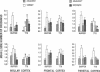Abstract
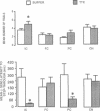
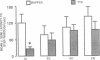
Tetrodotoxin (TTX; a voltage-sensitive sodium channel blocker) was microinjected bilaterally into the insular (IC), frontal (FC), or parietal (PC) cortex or the ventral caudate nucleus of rats either before or after they were trained in an inhibitory avoidance task. When administered either before or after training, injections of TTX into the IC impaired performance on a 48-hr retention test. Injections of TTX into the PC also impaired retention when administered before training. One week later, rats with cannulae in the IC, FC, and PC received microinjections of TTX either before or after training in a water maze (Morris) spatial learning task and retention was tested 24 hr later. TTX impaired retention when administered to the IC either before or after training. These findings indicate that a functionally intact IC during and after training in these tasks appears to be essential for the storage of long-term memory.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermúdez-Rattoni F., Fernández J., Sánchez M. A., Aguilar-Roblero R., Drucker-Colín R. Fetal brain transplants induce recuperation of taste aversion learning. Brain Res. 1987 Jul 21;416(1):147–152. doi: 10.1016/0006-8993(87)91507-1. [DOI] [PubMed] [Google Scholar]
- Buresová O., Bures J. Unilateral and bilateral cortical spreading depression interferes with radial maze performance in rats. Brain Res. 1985 Oct 7;344(2):365–368. doi: 10.1016/0006-8993(85)90816-9. [DOI] [PubMed] [Google Scholar]
- Colavita F. B., Szeligo F. V., Zimmer S. D. Temporal pattern discrimination in cats with insular-temporal lesions. Brain Res. 1974 Oct 11;79(1):153–156. doi: 10.1016/0006-8993(74)90575-7. [DOI] [PubMed] [Google Scholar]
- Colavita F. B., Weisberg D. H. Insular cortex and perception of temporal patterns. Physiol Behav. 1979 May;22(5):827–830. doi: 10.1016/0031-9384(79)90322-6. [DOI] [PubMed] [Google Scholar]
- Deyo R. A., Conner R. L. Microinjections of leupeptin in the frontal cortex or dorsal hippocampus block spatial learning in the rat. Behav Neural Biol. 1989 Sep;52(2):213–221. doi: 10.1016/s0163-1047(89)90327-0. [DOI] [PubMed] [Google Scholar]
- DiMattia B. D., Kesner R. P. Spatial cognitive maps: differential role of parietal cortex and hippocampal formation. Behav Neurosci. 1988 Aug;102(4):471–480. doi: 10.1037//0735-7044.102.4.471. [DOI] [PubMed] [Google Scholar]
- Fukuchi I., Kato S., Nakahiro M., Uchida S., Ishida R., Yoshida H. Blockade of cholinergic receptors by an irreversible antagonist, propylbenzilylcholine mustard (PrBCM), in the rat cerebral cortex causes deficits in passive avoidance learning. Brain Res. 1987 Jan 1;400(1):53–61. doi: 10.1016/0006-8993(87)90652-4. [DOI] [PubMed] [Google Scholar]
- Garcia J., Lasiter P. S., Bermudez-Rattoni F., Deems D. A. A general theory of aversion learning. Ann N Y Acad Sci. 1985;443:8–21. doi: 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- Gold P. E., Macri J., McGaugh J. L. Retrograde amnesia gradients: effects of direct cortical stimulation. Science. 1973 Mar 30;179(4080):1343–1345. doi: 10.1126/science.179.4080.1343. [DOI] [PubMed] [Google Scholar]
- Kiefer S. W. Neural mediation of conditioned food aversions. Ann N Y Acad Sci. 1985;443:100–109. doi: 10.1111/j.1749-6632.1985.tb27066.x. [DOI] [PubMed] [Google Scholar]
- Krushel L. A., van der Kooy D. Visceral cortex: integration of the mucosal senses with limbic information in the rat agranular insular cortex. J Comp Neurol. 1988 Apr 1;270(1):39-54, 62-3. doi: 10.1002/cne.902700105. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L., Introini-Collison I. B., Nagahara A. H. Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Res. 1988 Apr 12;446(1):37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Mechanism of action of tetrodotoxin and saxitoxin on excitable membranes. Fed Proc. 1972 May-Jun;31(3):1124–1132. [PubMed] [Google Scholar]
- Norgren R., Wolf G. Projections of thalamic gustatory and lingual areas in the rat. Brain Res. 1975 Jul 4;92(1):123–129. doi: 10.1016/0006-8993(75)90531-4. [DOI] [PubMed] [Google Scholar]
- Pérez-Ruiz C., Prado-Alcalá R. A. Retrograde amnesia induced by lidocaine injection into the striatum: protective effect of the negative reinforcer. Brain Res Bull. 1989 Apr;22(4):599–603. doi: 10.1016/0361-9230(89)90076-2. [DOI] [PubMed] [Google Scholar]
- Rothfeld J. M., Harlan R. E., Shivers B. D., Pfaff D. W. Reversible disruption of lordosis via midbrain infusions of procaine and tetrodotoxin. Pharmacol Biochem Behav. 1986 Oct;25(4):857–863. doi: 10.1016/0091-3057(86)90398-9. [DOI] [PubMed] [Google Scholar]
- Spanis C. W., Squire L. R. Stability of long temporal gradients of retrograde amnesia in mice. Behav Neural Biol. 1987 Sep;48(2):237–245. doi: 10.1016/s0163-1047(87)90794-1. [DOI] [PubMed] [Google Scholar]
- van der Kooy D., Koda L. Y., McGinty J. F., Gerfen C. R., Bloom F. E. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984 Mar 20;224(1):1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]