we refer to this gene as hlyD. Bioinformatical analyses using the Kyoto Encyclopedia of Genes and Genomes (KEGG) [24] showed that if hlyD is present in Bacillus spec. and Staphylococcus spec. it is always located in an operon with two other genes. Each of them possesses its own start codon but they share one Shine-Dalgarno sequence and one promotor region. The first gene (SCA_1996) encodes for a putative ABC transport system ATP-binding protein (K02003) with structural similarity to FtsE of E. coli. The second gene (SCA_1995) is predicted to produce an ABC transport system permease protein (K02004) with structural similarity to E. coli FtsX [30,41]. So far, there is no function described for the staphylococcal HlyD, FtsE and FtsX proteins.
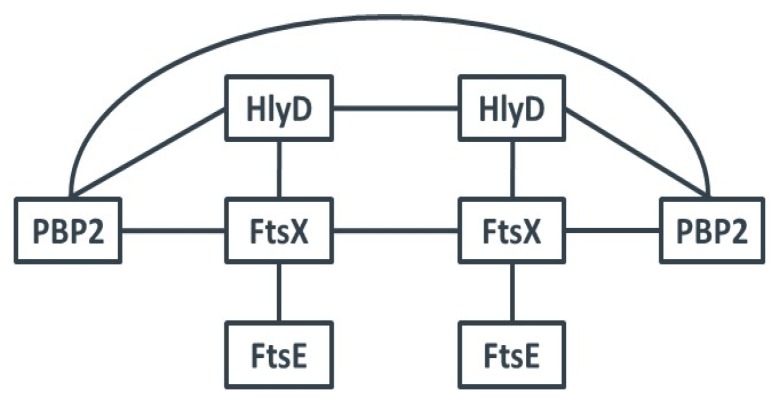
We cloned the genes of each of these proteins into the two vectors of the BTH system and performed interaction studies with all possible pairs (Table 2). PBP2 interacted with itself as well as with HlyD and FtsX. HlyD also interacted with FtsX. In addition, FtsX interacted with itself and with FtsE. There was no self-interaction of FtsE detected, and FtsE did not directly interact with HlyD (Figure 2). The interactions of the full length proteins confirmed our genetic screen results, and suggest that HlyD, FtsX and FtsE are involved in the same or in closely related biosynthetic pathways, most likely in cell wall biosynthesis and/or cell division.
Table 2.
BTH interaction of proteins related to PGN biosynthesis. Interactions between proteins putatively involved in PGN biosynthesis were tested by BTH experiments. The mean values of three independent experiments are given in Miller Units per mg dry weight bacteria. (Gray: <460; orange: 1500–3000; red: >3000)
| pUT18C | ||||||
|---|---|---|---|---|---|---|
| pKT25 | Units/mg | pbp2 | ftsX | ftsE | hlyD | zip |
| pbp2 | 3769 ± 626 | 4373 ± 105 | 136 ± 13 | 3488 ± 658 | 164 ± 28 | |
| ftsX | 3714 ± 833 | 2502 ± 491 | 2008 ± 289 | 2842 ± 287 | 165 ± 25 | |
| ftsE | 109 ± 21 | 4484 ± 279 | 354 ± 138 | 132 ± 27 | 201 ± 103 | |
| hlyD | 4020 ± 431 | 2280 ± 456 | 205 ± 29 | 3515 ± 113 | 156 ± 36 | |
| zip | 113 ± 20 | 155 ± 4 | 126 ± 7 | 157 ± 59 | 6608 ± 546 | |
Figure 2.
Protein-Protein interactions. BTH analyses revealed that PBP2 interacted with itself as well as with HlyD and FtsX. HlyD also interacted with FtsX. In addition, FtsX interacted with itself and with FtsE. There was no self-interaction of FtsE determined, and FtsE did not interact with HlyD or PBP2.
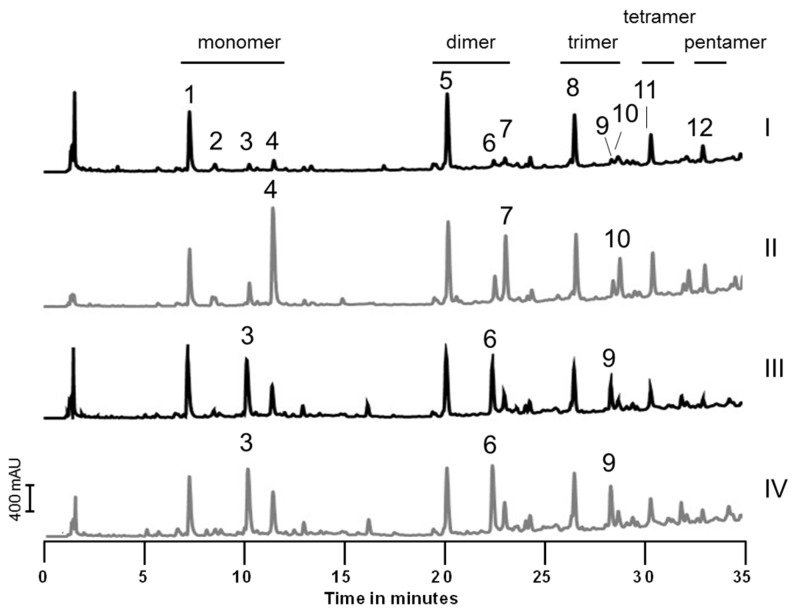
Therefore, we deleted the whole operon and examined the resulting mutant strain S. carnosus TM300 ΔhlyD-ftsEX. We did not observe any difference in growth behavior under standard BM conditions (Supplementary Materials Figure S2A), in minimal M9 medium (Supplementary Materials Figure S2B), or when the amount of NaCl in the medium was varied (Figure S2C). In addition, deletion of this operon did not have any effect on the muropeptide composition (Figure S3A). However, when the three genes were overexpressed from a plasmid harboring a xylose inducible promoter, all peaks with muropeptides containing a penta stem peptide were highly elevated while the tri muropeptides were still present in comparable amounts. Muropeptides with tetra stem peptides were slightly elevated as well. As 25 mM xylose itself had no effect on the wild type strain, the observed effect is due to overexpression of the hlyD-ftsEX operon (Figure 3) while overexpression of hlyD or ftsEX had no effect on muropeptide composition (Supplementary Materials Figure S3B). Astonishingly, we also observed elevated tetra and—to a lower extent—penta muropeptides when we repressed plasmid gene expression with 25 mM glucose, indicating that the PGN of S. carnosus is influenced by genetic as well as metabolic factors.
Figure 3.
Variations in muropeptide profile caused by genetic and metabolic factors. PGN from different S. carnosus TM300 variants was isolated, digested into muropeptides and analyzed by UPLC. (I) S. carnosus TM300 grown in the presence of 25 mM xylose; (II) S. carnosus TM300 pPTX-hlyD-ftsEX induced with 25 mM xylose; (III) S. carnosus TM300 pPTX-hlyD-ftsEX repressed with 25 mM glucose; (IV) S. carnosus TM300 grown in the presence of 25 mM glucose. Overexpression of hlyD-ftsEX led to an accumulation of penta muropeptides (peak 4, 7 and 10 in panel II) which was not observed when the wild type strain was grown in the presence of 25 mM xylose. Addition of 25 mM glucose in the medium lead to an increase in tetra muropeptides independent of the plasmid pPTX-hlyD-ftsEX (peaks 3, 6 and 9 in panel III and IV, respectively).
2.3. Precursor Analysis
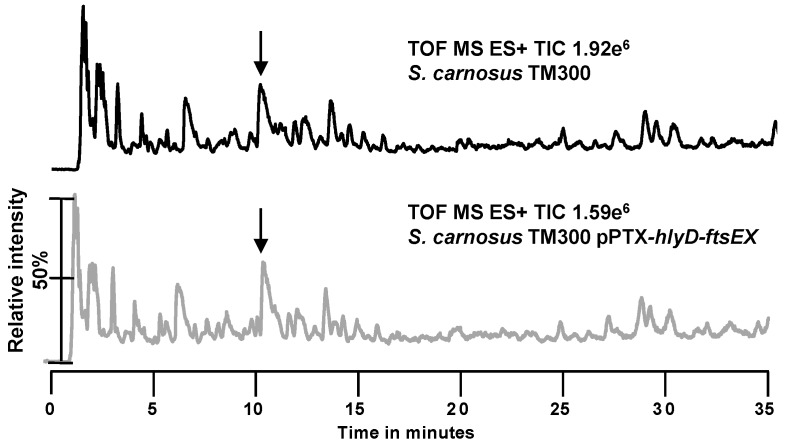
To examine if S. carnosus TM300 synthesizes already shortened variants of the classical PGN precursor UDP-MurNAc-pentapeptide, we accumulated and analyzed the PGN precursor of the wild type strain S. carnosus TM300 and of the overexpression strain S. carnosus TM300 pPTX-hlyD-ftsEX (Figure 4). In S. aureus, the PGN precursor is the UDP-MurNAc-pentapeptide with a mass of 1149.37 g/mol. In both tested S. carnosus strains, this mass was only found in a broad peak group at about 11 min in the TIC of the UPLC-MS. None of the other masses obtained correlated with the UDP-MurNAc-tripeptide (1007.2774 g/mol) or UDP-MurNAc-tetrapeptide (1078.3145 g/mol). This shows that S. carnosus TM300 only synthesizes the classical precursor UDP-MurNAc-pentapeptide and truncation to the tetra and tri stem peptide must occur at a later time point.
Figure 4.
UPLC/MS analysis of the PGN precursor. S. carnosus TM300 wild type and S. c. TM300 pPTX-hlyD-ftsEX were grown in B medium supplemented with 25 mM xylose. Precursors were concentrated, isolated and analyzed by UPLC/MS. Peaks highlighted by arrows were identified to harbor the same masses including the mass for the classical precursor UDP-MurNAc-pentapeptide (1149.37 g/mol). Masses for UDP-MurNAc-tripeptide (1007.2774 g/mol) or UDP-MurNAc-tetrapeptide (1078.3145 g/mol) were not detected in any of the analyses.
2.4. Investigation of a Putative l,d-Carboxypeptidase
A logical explanation for the observed truncation of the penta stem peptide to a tri stem peptide would be an l,d-carboxypeptidase (ld-CP) activity. Even though such an activity has never been described for staphylococci, we found one potential candidate in the genome of S. carnosus TM300: SCA_0214 is annotated as a cytoplasmic muramoyl-tetrapeptide carboxypeptidase that hydrolyses the bond between a di-basic amino acid and the C-terminal d-alanine in the tetra peptide moiety of PGN. An orthologous gene was not found in S. aureus and no paralog was annotated in S. carnosus TM300. Therefore, a deletion mutant of SCA_0214 was created in S. carnosus TM300. However, deletion of this gene did not alter the PGN pattern compared to the wild type strain (Supplementary Materials Figure S4A). When we overexpressed this gene, we found the resulting protein to accumulate in the cytoplasm and not be transported to the supernatant (Supplementary Materials Figure S4B). As truncation of the stem peptide most likely occurs outside the cell, we exclude SCA_0214 as the sought-after ld-CP.
2.5. Influence of Sugars on PGN Composition
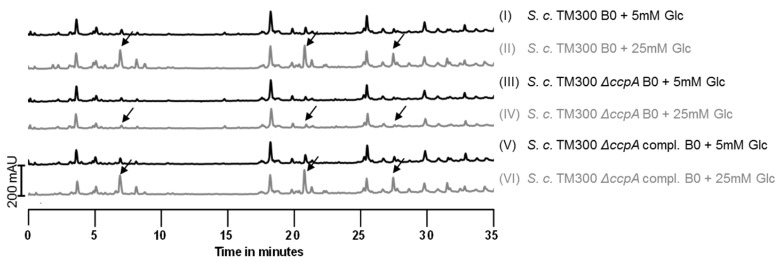
As we had observed that high concentrations of glucose (25 mM) led to an increase in muropeptides with tetra stem peptides, we tested other C-sources in this concentration as well. While fructose had the same effect, the muropeptide pattern in the presence of glycerol or ribose was unchanged. PGN of cells grown in B0 medium (B medium without an additional C-source) was also unaffected (Supplementary Materials Figure S5A). Therefore, we examined whether this observation was due to overflow metabolism and deleted the gene for the catabolite control protein A (ccpA). CcpA mainly functions as a gene repressor, but it is also involved in the transcription activation of genes involved in fermentation and overflow metabolism [42,43]. It is itself activated by glucose intermediates like glucose-6-phosphate and fructose-1,6-bisphosphate [44]. Indeed, the effect of high amounts of glucose on PGN composition was lost in the ΔccpA mutant (Figure 5). In the complemented mutant, the ccpA gene was constitutively expressed from a plasmid, and addition of 25 mM glucose to the medium again caused the appearance of muropeptides with tetra stem peptides in addition to tri stem peptides. Alterations in the PGN pattern of S. carnosus TM300 were not solely reduced to high glucose concentrations and were lost in a ccpA deletion mutant. Therefore, this effect can be explained by an overflow metabolism when an abundance of nutrients is present in the medium rather than solely by a glucose effect. In S. aureus, SA113 an increased glucose concentration showed no effect on the muropeptide pattern (Supplementary Materials Figure S6).
Figure 5.
Deletion of ccpA prevents the effect of high sugar concentrations. The catabolite repressor gene ccpA was deleted in S. carnosus TM300. PGN of the wild type, the mutant and the complemented strain grown with 5 or 25 mM glucose was isolated and analyzed by UPLC. In the ccpA deletion mutant, 25 mM glucose did not cause accumulation of tetra muropeptides (arrows in panel 4), while complementation with a constitutively expressed ccpA gene completely restored this effect (panel 6).
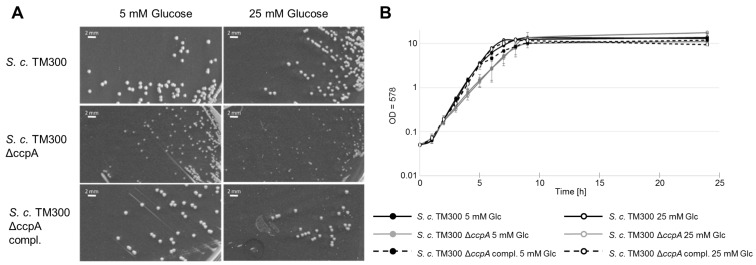
2.6. Deletion of ccpA Causes a Decrease in Colony Size and Growth Rate
While the observed variation in PGN composition was lost in the ccpA deletion mutant, this strain was influenced in its growth behavior. There was a decrease in colony size (Figure 6 and Table 3) of the S. carnosus TM300 ΔccpA mutant of ~60% compared to the wild type strain. Complementation restored the mutant phenotype completely. In liquid culture, the S. carnosus TM300 ΔccpA mutant showed a growth defect during log phase compared to the wild type strain that was restored again by complementation (Figure 6). Growth rate of the mutant was reduced to 60% in low glucose concentration and to 68% in high glucose concentrations compared to the wild type. This effect was restored by complementation (Table 4). Therefore, in these experiments, the glucose concentration itself had only minor influence, indicating observed effects on the PGN are specific.
Figure 6.
The ΔccpA mutant showed a decrease in colony size and a reduction in growth rate. (A) On solid media colonies, the deletion mutant appeared clearly smaller than the parental strains. The glucose concentration seemed to have only a minor influence on colony size; (B) In liquid media, S. carnosusTM300 ΔccpA also showed a growth defect compared to the wild type strain. In both cases, the effect could be complemented. The influence of the glucose concentration in both experiments was negligible. (Bar size: 2 mm; Glc: glucose).
Table 3.
Determination of colony size. The size of ten individual grown colonies was measured digitally. The ccpA mutant showed a 57% decrease in cell size that could be complemented again. The influence of glucose was only minor. The mean values of three independent experiments are given.
| Strain | 5 mM Glucose (mm) | 25 mM Glucose (mm) |
|---|---|---|
| S. carnosus TM300 | 0.91 ± 0.07 | 0.83 ± 0.09 |
| S. carnosus TM300 ΔccpA | 0.39 ± 0.07 | 0.33 ± 0.06 |
| S. carnosus TM300 ΔccpA compl. | 0.92 ± 0.04 | 0.89 ± 0.07 |
Table 4.
Calculation of growth rate. Growth rate for each strain was calculated for the mid exponential phase between time point 4 and 5 h and is given as doubling time per hour (td/h). S. carnosus TM300 ΔccpA showed a decreased doubling time compared to its parental strain. The influence of high glucose concentrations was only minor. The mean values of three independent experiments are given.
| Strain | 5 mM Glucose (td/h) | 25 mM Glucose (td/h) |
|---|---|---|
| S. carnosus TM300 | 1.82 ± 0.16 | 2.10 ± 0.35 |
| S. carnosus TM300 ΔccpA | 0.71 ± 0.31 | 0.67 ± 0.19 |
| S. carnosus TM300 ΔccpA compl. | 1.90 ± 0.40 | 2.00 ± 0.38 |
3. Discussion
3.1. The Peptidoglycan Composition of S. carnosus TM300
UPLC/MS analysis of the PGN of S. carnosus TM300 revealed a muropeptide pattern different from the one known of S. aureus. Instead of stem peptides containing the classical five amino acids l-Ala-d-iGln-l-Lys-d-Ala-d-Ala) typical for S. aureus, most stem peptides were degraded to tri peptides missing the two d-Ala. This is the case for monomeric muropeptides (Figure 1a, peak 1) as well as for all cross-linked muropeptides, in which the acceptor stem peptide was also degraded, while the former donor stem peptides are still classical tetra peptides (Figure 1a, peaks 5, 8, 11 and 12). We were able to identify the cross-linked muropeptides up to the pentamer (Tri-Tetra4). Acceptor stem peptides with penta peptides were also found (Figure 1a, peaks 4, 7 and 10), but in small amounts only. We also found tetra muropeptides containing five (Figure 1a, peak 3) or six to nine glycine residues in the interpeptide bridge (Figure 1a, peak 2). The latter indicates that these muropeptides had been released again from mature PGN. This peak was not influenced by the genetic and metabolic factors tested.
In S. aureus PGN—the best studied PGN in staphylococci—this degradation was never observed [37,38,45]. Tri stem peptides are a classical feature of E. coli [36] but also of other firmicutes like Bacillus subtilis, where the PGN is modified after cross-linking has occurred. Interestingly, we observed the length of the S. carnosus TM300 stem peptide not only to be influenced by genetic factors (in our study hlyD-ftsE-ftsX) but also by the composition of the growth medium (see below). Especially 25 mM glucose, a concentration frequently used for plasmid repression, caused an increase in muropeptides with tetra stem peptides. Changes in stem peptide composition due to medium ingredients have been reported for example for Caulobacter crescentus. It incorporates glycine at position five, even if only traces of free glycine are present in the growth medium [46]. When glycine is depleted in the medium, S. aureus PGN is directly cross-linked and lacks the interpeptide bridge [47]. Cells then enter the stationary phase, indicating this to be a protection mechanism.
3.2. The hlyD-ftsE-ftsX Operon
A gene for HlyD (hemolysin D) is present in e.g., Escherichia coli (E. coli) but the orthologue could not be found in staphylococci. The database KEGG and a Blast search for orthologous genes did not lead to any positive results. However, SCA_1997, which was identified in the BTH screen with a genomic library of S. carnosus TM300 as a putative interaction partner for PBP2, was annotated as a conserved hypothetical protein belonging to the HlyD family of secretion proteins. The nomenclature was maintained as SCA_1997 and orthologous genes possess a short HlyD motif that is eponymous for these proteins.
Like the E. coli HlyD, the staphylococcal HlyD is highly similar to the membrane fusion component of the RND family of transporters (RND: Resistance, Nodulation, Cell Division). As the proposed function is to facilitate and enable transport (e.g., resistance through efflux) by bringing the inner and outer membrane together, most members of this family are found in Gram-negative bacteria [48]. Nevertheless, also in Gram-positive bacteria, there are a few representatives of this family. But hlyD is not omnipresent in Staphylococcus. Only about 47% (34 out of 73) of all annotated staphylococci harbor a copy of hlyD. In contrast, in Bacillus, 98% of strains (112 out of 114 strains sequenced) possess this gene. There is also no correlation between hlyD and pathogenicity, as it can be found throughout the whole genus of staphylococci.
While the S. carnosus TM300 FtsEX complex has no sequence similarity to FtsEX of E. coli, the predicted structures of the respective proteins are very similar. An ftsEX depletion mutant in E. coli forms filaments, and growth in LB medium was shown to be salt dependent as the septal ring does not properly assemble in the absence of FtsEX and salt [30]. However, growth of the S. carnosus TM300 hlyD-ftsEX mutant was not affected by the absence or presence of NaCl nor by the use of minimal medium M9 (Supplementary Materials Figure S2) and we did not observe any obvious changes in cell appearance. This indicates the septal ring in staphylococci does not necessarily need ftsEX for stabilization.
Our BTH results are in accordance with known results for PBP2 of S. aureus which was also shown to self-interact [49]. In our experiments, FtsX also interacted with HlyD, with itself and with FtsE (Figure 2 and Table 2). We could not detect homodimerization of FtsE as was published for the Streptococcus pneumoniae orthologue. In this organism, both FtsE and FtsX formed homodimers and interacted with each other [31]. In various species, FtsE and FtsX have been shown to interact with each other and thereby activate murein hydrolases. This includes PcsB of Streptocccus pneumoniae [31,32,33], the endopeptidase CwlO of Bacillus subtilis [35] and an amidase of E. coli that was activated by FtsEX via EnvC [34]. Our results from S. carnosus point to a role for HlyD-FtsEX in the regulation of a carboxypeptidase, as the overexpression of the hlyD-ftsEX operon led to an increase in muropeptides containing penta stem peptides. To a minor extent, tetra stem peptides were also observed (Figure 3). Penta stem peptides of S. carnosus are normally degraded into tri stem peptides by a so far unknown enzyme(s). We propose that HlyD-FtsEX must have an inhibitory effect on this/these enzyme/s, as deletion of the whole operon did not alter the muropeptide pattern and therefore did not influence degradation activity. In addition, only overproduction of all three proteins caused an increase in penta and tetra muropeptides, indicating a necessity for complex formation for activity. As tri muropeptides are present in similar amounts to pentas, inhibition of the degradation enzymes is not complete and other factors might contribute to their regulation. This is corroborated by the notion that the hlyD-ftsEX operon is not essential and its deletion had no effect on bacterial growth under various conditions tested.
3.3. Investigation of a Putative l,d-Carboxypeptidase (SCA_0214)
The PGN of S. carnosus TM300 mainly consists of tri and tetra peptide structures (Table 1) suggesting l,d-carboxypeptidase (ld-CP) activity. Bioinformatical analysis led to only one possible candidate in S. carnosus TM300: SCA_0214, which is located between SCA_0213 (an NAD dependent epimerase/dehydratase family protein) and SCA_0215 (ilvE, a branched chain amino acid aminotransferase). In contrast to S. carnosus TM300, a gene for an ld-CP is missing from the genome of S. aureus. For instance, the genome of S. a. USA300 does not contain a homologous gene for SCA_0214. Instead, the two genes SCA_0213 and SCA_0215 are located directly next to each other. The absence of a putative ld-CP is in accordance with the presence of only penta and tetra stem peptides in S. aureus PGN [37]. However, deletion of SCA_0214 in S. carnosus TM300 had no effect at all on the muropeptide pattern. It still harbored mainly tri stem peptides (Figure S4A). Even if SCA_0214 was only able to cleave off d-Ala from a tetrapeptide but not from a pentapeptide, as could be expected from a carboxypeptidase, its deletion should have resulted in an accumulation of tetrapeptides. As this is not the case, we exclude SCA_0214 as being responsible for the occurrence of tripeptides in S. carnosus TM300.
But what could be the role of SCA_0214? Overproduction resulted in protein accumulation in the cytoplasm. This is in accordance with the ld-CP sequence analysis that revealed neither a TAT (Twin Arginine Translocation) nor a Sec (Secretion) sequence. This could point to a role in the cytoplasm, but could also be a mere side effect of protein overproduction.
But which enzyme(s) could cause the reduction from penta to tri stem peptides? As trimming of the stem-peptides during PGN maturation is widely seen in eubacteria, S. carnosus might be “normal”, while actually S. aureus is an exception by keeping its pentapeptide intact. Another bioinformatical search in KEGG revealed three annotated dd-CPs in S. carnosus: (1) SCA_0291, the homologue of S. aureus PBP4, which is actually a PGN synthase responsible for secondary cross-linking of PGN [22]; (2) SCA_2445, which is also assigned as an S. aureus PBP4 homologue, but with less homology; and (3) SCA_1643 that is orthologous to LdcB of Streptococcus pneumoniae and B. subtilis but has no orthologue in S. aureus. Both enzymes had originally been annotated as d,d-CP (dacA) but were recently shown to degrade tetra peptides from isolated PGN into tri peptides in vitro and were therefore renamed [50]. SCA_2445 could be the enzyme that creates tetra peptides, and tri peptides are then produced by SCA_1643. We tried to construct deletion mutants of SCA_1643 and SCA_2445 but were unsuccessful.
3.4. Influence of Sugars on the Muropeptide Composition
As we had observed an altered PGN pattern when we repressed plasmid gene expression with 25 mM glucose, we tested the influence of various carbon sources on the PGN pattern of S. carnosus TM300. We found an increase of tetra muropeptides when the cells of S. carnosus TM300 were grown with either 25 mM glucose (Figure 5, chromatogram 2) or fructose (Figure S5A). Again, tri muropeptides were still present in comparable amounts to these tetra muropeptides. No increase in tetra muropeptides was observed when supplemented with 25 mM xylose (Figure S5A), the standard gene induction conditions for plasmid pPTX [51,52] that served as a control.
In Gram-positive bacteria, the catabolite control protein A (CcpA), a global regulator of carbon source metabolism, regulates cellular processes that are influenced by the presence of glucose [53]. Therefore, we deleted the ccpA gene and analyzed the PGN composition again under low and high glucose concentrations. As expected, no elevated tetra peaks were observed in the ccpA deletion mutant under high glucose conditions. Instead, the muropeptide pattern was identical to the one obtained from cells grown in 5 mM glucose (Figure 5, chromatograms 3 and 4). Complementation with a plasmid constitutively expressing ccpA restored the original phenotype, showing that the appearance of tetra muropeptides can be traced back to the influence of glucose and fructose intermediate products on CcpA. Moreover, deletion of ccpA also affected cell growth. The deletion strain show a reduced growth rate in exponential phase and a severe decrease in colony size compared to the wild type strain, indicating a major role for CcpA in the metabolism of S. carnosus TM300.
Glucose and fructose are both metabolized to fructose-1,6-bisphosphate (FBP), an intermediate product of glycolysis. As alterations on the PGN pattern of S. carnosus TM300 did not only occur under high glucose concentrations, this effect could be rather explained by a general overflow metabolism instead of a glucose effect. However, glucose not only serves as a nutrient but also the expression of metabolic genes [54,55] and virulence factors [56] are influenced by glucose.
But how can glucose concentration influence the PGN composition? The increase in tetra muropeptides speaks again for a partial inhibition of a so far unknown ld-CP, although an increase in penta peptide structures was also detected. Taken together, this points to an influence of ccpA and hlyD-ftsEX on the stem peptide degrading enzyme(s) which is albeit not a complete inhibitory effect as tri stem peptides are also still present.
So far we do not understand why S. carnosus TM300 trims its stem peptides in a way that is also seen for other bacteria, but S. aureus does not. It was just shown that in E. coli the eight dd-CPs are active under different conditions. For example, PBP5 (dacA) is mainly active at neutral pH, while PBP6b is only expressed and active at acidic pH. Loss of either enzyme results in morphological defects when the cells are incubated at the respective pH. We, however, did not observe any obvious changes in the morphology of our strains during overexpression of the hlyD-ftsE-ftsX operon or under high glucose conditions. But the notion that even under these conditions tripeptides are present in similar amounts as the newly appearing penta or tetrapeptides can be interpreted as a hint for an important role of the tri peptides in the PGN of S. carnosus TM300.
4. Materials and Methods
For strains, plasmids and oligonucleotides used please refer to Supplementary Materials Table S1: Bacterial strains, Table S2: Plasmids and Table S3: Oligonucleotides.
4.1. Media
Basic medium (B medium) for Staphylococci consisted of Soy Peptone (10 g; Plato, Koblenz, Germany), Yeast Extract (5 g; Deutsche Hefewerke, Nuernberg, Germany), NaCl (5 g; Carl-Roth, Karlsruhe, Germany), Glucose (1 g; Carl Roth) and K2HPO4 (1 g; Applichem, Darmstadt, Germany). Deionized water was added to a final volume of 1 liter and pH was adjusted to 7.2.
LB medium for E. coli consisted of Peptone (10 g; Plato), Yeast Extract (5 g; Deutsche Hefewerke) and NaCl (5 g; Carl Roth). Deionized water was added to a final volume of 1 liter and pH was adjusted to 7.2. MacConkey agar was prepared according to the manufactures’ instructions (Carl Roth). Minimal media M9 for staphylococci consisted of Na2HPO4 (6 g; Carl Roth), KH2PO4 (3 g; Fisher Chemicals), NH4Cl (1 g; Merck Darmstadt, Germany) and NaCl (0.5 g; Carl Roth). Deionized water was added to a final volume of 1 L, the media was autoclaved and supplemented with thiamine (2 µg/mL; Sigma Aldrich, Munich, Germany), MgSO4 (1 mM; Carl Roth) and casamino acids (0.2%; BD Bioscience, Heidelberg, Germany). Cells were routinely grown at 37 °C either in liquid culture in baffled Erlenmeyer flasks (1:5 ratio) with shaking or on 1.5% agar plates.
4.2. Reagents
Unless otherwise stated, all reagents were bought from Sigma-Aldrich.
4.3. Plasmid Construction for Deletion Mutants and Overexpression
Chromosomal DNA from S. carnosus and S. aureus was isolated [57] using lysostaphin (0.5 mg/mL) for cell lysis. SCA_1995-1997(ftsX-ftsE-hlyD): For deletion of the operon SCA_1995-1997 ,the pBT2 [58] knockout vector was used. For overexpression of HlyD, FtsE and FtsX, the genes SCA_1997, SCA_1995-1996 and SCA_1995-1997 were cloned into the xylose inducible plasmid pPTX [52] and cut with the same enzymes. SCA_0214 (putative ld-CP): For deletion, the knockout vector pGS1 was used [59]. The overexpression plasmid was constructed in the xylose inducible plasmid pPTX [52] and cut with the same enzymes. SCA_1342 (ccpA): For deletion, knockout vector pGS1was used [59]. For constitutive complementation, SCA_1342 was ligated into pRAB11-EF-TU, cut with the same enzymes. SAOUHSC01850 (ccpA) was deleted from S. aureus SA113 Δspa using the pMAD knockout vector [60] and Gibson assembly [61] for cloning. For overexpression, pRAB11-EF-TU-SAOUHSC01850 was electroporated first into RN4220 and then into S. aureus SA113 Δspa [62]. The correctness of all plasmids was confirmed by sequencing before transformation. S. carnosus TM300 and S. aaureus SA113 Δspa were transformed by electroporation [62]; for E. coli, transformation chemocompetent cells were used [63].
4.4. Gene Deletion
All knockout procedures were performed according to the respective protocols of the vectors. While the pMAD system does not imply the usage of a resistance cassette, the other two knockout vectors were constructed with an ermB (erythromycin B) resistance cassette. Flanking of ermB with loxP sites enabled us to flox out the resistance cassette by the Cre recombinase constitutively produced by the vector pRAB1 [64]. This generated a marker-free mutant minimizing downstream effects caused by the resistance cassette.
4.5. Genomic Library
Chromosomal DNA from S. carnosus was isolated [57] using lysostaphin (0.5 mg/mL) for cell lysis. Genomic DNA was sheared using the nebulizer from the TOPO Shotgun Subcloning Kit (Invitrogen, Carlsbad, CA, USA) and separated on a preparative agarose gel. Five pools of DNA from 1.7 to 3 kbp were isolated, cleaned and treated with Klenow fragment to generate blunt ends. DNA from each pool was used for ligation into pKT25 [65], which had been cut with SmaI and dephosphorylated by rAPid Alkaline Phosphatase (Roche, Mannheim, Germany). The resulting plasmids were transformed into NEB 5-alpha electrocompetent E. coli cells (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s protocol. Ligation procedure was performed twice, resulting in ten transformations. Each transformation was plated on three LB/Kanamycin (50 µg/mL) plates (15 cm in diameter) and incubated overnight at 37 °C. All colonies from the same pool were combined and stored in 50% glycerol at −80 °C. From each pool, an aliquot was used to inoculate LB media for plasmid isolation (“prey” vectors). Over 80% of the plasmids contained genomic DNA inserts, and cell wall related genes like pbpA, pbpF, pbp4, rodA and murJ could each be amplified in at least one subpool. SCA_1084 (pbp2) was cloned into pUT18C [65] as “bait” vector.
4.6. Bacterial-Two-Hybrid Assays
The method of Karimova et al. was used [65]. The plasmids were co-transformed in E. coli BTH101, plated on LB agar plates containing 100 μg/mL ampicillin, 50 μg/mL kanamycin and 100 μg/mL streptomycin and incubated for 16 h at 37 °C. Ten colonies per each interaction tandem were transferred onto MacConkey agar plates (100 μg/mL ampicillin, 30 μg/mL kanamycin and 100 μg/mL streptomycin) and incubated for 24–48 h at 30 °C. To generate triplicates, the first three colonies per each interaction tandem were inoculated overnight in LB media containing 100 μg/mL ampicillin, 30 μg/mL kanamycin, 100 μg/mL streptomycin and 0.5 mM IPTG. Overnight cultures were diluted in H2Obidest (1:4) and cell density was measured at OD600. Of each cell suspension, 100 µL was mixed with 20 µL 0.1% SDS, 40 µL chloroform and 1 mL Z buffer (70 mM Na2HPO4 × 12 H2O, 30 mM NaH2PO4 H2O, 1 mM MgSO4, 0.2 mM MnSO4, pH 7, 100 mM β-mercaptoethanol). The cells were solubilized by pipetting 10–15 times. Of each supernatant, 100 µL was incubated with 20 µL ONPG solution (ortho-Nitrophenyl-β-galactoside; 4 mg/mL ONPG in Z-buffer without β-mercaptoethanol) for 10 min. ONPG served as substrate for the detection of β-galactosidase activity. The colorless substrate is cleaved to galactose and the yellow colored product ortho-nitrophenol. The reaction was stopped by adding 50 µL of 1 M Na2CO3 and the optical density was determined at 420 nm. The enzymatic activity of the β-galactosidase is given in units/mg dry weight bacteria and was calculated as follows:
Calculation of enzyme activity:
| A = 200 × (OD420 − OD420 in control tube)/minutes of incubation × dilution factor |
Calculation of bacteria dry weight:
| 1 mL of culture at OD600 = 1 corresponds to 300 μg dry weight bacteria |
A negative control was performed by co-transforming the empty plasmids pKT25 and pUT18C into the host strain E. coli BTH101. The cut-off value for positive interactions was four times the negative control.
4.7. Peptidoglycan Analysis
The cells were grown in 20 mL BM for 8 h and peptidoglycan was isolated and analyzed by UPLC and UPLC/MS as previously described [37]. Precursors were isolated as published [66] and analyzed by UPLC-MS using the PGN method.
4.8. Overproduction of SCA_0214
Main cultures (20 mL B0 media supplemented with either 5 mM glucose for repression or 25 mM xylose for induction) were inoculated with overnight cultures to OD578 = 0.05. After 8 h culture, supernatant was used to isolate extracellular proteins using StrataClean beads (Agilent Technologies, St. Clara, CA, USA). Intracellular proteins were extracted from pelleted cells. Cells were disrupted using a FastPrep®-24 (MP Biomedicals, Santa Ana, CA, USA) and 500 μL acid washed glass beads (0.22 mm in diameter, Sigma-Aldrich). Cells were treated 4 times at 6500 rpm for 30 s. After two cycles, the samples were placed on ice for 5 min to reduce potential heat that develops during FastPrep®-24 treatment and that could lead to protein denaturation. Afterwards, the tubes were centrifuged at 12,000 rpm for 5 min at RT. The supernatant contained the intracellular proteins. Proteins were analyzed by SDS-PAGE stained with Coomassie.
5. Conclusions
We could show that the PGN of the apathogenic bacterium S. carnosus TM300 differs from the well-studied PGN of the pathogen S. aureus. In TM300 free stem peptides are shortened to contain tri peptides only in contrast to the unmodified penta peptides of S. aureus. This suggests the activities of an ld- and a dd-CP. We could show, that the proposed dd-CP might be regulated by three enzymes that interact with the PGN synthase PBP2: HlyD, FtsE and FtsX. The overexpression of the respective operon results in the reappearance of penta stem peptides. The ld-CP seems to be regulated by the catabolite control protein A (ccpA) as high sugar concentrations result in the appearance of tetra stem peptides. Taken together, our results show that the PGN of S. carnosus can adapt to various conditions.
Acknowledgments
This work was supported by the DFG via the SFB 766 and the University of Tuebingen: Promotion of Junior Researchers Program to Ute Bertsche. We thank Darya Belikova for excellent technical assistance, Friedrich Götz for helpful discussions and Christopher Schuster for critical reading of the manuscript.
Abbreviations
The following abbreviations are used in this manuscript:
| BTH | Bacterial-Two-Hybrid |
| PGN | peptidoglycan |
| UPLC/MS | Ultra Performance Liquid Chromatography coupled to mass spectrometry |
| MurNAc | N-acetylmuramic acid |
| GlcNAc | N-acetylglucosamine |
| CP | carboxypeptidase |
Supplementary Materials
The following are available online at www.mdpi.com/2079-6382/5/4/33/s1. Figure S1: Muropeptide structures of S. aureus and S. carnosus TM300; Figure S2: Growth behavior of S. carnosus TM300 ΔhlyD-ftsEX; Figure S3: Deletion of hlyD-ftsEX and overexpression of single genes does not influence the peptidoglycan pattern; Figure S4: Analysis of the S. carnosus TM300 Δ0214::ermB mutant and localization of the encoded protein; Figure S5: Influence of different sugars on the peptidoglycan of S. carnosus TM3; Figure S6: Influence of glucose on the peptidoglycan of S. aureus SA113 Δspa; Table S1: Bacterial strains; Table S2: Plasmids; Table S3: Oligonucleotides.
Author Contributions
Julia Deibert and Ute Bertsche conceived and designed the experiments; Julia Deibert and Elif Koeksoy performed the experiments; Julia Deibert, Daniel Kühner, Mark Stahl and Ute Bertsche analyzed the data; Ute Bertsche wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schleifer K.H., Fischer U. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int. J. Syst. Bacteriol. 1982;32:153–156. doi: 10.1099/00207713-32-2-153. [DOI] [Google Scholar]
- 2.Niinivaara F.P., Pohja M.S. Über die Reifung der Rohwurst. I. Mitt: Die Veränderung der Bakterienflora während der Reifung. Z. Lebensm. Unters. Forsch. 1956;104:413–422. doi: 10.1007/BF01092489. (In German) [DOI] [Google Scholar]
- 3.Rosenstein R., Götz F. Genomic differences between the food-grade Staphylococcus carnosus and pathogenic staphylococcal species. Int. J. Med. Microbiol. 2010;300:104–108. doi: 10.1016/j.ijmm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstein R., Nerz C., Biswas L., Resch A., Raddatz G., Schuster S.C., Götz F. Genome analysis of the meat starter culture bacterium Staphylococcus carnosus TM300. Appl. Environ. Microbiol. 2009;75:811–822. doi: 10.1128/AEM.01982-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schleifer K.H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bera A., Biswas R., Herbert S., Götz F. The presence of peptidoglycan O-acetyltransferase in various staphylococcal species correlates with lysozyme resistance and pathogenicity. Infect. Immun. 2006;74:4598–4604. doi: 10.1128/IAI.00301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bera A., Herbert S., Jakob A., Vollmer W., Götz F. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol. Microbiol. 2005;55:778–787. doi: 10.1111/j.1365-2958.2004.04446.x. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz N. Lipid flippases for bacterial peptidoglycan biosynthesis. Lipid Insights. 2015;8:21–31. doi: 10.4137/LPI.S31783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammadi T., van Dam V., Sijbrandi R., Vernet T., Zapun A., Bouhss A., Diepeveen-de Bruin M., Nguyen-Disteche M., de Kruijff B., Breukink E. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. EMBO J. 2011;30:1425–1432. doi: 10.1038/emboj.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammadi T., Sijbrandi R., Lutters M., Verheul J., Martin N., den Blaauwen T., de Kruijff B., Breukink E. Specificity of the transport of Lipid II by FtsW in Escherichia coli. J. Biol. Chem. 2014 doi: 10.1074/jbc.M114.557371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sham L.-T., Butler E.K., Lebar M.D., Kahne D., Bernhardt T.G., Ruiz N. MurJ is the flippase of lipid-linked precursors for peptidoglycan biogenesis. Science. 2014;345:220–222. doi: 10.1126/science.1254522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler E.K., Tan W.B., Joseph H., Ruiz N. Charge requirements of Lipid II flippase activity in Escherichia coli. J. Bacteriol. 2014;196:4111–4119. doi: 10.1128/JB.02172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueiredo T.A., Sobral R.G., Ludovice A.M., Almeida J.M., Bui N.K., Vollmer W., de Lencastre H., Tomasz A. Identification of genetic determinants and enzymes involved with the amidation of glutamic acid residues in the peptidoglycan of Staphylococcus aureus. PLoS Pathog. 2012;8:e1002508. doi: 10.1371/journal.ppat.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Münch D., Roemer T., Lee S.H., Engeser M., Sahl H.G., Schneider T. Identification and in vitro analysis of the GatD/MurT enzyme-complex catalyzing lipid II amidation in Staphylococcus aureus. PLoS Pathog. 2012;8:e1002509. doi: 10.1371/journal.ppat.1002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakel M., Ghuysen J.M., Kandler O. Wall peptidoglycan in Aerococcus viridans strains 201 Evans and ATCC 11563 and in Gaffkya homari strain ATCC 10400. Biochemistry. 1971;10:2170–2175. doi: 10.1021/bi00787a033. [DOI] [PubMed] [Google Scholar]
- 16.Goffin C., Ghuysen J.M. Multimodular penicillin-binding proteins: An enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 1998;62:1079–1093. doi: 10.1128/mmbr.62.4.1079-1093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinho M.G., Errington J. Recruitment of penicillin-binding protein PBP2 to the division site of Staphylococcus aureus is dependent on its transpeptidation substrates. Mol. Microbiol. 2005;55:799–807. doi: 10.1111/j.1365-2958.2004.04420.x. [DOI] [PubMed] [Google Scholar]
- 18.Pinho M.G., Filipe S.R., de Lencastre H., Tomasz A. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 2001;183:6525–6531. doi: 10.1128/JB.183.22.6525-6531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinho M.G., de Lencastre H., Tomasz A. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. USA. 2001;98:10886–10891. doi: 10.1073/pnas.191260798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira S.F.F., Henriques A.O., Pinho M.G., de Lencastre H., Tomasz A. Role of PBP1 in cell division of Staphylococcus aureus. J. Bacteriol. 2007;189:3525–3531. doi: 10.1128/JB.00044-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinho M.G., de Lencastre H., Tomasz A. Cloning, characterization, and inactivation of the gene pbpC, encoding penicillin-binding protein 3 of Staphylococcus aureus. J. Bacteriol. 2000;182:1074–1079. doi: 10.1128/JB.182.4.1074-1079.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leski T.A., Tomasz A. Role of penicillin-binding protein 2 (PBP2) in the antibiotic susceptibility and cell wall cross-linking of Staphylococcus aureus: Evidence for the cooperative functioning of PBP2, PBP4, and PBP2A. J. Bacteriol. 2005;187:1815–1824. doi: 10.1128/JB.187.5.1815-1824.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memmi G., Filipe S.R., Pinho M.G., Fu Z., Cheung A. Staphylococcus aureus PBP4 is essential for β-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob. Agents Chemother. 2008;52:3955–3966. doi: 10.1128/AAC.00049-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.KEGG: Kyoto Encyclopedia of Genes and Genomes. [(accessed on 6 August 2016)]. Available online: http://www.genome.jp/kegg/
- 25.Omasits U., Ahrens C.H., Müller S., Wollscheid B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884–886. doi: 10.1093/bioinformatics/btt607. [DOI] [PubMed] [Google Scholar]
- 26.Tzagoloff H., Novick R. Geometry of cell division in Staphylococcus aureus. J. Bacteriol. 1977;129:343–350. doi: 10.1128/jb.129.1.343-350.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteiro J.M., Fernandes P.B., Vaz F., Pereira A.R., Tavares A.C., Ferreira M.T., Pereira P.M., Veiga H., Kuru E., VanNieuwenhze M.S., et al. Cell shape dynamics during the staphylococcal cell cycle. Nat. Commun. 2015 doi: 10.1038/ncomms9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarsman M.E., Piette A., Fraipont C., Vinkenvleugel T.M., Nguyen-Disteche M., den Blaauwen T. Maturation of the Escherichia coli divisome occurs in two steps. Mol. Microbiol. 2005;55:1631–1645. doi: 10.1111/j.1365-2958.2005.04502.x. [DOI] [PubMed] [Google Scholar]
- 29.Söderström B., Daley D.O. The bacterial divisome: More than a ring? Curr. Genet. 2016 doi: 10.1007/s00294-016-0630-2. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K.L., Peterson N.D., Kustusch R.J., Wissel M.C., Graham B., Phillips G.J., Weiss D.S. A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. J. Bacteriol. 2004;186:785–793. doi: 10.1128/JB.186.3.785-793.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajaj R., Bruce K.E., Davidson A.L., Rued B.E., Stauffacher C.V., Winkler M.E. Biochemical characterization of essential cell division proteins FtsX and FtsE that mediate peptidoglycan hydrolysis by PcsB in Streptococcus pneumoniae. Microbiol. Open. 2016 doi: 10.1002/mbo3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sham L.-T., Jensen K.R., Bruce K.E., Winkler M.E. Involvement of FtsE ATPase and FtsX extracellular loops 1 and 2 in FtsEX-PcsB complex function in cell division of Streptococcus pneumoniae D39. mBio. 2013 doi: 10.1128/mBio.00431-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sham L.T., Barendt S.M., Kopecky K.E., Winkler M.E. Essential PcsB putative peptidoglycan hydrolase interacts with the essential FtsXSpn cell division protein in Streptococcus pneumoniae D39. Proc. Natl. Acad. Sci. USA. 2011;108:E1061–E1069. doi: 10.1073/pnas.1108323108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang D.C., Peters N.T., Parzych K.R., Uehara T., Markovski M., Bernhardt T.G. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc. Natl. Acad. Sci. USA. 2011;108:E1052–E1060. doi: 10.1073/pnas.1107780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meisner J., Montero Llopis P., Sham L.T., Garner E., Bernhardt T.G., Rudner D.Z. FtsEX is required for CwlO peptidoglycan hydrolase activity during cell wall elongation in Bacillus subtilis. Mol. Microbiol. 2013;89:1069–1083. doi: 10.1111/mmi.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glauner B., Höltje J.-V., Schwarz U. The composition of the murein of Escherichia coli. J. Biol. Chem. 1988;263:10088–10095. [PubMed] [Google Scholar]
- 37.Kühner D., Stahl M., Demircioglu D.D., Bertsche U. From cells to muropeptide structures in 24 h: Peptidoglycan mapping by UPLC-MS. Sci. Rep. 2014 doi: 10.1038/srep07494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Jonge B.L., Chang Y.S., Gage D., Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J. Biol. Chem. 1992;267:11248–11254. [PubMed] [Google Scholar]
- 39.Murakami K., Fujimura T., Doi M. Nucleotide sequence of the structural gene for the penicillin-binding protein 2 of Staphylococcus aureus and the presence of a homologous gene in other staphylococci. FEMS Microbiol. Lett. 1994;117:131–136. doi: 10.1111/j.1574-6968.1994.tb06754.x. [DOI] [PubMed] [Google Scholar]
- 40.Lovering A.L., de Castro L.H., Lim D., Strynadka N.C. Structural insight into the transglycosylation step of bacterial cell-wall biosynthesis. Science. 2007;315:1402–1405. doi: 10.1126/science.1136611. [DOI] [PubMed] [Google Scholar]
- 41.De Leeuw E., Graham B., Phillips G.J., ten Hagen-Jongman C.M., Oudega B., Luirink J. Molecular characterization of Escherichia coli FtsE and FtsX. Mol. Microbiol. 1999;31:983–993. doi: 10.1046/j.1365-2958.1999.01245.x. [DOI] [PubMed] [Google Scholar]
- 42.Shivers R.P., Dineen S.S., Sonenshein A.L. Positive regulation of Bacillus subtilis ackA by CodY and CcpA: Establishing a potential hierarchy in carbon flow. Mol. Microbiol. 2006;62:811–822. doi: 10.1111/j.1365-2958.2006.05410.x. [DOI] [PubMed] [Google Scholar]
- 43.Sonenshein A.L. Control of key metabolic intersections in Bacillus subtilis. Nat. Rev. Microbiol. 2007;5:917–927. doi: 10.1038/nrmicro1772. [DOI] [PubMed] [Google Scholar]
- 44.Lopez J.M., Thoms B. Role of sugar uptake and metabolic intermediates on catabolite repression in Bacillus subtilis. J. Bacteriol. 1977;129:217–224. doi: 10.1128/jb.129.1.217-224.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Jonge B.L., Chang Y.S., Gage D., Tomasz A. Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J. Biol. Chem. 1992;267:11255–11259. [PubMed] [Google Scholar]
- 46.Takacs C.N., Hocking J., Cabeen M.T., Bui N.K., Poggio S., Vollmer W., Jacobs-Wagner C. Growth medium-dependent glycine incorporation into the peptidoglycan of Caulobacter crescentus. PLoS ONE. 2013;8:e57579. doi: 10.1371/journal.pone.0057579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou X., Cegelski L. Nutrient-dependent structural changes in S. aureus peptidoglycan revealed by solid-state NMR spectroscopy. Biochemistry. 2012;51:8143–8153. doi: 10.1021/bi3012115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schülein R., Gentschev I., Möllenkopf H.J., Goebel W. A topological model for the haemolysin translocator protein HlyD. Mol. Gen. Genet. 1992;234:155–163. doi: 10.1007/BF00272357. [DOI] [PubMed] [Google Scholar]
- 49.Steele A.L., Young S. A descriptive study of Myers-Briggs personality types of professional music educators and music therapists with comparisons to undergraduate majors. J. Music Ther. 2011;48:55–73. doi: 10.1093/jmt/48.1.55. [DOI] [PubMed] [Google Scholar]
- 50.Hoyland C.N., Aldridge C., Cleverley R.M., Duchene M.C., Minasov G., Onopriyenko O., Sidiq K., Stogios P.J., Anderson W.F., Daniel R.A., et al. Structure of the LdcB LD-carboxypeptidase reveals the molecular basis of peptidoglycan recognition. Structure. 2014;22:949–960. doi: 10.1016/j.str.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peschel A., Ottenwalder B., Götz F. Inducible production and cellular location of the epidermin biosynthetic enzyme EpiB using an improved staphylococcal expression system. FEMS Microbiol. Lett. 1996;137:279–284. doi: 10.1111/j.1574-6968.1996.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 52.Popella P., Krauss S., Ebner P., Nega M., Deibert J., Götz F. VraH is a third component of the Staphylococcus aureus VraDEH system involved in gallidermin and daptomycin resistance and pathogenicity. Antimicrob. Agents Chemother. 2016 doi: 10.1128/AAC.02865-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henkin T.M., Grundy F.J., Nicholson W.L., Chambliss G.H. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors. Mol. Microbiol. 1991;5:575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 54.Collins F.M., Lascelles J. The effect of growth conditions on oxidative and dehydrogenase activity in Staphylococcus aureus. J. Gen. Microbiol. 1962;29:531–535. doi: 10.1099/00221287-29-3-531. [DOI] [PubMed] [Google Scholar]
- 55.Strasters K.C., Winkler K.C. Carbohydrate Metabolism of Staphylococcus aureus. J. Gen. Microbiol. 1963;33:213–229. doi: 10.1099/00221287-33-2-213. [DOI] [PubMed] [Google Scholar]
- 56.Seidl K., Stucki M., Ruegg M., Goerke C., Wolz C., Harris L., Berger-Bächi B., Bischoff M. Staphylococcus aureus CcpA affects virulence determinant production and antibiotic resistance. Antimicrob. Agents Chemother. 2006;50:1183–1194. doi: 10.1128/AAC.50.4.1183-1194.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 1961;3:208–218. doi: 10.1016/S0022-2836(61)80047-8. [DOI] [Google Scholar]
- 58.Brückner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 1997;151:1–8. doi: 10.1016/S0378-1097(97)00116-X. [DOI] [PubMed] [Google Scholar]
- 59.Krismer B., Nega M., Thumm G., Götz F., Peschel A. Highly efficient Staphylococcus carnosus mutant selection system based on suicidal bacteriocin activation. Appl. Environ. Microbiol. 2012;78:1148–1156. doi: 10.1128/AEM.06290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnaud M., Chastanet A., Debarbouille M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 2004;70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gibson D.G., Young L., Chuang R.Y., Venter J.C., Hutchison C.A., 3rd, Smith H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 62.Lofblom J., Kronqvist N., Uhlen M., Stahl S., Wernerus H. Optimization of electroporation-mediated transformation: Staphylococcus carnosus as model organism. J. Appl. Microbiol. 2007;102:736–747. doi: 10.1111/j.1365-2672.2006.03127.x. [DOI] [PubMed] [Google Scholar]
- 63.Dagert M., Ehrlich S.D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979;6:23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- 64.Leibig M., Krismer B., Kolb M., Friede A., Götz F., Bertram R. Marker removal in Staphylococci via Cre recombinase and different lox sites. Appl. Environ. Microbiol. 2008;74:1316–1323. doi: 10.1128/AEM.02424-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karimova G., Pidoux J., Ullmann A., Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohlrausch U., Höltje J.-V. One-step purification procedure for UDP-N-acetylmuramyl-peptide murein precursors from Bacillus cereus. FEMS Microbiol. Lett. 1991;62:253–257. doi: 10.1111/j.1574-6968.1991.tb04451.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.