Abstract
Lamins are mechanosensitive and elastic components of the nuclear lamina that respond to external mechanical cues by altering gene regulation in a feedback mechanism. Numerous mutations in A-type lamins cause a plethora of diverse diseases collectively termed as laminopathies, the majority of which are characterized by irregularly shaped, fragile, and plastic nuclei. These nuclei are challenged to normal mechanotransduction and lead to disease phenotypes. Here, we review our current understanding of the nucleocytoskeleton coupling in mechanotransduction mediated by lamins. We also present an up-to-date understanding of the methods used to determine laminar elasticity both at the bulk and single molecule level.
Keywords: lamins, mechanotransduction, elasticity, single molecule force spectroscopy
1. Introduction
The nucleus of a eukaryotic cell plays a deterministic role in cellular fate and homeostasis. It is a double membrane bound organelle comprising of an inner nuclear membrane (INM), an outer nuclear membrane (ONM), and a nuclear pore complex (NPC) and confines the tightly packed chromatin. A dense meshwork of proteins underneath the INM imparts proper structure, shape, and rigidity to the nuclear membrane and acts as sites of attachment for NPCs and chromosome tethering [1,2]. Lamin proteins are type V intermediate filament proteins and are principal components of nuclear lamina. There has been an upsurge of interest in lamin biology since the discovery of an increasing number of mutations associated with lamin A, leading to at least 14 different diseases collectively referred to as laminopathies (http://www.umd.be/LMNA/). Insights into different laminopathies and the roles of lamins thereof necessitate revisiting the elastic properties of nuclear lamina. This review focuses on the structural perturbations of laminopathic nuclei associated with the mutations of lamin A corresponding to changes in viscoelastic behavior. We have presented an up-to-date status of our understanding of the role of lamins in modulating the elastic behavior of the nucleus.
2. Structure and Properties of Lamins
Lamin proteins were first isolated from rat liver and chicken erythrocyte nuclei as components of nuclear lamina [2,3] and later classified as type V intermediate filament proteins [4,5,6]. Lamin proteins are found in all metazoan cells with the sole exception of plants and unicelular organisms [7,8]. Based on sequence homologies, lamins can be classified into A- and B-types. In mammals, two A-type lamins (lamin A and C) are derived from the LMNA gene located on chromosome 1, whereas B-type lamins (lamin B1 and B2) are derived from LMNB1 & LMNB2 on chromosomes 5 and 19, respectively [9,10]. Other minor isoforms like lamin A∆10 [11] and germ line-specific Lamin C2 [12] are obtained from LMNA, and B3 is obtained from LMNB2 [13]. All lamins carry a tripartite structure consisting of a long central alpha helical rod domain, which are flanked by N terminal head and C terminal tail domains. The C terminal tail domain is grossly unstructured and contains a nuclear localization signal NLS [14] followed by the immunoglobulin fold (Ig-fold) [15,16] while terminating in a CAAX box that is necessary for farnesylation [17] Interestingly, B-type lamins are permanently farnesylated, whereas A-type lamins do not retain the farnesyl group.
Lamins have a propensity to self assemble into higher order oligomeric structures that are initiated by a dimer formation and propagated by parallel in-register associations at the molecular level [8,18,19]. It is now established that the head, rod, and tail domains play active roles in lamin assembly [18,20]. Inside the nucleus, A- and B-type lamins form separate networks which interact amongst themselves [21] However, the means by which individual lamins in a cell polymerize to render the final laminar structure is still shrouded in mystery. Near orthogonal meshwork of lamin fibers were visualized in Xenopus oocytes [4], while mammalian nuclei almost always exhibit irregular filamentous networks [22,23]. Recent studies on lamin assembly in xenopus oocytes have shown a near orthogonal regular network of B-type lamins, superposed by irregular bundles of A-type lamins [24]. Therefore, it can be conjectured that B-type lamins, especially B1, form the plinth on which the laminar superstructure is assembled. This view finds support from the knockdown experiments of lamin B1, which culminates into an increased mesh size of lamin A/C and B2 with an additional formation of nuclear blebs [21]. The lamin proteins form an elastic component of the lamina and act as “shock absorbers” [25,26], which therefore regulate the mechanical behavior of the nucleus. It is now established that viscoelasticity of the nucleus depends entirely on A-type lamins, while B-type lamins resist nuclear deformation in response to force [27].
3. Expression of Nuclear Lamins during Development and Differentiation
A- and B-type lamins not only differ in their structural features but also in their expression patterns as well. B-type lamins are expressed as the first intermediate filament protein of the nucleus in most embryonic stem cell types, except in one instance where lamin B was shown to be superfluous in differentiation [28], while lamin A sets in during differentiation, although the expression patterns varies among animal to animal [29]. Xenopous oocytes contain LB3 as the principal, whereas minor forms LB1 and LB2 express at the time of blastula transition and gastrulation [30,31,32]. Drosophila and chicken embryos exhibit a similar trend of lamin expression [33,34]. In mice, lamin A/C first appear at the trophoblast stage (E.D 9), followed by expression in myoblasts and mesenchymal tissues on embryonic days 10 and 11, respectively [35]. Thus, it is only lamins B1 and B2—not lamin A/C—that are expressed in undifferentiated mouse and human embryonic stem cells [36]. B-type lamins are very essential during animal development. Site-directed mutations on B-type lamins of drosophila cause fatality at different embryonic or late pupal stages [37,38]. Lmnb1-/- and Lmnb1-/-/Lmnb2-/- are born smaller in size and succumb to death within minutes after birth due to lung and brain developmental defects [28,39,40]. On the other hand, animals with deficiency of lamin A/C suffer from postnatal retardation of growth [41] associated with skeletal and cardiac muscle disorder [41,42]. Again, stiffness of a tissue is largely dependent on the relative expression ratio of A- and B-type lamins. Soft tissues such as the brain exhibit a low lamin A:lamin B ratio that is reversed for harder load-bearing tissues such as muscles, bone, and cartilage [43]. Correspondingly, embryonic stem cells (ESCs) expressing low lamin A/C levels exhibit higher plasticity compared to differentiated cells [44,45].
4. Laminopathies and Role of Lamin A in Mechanotransduction
Lamins have gained fresh impetus due to the uncovering of over 450 mutations only in lamin A, which are associated with 14 human diseases termed as laminopathies. Surprisingly, only a handful of diseases have been identified with lamin B1 and lamin B2 mutations [46], which further support the notion that B-type mutations are embryonically lethal, while A-type lamins are not. Some of the laminopathies, including dilated cardiomyopathy (DCM), Emery–Dreifuss muscular dystrophy (EDMD), Hutchinson–Gilford progeria syndrome (HGPS), and familial partial lipodystrophy (FPLD), are characterized by mechanically compromised nuclei that are often deformed and lack integrity, as observed in tissue samples from patients [47,48]. Heart muscle tissues were obtained as biopsy samples from DCM patients [49] and from mouse models [50,51] as well as DCM and EDMD models in Drosophila, where body wall muscle showed abnormally elongated nuclei with a concomitant reduction in nuclear stiffness [52,53]. Moreover, the human and murine muscle tissues bearing DCM mutations showed increased nuclear fragility and discontinuity in nuclear lamina, as visualized by electron microscopic analyses [54,55]. On the contrary, LMNA mutations cause a HGPS result in stiffened nuclei as observed in patient fibroblasts [56,57]. Thus, the mechanical model comes in the scenario that explains that sensing and response to external mechanical cues by the nucleus is mediated by nuclear lamins. According to the mechanical model, aberrant mechanical response from the nucleus due to lamin A mutations causes increased vulnerability of the nuclei of load bearing tissues encountering mechanical stress. As a consequence, the misshapen nuclei of the laminopathic cells respond poorly to stretching forces. ”Mechanosensing” occurs at the level of the extracellular matrix (ECM), which generates tensional force on the cell. This in turn is sensed and processed by the cytoskeleton as biochemical signals and mechanical forces that terminate into the nucleus, thereby eliciting effector responses [58]. The nuclear lamina is physically connected to the cytoskeleton via the linker of nucleoskeleton and cytoskeleton (LINC) complex, which plays a crucial role in mechanotransduction [59]. The LINC complex is comprised of SUN ( Sad1 & UNC-84) domain proteins spanning the INM [60] and is connected to nesprins (also referred to as Syne, Myne, and NUANCE) at the ONM [59,61,62]. Different isoforms of nesprin also bind actin and microtubules and cytoplasmic intermediate filaments [63,64]. Silencing or depleting the components of the LINC complex results in nuclear envelop deformities, thereby suggesting that these components are necessary to maintain proper shape and structure of the nucleus in response to mechanical forces [65,66]. The support for this statement comes from the observations that defective nucleocytoskeletal coupling is observed in cells expressing mutant lamin A proteins [52], thereby disrupting the proper mechanotransduction pathway. Recent studies in C. elegans models have shown that L535P causing EDMD perturbs the mechanical response of muscle nuclei in response to strain. As expected, the LINC complex and emerin are required to elicit this mechanical response of the nuclei [67]. This is an important addition in the field, as the experimental procedures determining nuclear strain are based on the whole living animal. This is reminiscent of the mammalian cells, which are also characterized by improper localization of various components of the LINC complex [68,69]. At the same time, the interaction of various downstream signaling molecules with lamin A might be perturbed [70]. Load-bearing tissues such as muscles, cartilage, and bones containing elevated levels of lamin A are worst affected in diseases such as EDMD, DCM, and HGPS and are characterized by aberrant nucleocytoskeletal coupling [52].
5. Measurement of Viscoelasticity of Lamin A Proteins
Nuclear deformations observed in different laminopathies include nuclear envelop blebbing, elongated nuclei, lamina thickening, and overall fragility of the lamina [71]. These phenotypes can result from an alteration of viscoelastic behavior of the nuclear lamina. Several biomechanical techniques such as micropipette aspiration, cell strain and compression, and atomic force microscopy have aimed to probe mechanical properties of nuclei [44,72,73], where chromatin contributions also had to be taken into consideration [25]. Similar viscoelastic properties of the actin cytoskeleton have been shown earlier via particle tracking microrheology experiments in the cytoplasm of stem cells and differentiated fibroblasts. It must be borne in mind that stem cells due to their intrinsic plasticity are assumed to be an ideal viscous system, whereas differentiated fibroblasts (as used in the study) possess both viscous and elastic components [74]. However, how the viscoelasticity of the individual proteins contribute to the bulk behavior of the cell posed an interesting problem to biophysicists and cell biologists. Likewise, the viscoelastic properties of the actin protein have been studied in great detail via rheological measurements [75,76,77]. Similar viscoelastic studies also exist for cytoplasmic intermediate filament proteins such as desmin, keratin, and vimentin [78,79,80,81,82]. Both desmin and vimentin have shown strain stiffening behavior from mechanical rheometry [83]. Pioneering studies have also focused on quantitative rheological measurements of lamin B1, where the stiffness of the matrix increased with strain. This strain hardening was hypothesized to resist forces leading to nuclear deformation prior to nuclear envelope rupture in prophase [83]. However, lamin B1-depleted cells retained normal mechanical functions despite occasional nuclear blebbings, while A-type lamin-deficient cells had attenuated nuclear stiffness [84]. Lamin A plays a pivotal role in the pathophysiology of diseases linked to muscles where mechanical force transmission is the prerequisite for their proper functioning. Therefore, the effect of the viscoelasticity of lamin A is an important avenue to explore. However, such study with lamin A was largely plaguing the field until 2013, when the viscoelasticity of human lamin A protein, along with some of its mutants, was first elucidated [27]. The hallmark of this study lies in the unique viscoelastic response of the lamin A networks deviating from strain hardening behavior of lamin B1. This is not surprising, as there is only 50% homology between human lamins A and B1, and they have been shown to possess distinct higher order associations [85]. The mutations E161K and R190W, which cause DCM in patients [86,87,88], lead to a fragile and poorly elastic matrix, which is unable to bear shear strain deformation [27]. This clearly explains why such lamin A mutations lead to abnormally elongated nuclei with concomitant changes in elasticity.
6. Determination of Lamin A Elasticity at Single Molecule Level
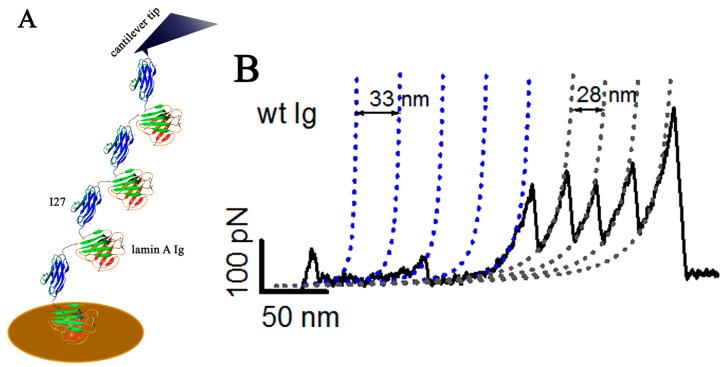
So far, we have reviewed the bulk properties of the lamin A and how mutations affect the viscoelasticity of the lamina. However, it is of interest to elucidate how the mutations that induce secondary and tertiary structural changes at the molecular level [89] play a role in altering the stretching of the domains in response to external forces. Although earlier reports elucidating the mechanical properties of a single vimentin filament had been addressed by atomic force microscopy [90], single molecule pulling experiments on nuclear intermediate filaments using atomic force microscopy were lacking, which has been adequately addressed by Bera et al. [91,92]. Single molecule pulling experiments by atomic force microscope cantilevers of defined spring constants have been elegantly used to show a stretching of molecular domains on the application of picoNewton forces. The stretching experiments using the I27 domain of the titin giant protein enables one to plot force-extension traces by using a worm-like chain model for polymer elasticity [93] and calculate its mechanical properties such as persistence length and contour length with utmost precision [94,95]. Although it has been speculated that the uncoupling of the nucleoskeletal framework corresponds to poor force transduction from the exterior to the nuclear interior, no report has ever shown how the mutations in lamin A that cause laminopathies alter the “springiness” of the otherwise spring-like elastic protein. Recently, how lamin A protein domains unfold in response to externally applied force (Figure 1) and that response is abrogated as a consequence of mutations has been demonstrated via single molecule force spectroscopy [91,92]. In separate experiments, rods 1B and 2B, and Ig-fold domains, were pulled at an average of 1000 nm/s rate. R453W in the Ig-fold unfolded at lower force, and this data was corroborated with a steered molecular dynamic simulation where the sequential unfolding of 9 beta strands was observed. It was conclusively shown that R453W, the mutant causing AD-EDMD was intrinsically unfolded, thereby rendering the network weaker and less resilient to force [91]. Interestingly, statistics reveal that the majority of the mutations in the central alpha helical rod domain are principally concentrated in the 1B domain, and this domain possesses a mechanical resilience of up to 200% strain. This might explain why mutations in the rod 1B leads to severe phenotypes in patients afflicted with laminopathies [92]. The single molecule force spectroscopy is an excellent method to study the stretch response of a protein but is limited by the choice of the protein. As lamin proteins have a high propensity to aggregate, these are not suitable to be studied as a full-length protein. Nevertheless, it can be broken up into smaller independent fragments to generate force-extension curves, which are additive over the entire length of the protein.
Figure 1.
Schematic diagram of stretching of the Ig-fold domain in single molecule force spectroscopy (SMFS) and the force-extension curve. (A) Illustration showing the construct of alternating units of Ig and I27, which are pulled by a cantilever tip from the gold coated cover slip containing a drop of the protein; (B) Force extension curves plotting the unfolding force and change in contour length. The curve is fitted with a worm-like chain model of polymer elasticity. (Reproduced with permission from Bera et. al, Biochemistry, 2014).
7. Conclusions
The nuclear lamina can thus be viewed as a mechanoresponsive element of the cell where the mechanical cues are transmitted from ECM with the help of LINC complexes. Lamins being principal components are also effectors in this mechanotransduction relay by dint of its structural fluctuations and chromatin reorganization. Lamin A is of paramount importance in this relay as a large number of mutations associated with it lead to a plethora of diseases termed as laminopathies. Some of the causative factors behind these mutations are being uncovered by applying principles of physics in biology in the form of rheology and single molecule force spectroscopy. However, further investigations are underway to enable us to delineate the signaling pathways leading to the pathophysiology of laminopathies.
Acknowledgments
We sincerely apologize to all of the authors whom we could not cite due to space constraints. The content has been kept concise but informative to the best of our knowledge.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fawcett D.W. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am. J. Anat. 1966;119:129–145. doi: 10.1002/aja.1001190108. [DOI] [PubMed] [Google Scholar]
- 2.Aaronson R.P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc. Natl. Acad. Sci. USA. 1975;72:1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J. Cell Biol. 1978;79 Pt 1:546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aebi U., Cohn J., Buhle L., Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 5.Goldman A.E., Maul G., Steinert P.M., Yang H.Y., Goldman R.D. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc. Natl. Acad. Sci. USA. 1986;83:3839–3843. doi: 10.1073/pnas.83.11.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeon F.D., Kirschner M.W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 7.Meier I. The plant nuclear envelope. Cell. Mol. Life Sci. 2001;58:1774–1780. doi: 10.1007/PL00000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melcer S., Gruenbaum Y., Krohne G. Invertebrate lamins. Exp. Cell Res. 2007;313:2157–2166. doi: 10.1016/j.yexcr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Broers J.L.V., Ramaekers F.C.S., Bonne G., Yaou R.B., Hutchison C.J. Nuclear lamins: Laminopathies and their role in premature ageing. Physiol. Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 10.Verstraeten V.L., Broers J.L., Ramaekers F.C., van Steensel M.A. The nuclear envelope, a key structure in cellular integrity and gene expression. Curr. Med. Chem. 2007;14:1231–1248. doi: 10.2174/092986707780598032. [DOI] [PubMed] [Google Scholar]
- 11.Machiels B.M., Zorenc A.H., Endert J.M., Kuijpers H.J., van Eys G.J., Ramaekers F.C., Broers J.L. An alternative splicing product of the lamin A/C gene lacks exon 10. J. Biol. Chem. 1996;271:9249–9253. doi: 10.1074/jbc.271.16.9249. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa K., Inagaki H., Hotta Y. Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp. Cell Res. 1994;212:426–430. doi: 10.1006/excr.1994.1164. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa K., Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J. 1993;12:97–106. doi: 10.1002/j.1460-2075.1993.tb05635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewinger L., McKeon F. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. EMBO J. 1988;7:2301–2309. doi: 10.1002/j.1460-2075.1988.tb03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhe-Paganon S., Werner E.D., Chi Y.I., Shoelson S.E. Structure of the globular tail of nuclear lamin. J. Biol. Chem. 2002;277:17381–17384. doi: 10.1074/jbc.C200038200. [DOI] [PubMed] [Google Scholar]
- 16.Krimm I., Ostlund C., Gilquin B., Couprie J., Hossenlopp P., Mornon J.P., Bonne G., Courvalin J.C., Worman H.J., Zinn-Justin S. The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure. 2002;10:811–823. doi: 10.1016/S0969-2126(02)00777-3. [DOI] [PubMed] [Google Scholar]
- 17.Rusinol A.E., Sinensky M.S. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J. Cell Sci. 2006;119 Pt 16:3265–3272. doi: 10.1242/jcs.03156. [DOI] [PubMed] [Google Scholar]
- 18.Stuurman N., Heins S., Aebi U. Nuclear lamins: Their structure, assembly, and interactions. J. Struct. Biol. 1998;122:42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann H., Foisner R. Intermediate filaments: Novel assembly models and exciting new functions for nuclear lamins. Cell. Mol. Life Sci. 2003;60:1607–1612. doi: 10.1007/s00018-003-3004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shumaker D.K., Lopez-Soler R.I., Adam S.A., Herrmann H., Moir R.D., Spann T.P., Goldman R.D. Functions and dysfunctions of the nuclear lamin Ig-fold domain in nuclear assembly, growth, and Emery-Dreifuss muscular dystrophy. Proc. Natl. Acad. Sci. USA. 2005;102:15494–15499. doi: 10.1073/pnas.0507612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimi T., Pfleghaar K., Kojima S., Pack C.G., Solovei I., Goldman A.E., Adam S.A., Shumaker D.K., Kinjo M., Cremer T., et al. The A- and B-type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capco D.G., Wan K.M., Penman S. The nuclear matrix: Three-dimensional architecture and protein composition. Cell. 1982;29:847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- 23.Belmont A.S., Zhai Y., Thilenius A. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopy tomography. J. Cell Biol. 1993;123 Pt 2:1671–1685. doi: 10.1083/jcb.123.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg M.W., Huttenlauch I., Hutchison C.J., Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J. Cell Sci. 2008;121 Pt 2:215–225. doi: 10.1242/jcs.022020. [DOI] [PubMed] [Google Scholar]
- 25.Dahl K.N., Kahn S.M., Wilson K.L., Discher D.E. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 2004;117 Pt 20:4779–4786. doi: 10.1242/jcs.01357. [DOI] [PubMed] [Google Scholar]
- 26.Panorchan P., Wirtz D., Tseng Y. Structure-function relationship of biological gels revealed by multiple-particle tracking and differential interference contrast microscopy: The case of human lamin networks. Phys. Rev. E. 2004;70 Pt 1:041906. doi: 10.1103/PhysRevE.70.041906. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee A., Rathee V., Krishnaswamy R., Bhattacharjee P., Ray P., Sood A.K., Sengupta K. Viscoelastic behavior of human lamin A proteins in the context of dilated cardiomyopathy. PLoS ONE. 2013;8:e83410. doi: 10.1371/journal.pone.0083410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y., Sharov A.A., McDole K., Cheng M., Hao H., Fan C.M., Gaiano N., Ko M.S., Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–1710. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rober R.A., Weber K., Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: A developmental study. Development. 1989;105:365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- 30.Benavente R., Krohne G., Franke W.W. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell. 1985;41:177–190. doi: 10.1016/0092-8674(85)90072-8. [DOI] [PubMed] [Google Scholar]
- 31.Lourim D., Kempf A., Krohne G. Characterization and quantitation of three B-type lamins in Xenopus oocytes and eggs: Increase of lamin LI protein synthesis during meiotic maturation. J. Cell Sci. 1996;109 Pt 7:1775–1785. doi: 10.1242/jcs.109.7.1775. [DOI] [PubMed] [Google Scholar]
- 32.Stick R., Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell. 1985;41:191–200. doi: 10.1016/0092-8674(85)90073-X. [DOI] [PubMed] [Google Scholar]
- 33.Riemer D., Stuurman N., Berrios M., Hunter C., Fisher P.A., Weber K. Expression of Drosophila lamin C is developmentally regulated: Analogies with vertebrate A-type lamins. J. Cell Sci. 1995;108 Pt 10:3189–3198. doi: 10.1242/jcs.108.10.3189. [DOI] [PubMed] [Google Scholar]
- 34.Lehner C.F., Stick R., Eppenberger H.M., Nigg E.A. Differential expression of nuclear lamin proteins during chicken development. J. Cell Biol. 1987;105:577–587. doi: 10.1083/jcb.105.1.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart C., Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987;51:383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- 36.Constantinescu D., Gray H.L., Sammak P.J., Schatten G.P., Csoka A.B. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- 37.Gruenbaum Y., Goldman R.D., Meyuhas R., Mills E., Margalit A., Fridkin A., Dayani Y., Prokocimer M., Enosh A. The nuclear lamina and its functions in the nucleus. Int. Rev. Cytol. 2003;226:1–62. doi: 10.1016/s0074-7696(03)01001-5. [DOI] [PubMed] [Google Scholar]
- 38.Osouda S., Nakamura Y., de Saint Phalle B., McConnell M., Horigome T., Sugiyama S., Fisher P.A., Furukawa K. Null mutants of Drosophila B-type lamin Dm(0) show aberrant tissue differentiation rather than obvious nuclear shape distortion or specific defects during cell proliferation. Dev. Biol. 2005;284:219–232. doi: 10.1016/j.ydbio.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Coffinier C., Chang S.Y., Nobumori C., Tu Y., Farber E.A., Toth J.I., Fong L.G., Young S.G. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc. Natl. Acad. Sci. USA. 2010;107:5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coffinier C., Jung H.J., Nobumori C., Chang S., Tu Y., Barnes R.H., 2nd, Yoshinaga Y., de Jong P.J., Vergnes L., Reue K., et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol. Biol. Cell. 2011;22:4683–4693. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan T., Escalante-Alcalde D., Bhatt H., Anver M., Bhat N., Nagashima K., Stewart C.L., Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubben N., Voncken J.W., Konings G., van Weeghel M., van den Hoogenhof M.M., Gijbels M., van Erk A., Schoonderwoerd K., van den Bosch B., Dahlmans V., et al. Post-natal myogenic and adipogenic developmental: Defects and metabolic impairment upon loss of A-type lamins. Nucleus. 2011;2:195–207. doi: 10.4161/nucl.2.3.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C.D.P., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.-W., Tewari M., et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowat A.C., Lammerding J., Ipsen J.H. Mechanical properties of the cell nucleus and the effect of emerin deficiency. Biophys. J. 2006;91:4649–4664. doi: 10.1529/biophysj.106.086454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lammerding J., Fong L.G., Ji J.Y., Reue K., Stewart C.L., Young S.G., Lee R.T. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 46.Schreiber K.H., Kennedy B.K. When lamins go bad: Nuclear structure and disease. Cell. 2013;152:1365–1375. doi: 10.1016/j.cell.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folker E.S., Östlund C., Luxton G.G., Worman H.J., Gundersen G.G. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc. Natl. Acad. Sci. USA. 2011;108:131–136. doi: 10.1073/pnas.1000824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Worman H.J., Ostlund C., Wang Y. Diseases of the nuclear envelope. Cold Spring Harb. Perspect. Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roncarati R., Viviani Anselmi C., Krawitz P., Lattanzi G., von Kodolitsch Y., Perrot A., di Pasquale E., Papa L., Portararo P., Columbaro M., et al. Doubly heterozygous LMNA and TTN mutations revealed by exome sequencing in a severe form of dilated cardiomyopathy. Eur. J. Hum. Genet. 2013;21:1105–1111. doi: 10.1038/ejhg.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muchir A., Shan J., Bonne G., Lehnart S.E., Worman H.J. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum. Mol. Genet. 2009;18:241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mounkes L.C., Kozlov S.V., Rottman J.N., Stewart C.L. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 2005;14:2167–2180. doi: 10.1093/hmg/ddi221. [DOI] [PubMed] [Google Scholar]
- 52.Zwerger M., Jaalouk D.E., Lombardi M.L., Isermann P., Mauermann M., Dialynas G., Herrmann H., Wallrath L.L., Lammerding J. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum. Mol. Genet. 2013;22:2335–2349. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dialynas G., Speese S., Budnik V., Geyer P.K., Wallrath L.L. The role of Drosophila Lamin C in muscle function and gene expression. Development. 2010;137:3067–3077. doi: 10.1242/dev.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta P., Bilinska Z.T., Sylvius N., Boudreau E., Veinot J.P., Labib S., Bolongo P.M., Hamza A., Jackson T., Ploski R. Genetic and ultrastructural studies in dilated cardiomyopathy patients: A large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res. Cardiol. 2010;105:365–377. doi: 10.1007/s00395-010-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cattin M.E., Bertrand A.T., Schlossarek S., Le Bihan M.C., Skov Jensen S., Neuber C., Crocini C., Maron S., Lainé J., Mougenot N., et al. Heterozygous LmnadelK32 mice develop dilated cardiomyopathy through a combined pathomechanism of haploinsufficiency and peptide toxicity. Hum. Mol. Genet. 2013;22:3152–3164. doi: 10.1093/hmg/ddt172. [DOI] [PubMed] [Google Scholar]
- 56.Verstraeten V.L., Ji J.Y., Cummings K.S., Lee R.T., Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: Effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–393. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dahl K.N., Dahl K.N., Scaffidi P., Islam M.F., Yodh A.G., Wilson K.L., Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffman B.D., Grashoff C., Schwartz M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature. 2011;475:316–323. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crisp M., Liu Q., Roux K., Rattner J.B., Shanahan C., Burke B., Stahl P.D., Hodzic D. Coupling of the nucleus and cytoplasm: Role of the LINC complex. J. Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ketema M., Kreft M., Secades P., Janssen H., Sonnenberg A. Nesprin-3 connects plectin and vimentin to the nuclear envelope of Sertoli cells but is not required for Sertoli cell function in spermatogenesis. Mol. Biol. Cell. 2013;24:2454–6246. doi: 10.1091/mbc.E13-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haque F., Lloyd D.J., Smallwood D.T., Dent C.L., Shanahan C.M., Fry A.M., Trembath R.C., Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang W., Worman H.J., Gundersen G.G. Accessorizing and anchoring the LINC complex for multifunctionality. J. Cell Biol. 2015;208:11–22. doi: 10.1083/jcb.201409047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mejat A., Misteli T. LINC complexes in health and disease. Nucleus. 2010;1:40–52. doi: 10.4161/nucl.1.1.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilhelmsen K., Litjens S.H.M., Kuikman I., Tshimbalanga N., Janssen H., van den Bout I., Raymond K., Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luke Y., Zaim H., Karakesisoglou I., Jaeger V.M., Sellin L., Lu W., Schneider M., Neumann S., Beijer A., Munck M., et al. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J. Cell Sci. 2008;121:1887–1898. doi: 10.1242/jcs.019075. [DOI] [PubMed] [Google Scholar]
- 66.Cain N.E., Tapley E.C., McDonald K.L., Cain B.M., Starr D.A. The SUN protein UNC-84 is required only in force-bearing cells to maintain nuclear envelope architecture. J. Cell Biol. 2014;206:163–172. doi: 10.1083/jcb.201405081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuela N., Zwerger M., Levin T., Medalia O., Gruenbaum Y. Impaired mechanical response of an EDMD mutation leads to motility phenotypes that are repaired by loss of prenylation. J. Cell Sci. 2016;129:1781–1791. doi: 10.1242/jcs.184309. [DOI] [PubMed] [Google Scholar]
- 68.Hale C.M., Shrestha A.L., Khatau S.B., Stewart-Hutchinson P.J., Hernandez L., Stewart C.L., Hodzic D., Wirtz D. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys. J. 2008;95:5462–5475. doi: 10.1529/biophysj.108.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Z.J., Wang W.P., Chen Y.C., Wang J.Y., Lin W.H., Tai L.A., Liou G.G., Yang C.S., Chi Y.H. Dysregulated interactions between lamin A and SUN1 induce abnormalities in the nuclear envelope and endoplasmic reticulum in progeric laminopathies. J. Cell Sci. 2014;127 Pt 8:1792–804. doi: 10.1242/jcs.139683. [DOI] [PubMed] [Google Scholar]
- 70.Dechat T., Gesson K., Foisner R. Lamina-independent lamins in the nuclear interior serve important functions. Cold Spring Harb. Symp. Quant. Biol. 2010;75:533–543. doi: 10.1101/sqb.2010.75.018. [DOI] [PubMed] [Google Scholar]
- 71.Dechat T., Pfleghaar K., Sengupta K., Shimi T., Shumaker D.K., Solimando L., Goldman R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radmacher M. Studying the mechanics of cellular processes by atomic force microscopy. Methods Cell Biol. 2007;83:347–372. doi: 10.1016/S0091-679X(07)83015-9. [DOI] [PubMed] [Google Scholar]
- 73.Lammerding J., Dahl K.N., Discher D.E., Kamm R.D. Nuclear mechanics and methods. Methods Cell Biol. 2007;83:269–294. doi: 10.1016/S0091-679X(07)83011-1. [DOI] [PubMed] [Google Scholar]
- 74.Khatau S.B., Kim D.H., Hale C.M., Bloom R.J., Wirtz D. The perinuclear actin cap in health and disease. Nucleus. 2010;1:337–342. doi: 10.4161/nucl.1.4.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gardel M.L., Shin J.H., MacKintosh F.C., Mahadevan L., Matsudaira P., Weitz D.A. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 76.Chaudhuri O., Parekh S.H., Fletcher D.A. Reversible stress softening of actin networks. Nature. 2007;445:295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Semmrich C., Storz T., Glaser J., Merkel R., Bausch A.R., Kroy K. Glass transition and rheological redundancy in F-actin solutions. Proc. Natl. Acad. Sci. USA. 2007;104:20199–20203. doi: 10.1073/pnas.0705513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamada S., Wirtz D., Coulombe P.A. The mechanical properties of simple epithelial keratins 8 and 18: Discriminating between interfacial and bulk elasticities. J. Struct. Biol. 2003;143:45–55. doi: 10.1016/S1047-8477(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 79.Lin Y.C., Yao N.Y., Broedersz C.P., Herrmann H., MacKintosh F.C., Weitz D.A. Origins of elasticity in intermediate filament networks. Phys. Rev. Lett. 2010;104:58101. doi: 10.1103/PhysRevLett.104.058101. [DOI] [PubMed] [Google Scholar]
- 80.Janmey P.A., Euteneuer U., Traub P., Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schopferer M., Bär H., Hochstein B., Sharma S., Mücke N., Herrmann H., Willenbacher N. Desmin and vimentin intermediate filament networks: Their viscoelastic properties investigated by mechanical rheometry. J. Mol. Biol. 2009;388:133–143. doi: 10.1016/j.jmb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Hofmann I., Franke W.W. Heterotypic interactions and filament assembly of type I and type II cytokeratins in vitro: Viscometry and determinations of relative affinities. Eur. J. Cell Biol. 1997;72:122–132. [PubMed] [Google Scholar]
- 83.Panorchan P., Schafer B.W., Wirtz D., Tseng Y. Nuclear envelope breakdown requires overcoming the mechanical integrity of the nuclear lamina. J. Biol. Chem. 2004;279:43462–43467. doi: 10.1074/jbc.M402474200. [DOI] [PubMed] [Google Scholar]
- 84.Lammerding J., Lee R.T. The nuclear membrane and mechanotransduction: Impaired nuclear mechanics and mechanotransduction in lamin A/C deficient cells. Novartis Found. Symp. 2005;264:264–278. [PubMed] [Google Scholar]
- 85.Goldberg M.W., Fiserova J., Huttenlauch I., Stick R. A new model for nuclear lamina organization. Biochem. Soc. Trans. 2008;36 Pt 6:1339–1343. doi: 10.1042/BST0361339. [DOI] [PubMed] [Google Scholar]
- 86.Perrot A., Hussein S., Ruppert V., Schmidt H.H., Wehnert M.S., Duong N.T., Posch M.G., Panek A., Dietz R., Kindermann I., et al. Identification of mutational hot spots in LMNA encoding lamin A/C in patients with familial dilated cardiomyopathy. Basic Res. Cardiol. 2009;104:90–99. doi: 10.1007/s00395-008-0748-6. [DOI] [PubMed] [Google Scholar]
- 87.Arbustini E., Pilotto A., Repetto A., Grasso M., Negri A., Diegoli M., Campana C., Scelsi L., Baldini E., Gavazzi A., et al. Autosomal dominant dilated cardiomyopathy with atrioventricular block: A lamin A/C defect-related disease. J. Am. Coll. Cardiol. 2002;39:981–990. doi: 10.1016/S0735-1097(02)01724-2. [DOI] [PubMed] [Google Scholar]
- 88.Pasotti M., Klersy C., Pilotto A., Marziliano N., Rapezzi C., Serio A., Mannarino S., Gambarin F., Favalli V., Grasso M., et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J. Am. Coll. Cardiol. 2008;52:1250–1260. doi: 10.1016/j.jacc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 89.Bhattacharjee P., Banerjee A., Banerjee A., Dasgupta D., Sengupta K. Structural alterations of Lamin A protein in dilated cardiomyopathy. Biochemistry. 2013;52:4229–4241. doi: 10.1021/bi400337t. [DOI] [PubMed] [Google Scholar]
- 90.Guzman C., Jeney S., Kreplak L., Kasas S., Kulik A.J., Aebi U., Forro L. Exploring the mechanical properties of single vimentin intermediate filaments by atomic force microscopy. J. Mol. Biol. 2006;360:623–630. doi: 10.1016/j.jmb.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 91.Bera M., Kotamarthi H.C., Dutta S., Ray A., Ghosh S., Bhattacharyya D., Ainavarapu S.R., Sengupta K. Characterization of unfolding mechanism of human lamin A Ig fold by single-molecule force spectroscopy—Implications in EDMD. Biochemistry. 2014;53:7247–7258. doi: 10.1021/bi500726f. [DOI] [PubMed] [Google Scholar]
- 92.Bera M., Ainavarapu S.R., Sengupta K. Significance of 1B and 2B domains in modulating elastic properties of lamin A. Sci. Rep. 2016;6:27879. doi: 10.1038/srep27879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bustamante C., Marko J.F., Siggia E.D., Smith S. Entropic elasticity of lambda-phage DNA. Science. 1994;265:1599–1600. doi: 10.1126/science.8079175. [DOI] [PubMed] [Google Scholar]
- 94.Oberhauser A.F., Marszalek P.E., Carrion-Vazquez M., Fernandez J.M. Single protein misfolding events captured by atomic force microscopy. Nat. Struct. Biol. 1999;6:1025–1028. doi: 10.1038/14907. [DOI] [PubMed] [Google Scholar]
- 95.Marszalek P.E., Lu H., Li H., Carrion-Vazquez M., Oberhauser A.F., Schulten K., Fernandez J.M. Mechanical unfolding intermediates in titin modules. Nature. 1999;402:100–103. doi: 10.1038/47083. [DOI] [PubMed] [Google Scholar]