Abstract
Statins are a mainstay of hyperlipidemia treatment. These drugs inhibit the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase and have beneficial effects on atherosclerosis including plaque stabilization, reduction of platelet activation, and reduction of plaque proliferation and inflammation. Statins also have a benefit beyond atherosclerotic plaque, including anticoagulation, vasodilatation, antioxidant effects, and reduction of mediators of inflammation. In the perioperative period, statins appear to contribute to improved outcomes via these mechanisms. Both vascular and nonvascular surgery patients have been shown in prospective studies to have lower risk of adverse cardiac outcomes when initiated on statins preoperatively. However, not all patients can tolerate statins; the search for novel lipid-lowering therapies led to the discovery of the proprotein convertase subtilisin/kexin Type 9 (PCSK9) inhibitors. These drugs are fully-humanized, injectable monoclonal antibodies. With lower PCSK9 activity, low-density lipoprotein cholesterol (LDL-C) receptors are more likely to be recycled to the hepatocyte surface, where they serve to clear plasma LDL-C. Evidence from several prospective studies shows that these new agents can significantly lower LDL-C levels. While PCSK9 inhibitors offer hope of effective therapy for patients with familial hyperlipidemia or intolerance of statins, several important questions remain, including the results of long term cardiovascular outcome studies. The perioperative effects of new LDL-C-lowering drugs are unknown at present but are likely to be similar to the older agents.
Key words: Atherosclerosis, hyperlipidemia, perioperative outcomes, proprotein convertase subtilisin/kexin Type 9 inhibitors, statins
Introduction
The leading cause of death in the United States is cardiac disease, accounting for more than 600,000 deaths each year. Further, the most frequent type of heart disease in the United States is coronary artery disease (CAD). The presence of hypercholesterolemia predisposes the patient to CAD via entry of low-density lipoprotein cholesterol (LDL-C) particles into arterial subendothelial spaces. Hyperlipidemia treatments include diet and exercise, fibrates, bile acid sequestrants, nicotinic acid, statins, and selective inhibitors of cholesterol absorption.[1] Statin therapy is effective for both primary and secondary prevention for CAD and also improves a number of perioperative outcomes. Significant reductions in cardiovascular morbidity and mortality from lipid-lowering as well as pleiotropic effects have been demonstrated.
However, statins are not adequate therapy for patients with elevated lipoprotein (a) and for patients with very high levels of LDL-C, such as those with familial hyperlipidemia.[2] Further, some patients are intolerant of statins and might benefit from alternatives and/or combination therapies and even lower LCL-C levels. The purpose of this manuscript is to review the mechanism of action of statins and their favorable effects on the lipid profile and perioperative outcomes; to review issues of compliance and cost associated with the statins; and to introduce the reader to the newest type of lipid-lowering agents: the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. These monoclonal antibodies reduce LDL-C, apolipoprotein B and non-high-density lipoprotein-cholesterol (HDL-C) with minimal effects on triglycerides and HDL-C.
Statin Mechanism of Action
For the past three decades, the mainstay of treatment for CAD has been statin therapy. Statins inhibit the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase. This inhibition occurs early in the hepatic pathway that produces not only cholesterol but also other vital metabolic products [Figure 1].[3] Cholesterol itself is an intermediate product in pathways that produce corticosteroids, sex steroids, Vitamin D, and bile acids.
Figure 1.
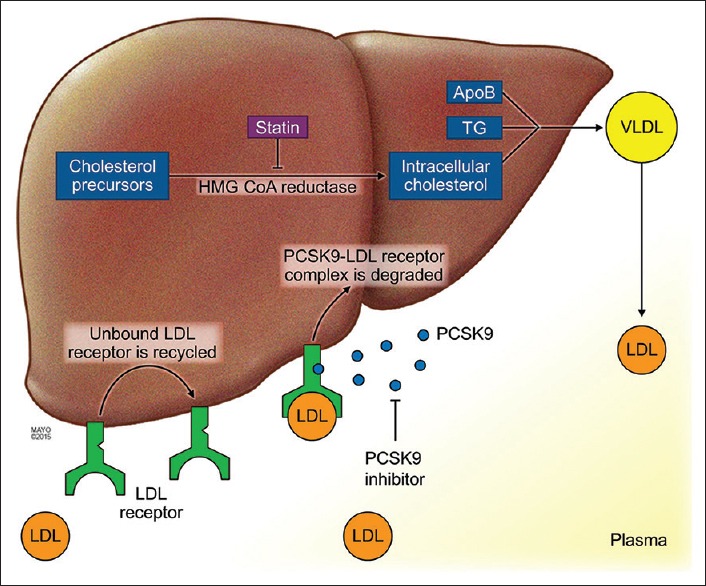
Metabolic pathways impacted by statins and proprotein convertase subtilisin/kexin Type 9 inhibitors. Decreased cholesterol synthesis results in upregulation of low-density lipoprotein cholesterol receptors and greater clearance of low-density lipoprotein cholesterol. When proprotein convertase subtilisin/kexin Type 9 is bound to the low-density lipoprotein cholesterol receptor, the internalized receptor is more likely to be degraded. With lower proprotein convertase subtilisin/kexin Type 9 activity due to proprotein convertase subtilisin/kexin Type 9 inhibitors, low-density lipoprotein receptors are more likely to be recycled to the cell surface to clear plasma low-density lipoprotein cholesterol. VLDL = Very low-density lipoprotein, TG = Triglyceride, ApoB = Apolipoprotein B. By permission of Mayo Foundation for Medical Education and Research
Decreased cholesterol synthesis results in upregulation of LDL-C receptors on the surface of the hepatocyte and greater clearance of LDL-C from the plasma. Effective statin therapy will reduce LDL-C, reduce triglycerides, and modestly raise HDL levels. In the past, guidelines to prevent CAD suggested patients achieve a LDL-C level <100 mg/dL, which limits the formation of plaque in coronary arteries. Newer guidelines do not address a targeted LDL-C level but target the types of patients that should lower their LDL-C levels.[4]
In addition to favorable changes of the lipid profile, statins have other beneficial effects on atherosclerosis including plaque stabilization, reduction of platelet activation, and reduction of plaque proliferation and inflammation. Their net effect is to decrease plaque thrombogenicity.[3] Statins also have a benefit beyond atherosclerotic plaque, including anticoagulation, vasodilatation and antioxidant effects, and reduction of mediators of inflammation.[5]
According to several national guidelines,[4,6] statins are recommended as first-line therapy for CAD because a significant body of literature supports their use for primary prevention, particularly in higher risk patients. Statin therapy is also supported for secondary prevention in known cardiovascular disease patients or those with the risk equivalent.[7] A meta-analysis of over 90,000 participants determined that reducing LDL-C levels by an average of 39 mg/dL will yield a 23% reduction in cardiovascular risk over 5 years.[8]
Newer, so-called high-intensity statin therapy (second or third generation statins, e.g., 20–40 mg/day of rosuvastatin; 40–80 mg/day of atorvastatin) was developed and has had even more success in lowering LDL-C levels.[9,10] In addition, ezetimibe (Zetia®) was approved in the class of cholesterol absorption inhibitors which when used alone or with a high-intensity statin have proved effective in those patients with extremely high LDL-C levels.[1,11]
Compliance and Cost
Over three decades of experience with statin therapies have shown that most patients tolerate them well, but a sub-population may be intolerant. Muscle adverse events (myalgias, muscle weakness, myositis) are the most commonly reported side effects of statins, with a clear dose-dependent relationship between many, but not all adverse events.[12] Overall, 5–10% of patients on statins will have an adverse event, and 10–20% on high-dose statins will have a muscle-related complaint.[13,14] Cognitive events (memory problems, confusion, and cognition changes) are second in frequency to muscle complaints.
Multiple studies indicate that patients previously intolerant of statins can tolerate them when reinitiated at a later time and possibly with a different agent.[15,16,17,18,19] Statin-intolerant patients can be managed with alternative therapies, including switching to another statin or use of intermittent dosage regimens, as well as nonstatin lipid-lowering drugs (e.g., ezetimibe and fibrates).[20]
Lazar et al. concluded that the use of low-cost generic statin therapy in the primary prevention of CAD was cost-effective, but suggested under certain conditions expanding to more aggressive prescribing would be cost-effective.[21] Their analysis used the benchmark of $4.00 USD per month for statin therapy based on the fact that the majority of statins are now generic; however, some of the high-intensity statins and ezetimibe are considerably more expensive (approximately $250 USD per month). Lazar and his team took into consideration the incidence of cardiovascular accidents, death rates, and the costs of medical treatment associated with these events compared to the current low cost of statin therapies.
Statin medications are taken orally, usually once a day. Even with that simplistic schedule, many patients are noncompliant.[22,23] Statin compliance may be as low as 54%,[24] and 50% or more of patients initiated on statin therapy will discontinue within 1 year.[25]
Parris et al. showed that an adherence rate above 80% produced a significantly higher number of patients who were able to achieve a goal of <100 mg/dL of LDL-C.[26] A number of interventions may help to improve compliance, including switching from brand name to generic drugs, reducing out-of-pocket costs, and eliminating co-pays.[22] Although statin therapy is effective in lowering cholesterol and lowering the risk of CAD at relatively low cost, adherence remains a challenge.
Statins and Perioperative Outcomes
After noncardiac surgery, cardiovascular complications are the leading cause of morbidity and mortality, affecting 3% of patients.[27,28] Statins may improve outcomes simply by lowering cholesterol levels and decreasing the incidence of atherogenesis and endothelial dysfunction. However, it appears that statins provide beneficial effects via nonlipid-lowering mechanisms as well. These pleiotropic effects include vasodilatation, reduced inflammation and thrombogenicity, antioxidant effects, and reduction in C-reactive protein (CRP).[29] Further, statins help to stabilize atherosclerotic plaque and produce antiplatelet effect.
In the perioperative period, statins have been shown to improve outcomes,[30,31,32] although much of the data is retrospective.[33,34] For instance, in a retrospective cohort study of over 780,000 patients undergoing noncardiac surgery, treatment with statins was associated with lower mortality.[35] The authors concluded that among highest risk patients, the number needed to treat (with statins) to prevent a perioperative death was 30.
In a randomized, prospective study of 100 vascular surgery patients, 20 mg of daily atorvastatin was compared to placebo.[31] Patients were initiated on therapy (or placebo) for 30 days prior to surgery and continued for 15 days afterward, for a total of 45 days of treatment. The patients were followed for 180 days for a composite of cardiovascular complications, including nonfatal myocardial infarction (MI), stroke, unstable angina, and death. There was a significant reduction in events for the statin group, 8% versus 26%, P = 0.031.
In a prospective study of 497 statin-naïve patients prior to major vascular surgery, patients were randomized to fluvastatin 80 mg or placebo plus a beta-blocker.[32] The patients were treated for a mean of 37 days before surgery and 30 days postoperatively. The occurrence of myocardial ischemia was significantly less in the treatment group within 30 days of surgery, 10.8% versus 19%, P = 0.01; the number needed to treat to prevent myocardial ischemia in one patient was 12. Of note in this study, the fluvastatin was also associated with a significant reduction in inflammatory markers including interlukin-6 and CRP.
Finally, in a prospective study that included noncardiovascular surgery patients considered to be intermediate-risk, bisoprolol and fluvastatin versus placebo was initiated about 35 days preoperatively and continued for 30 days postoperatively.[30] Similar to the previous prospective studies, a 30-day composite outcome of MI and cardiac death was less in the treatment group, 3.2% versus 4.9%, but statistical significance was not met (P = 0.17). Overall, one could conclude that the greater the cardiovascular risk, the greater the benefit of perioperative statins.
Other perioperative outcomes of interest to anesthesiologists include atrial fibrillation and renal function. In a prospective study of 131 thoracic surgery (cancer) patients, new atrial fibrillation was reduced 3-fold in patients taking statins preoperatively.[36] Interestingly, although atrial fibrillation/flutter patients have elevated CRP levels, the reduction in the incidence of atrial fibrillation in the statin group was independent of CRP. Putative beneficial effects of statins on dysrhythmias include reduced postoperative inflammation and lipid-modulating effects on ion channel membranes.[37]
In terms of renal function, there is a paucity of prospective data to inform practice. Retrospective data suggests that statins can reduce renal injury in patients undergoing cardiovascular procedures requiring aortic cross-clamping.[32,38] In one study, statin use was associated with increased odds of complete renal function recovery after lower extremity vascular or abdominal aortic procedures;[34] while another retrospective study showed no improvement in renal outcomes after major vascular interventions.[39]
A recent meta-analysis that included 2275 statin-naïve patients in 16 randomized, controlled trials concluded that statins favorably impact perioperative outcomes.[5] The patients in these trials were initiated on statin therapy versus placebo after randomization and before surgery. Outcomes included reduced mortality (P = 0.03), MI (P < 0.001), perioperative atrial fibrillation (P < 0.001), and hospital length of stay (P < 0.001). The outcomes were most beneficial in patients undergoing cardiac surgery, but noncardiac surgery patients also benefitted from initiation of statin therapy before surgery.
No current guidelines recommend initiating patients on statins in the perioperative period, and a number of questions remain unanswered regarding perioperative statin therapy. For instance, if statins are initiated preoperatively, how long before surgery should this be done, and at what dose? What impact, if any, would side-effects of statins have on surgery scheduling and perioperative management? Despite these and other questions, patients taking statins preoperatively should be restarted on their statin therapy postoperatively as soon as possible.[40] Perioperative discontinuation of statin therapy is associated with worse cardiac outcomes.[41] Unfortunately, many patients on preoperative statins are not aware of the benefit and may stop them before surgery, and surgeons rarely discuss the importance of continuing statins perioperatively.[42]
At present, there is no clear evidence of harm from administering succinylcholine to patients with statin-related myalgias, and no reason to monitor parameters associated with stain use such as myoglobin, potassium, or creatinine kinase.[43] Further, there is no literature to suggest statins slow or negatively impact cognitive recovery from anesthesia, despite patient complaints of cognitive changes from these drugs. In fact, there is literature to suggest that statins are neuroprotective in asymptomatic carotid stenosis patients undergoing carotid endarterectomy.[44]
New Therapies
With multiple studies demonstrating that lower LDL-C correlates with reduced cardiovascular events, interest in novel therapies led to the discovery of a new class of drugs known as PCSK9 inhibitors. First recognized in 2003, these drugs are fully-humanized, injectable monoclonal antibodies. Observations that led to the development of PCSK9 inhibitors include the fact that some missense mutations of PCSK9 are associated with hyperlipidemia.[45] In these mutations, increased LDL receptor degradation occurs in the hepatocyte; fewer LDL receptors mean that less LDL-C is cleared from the plasma.
Conversely, other PCSK9 gene sequence variations result in decreased PCSK9 activity; these patients have lower LDL-C and fewer lifetime cardiovascular events.[46] When PCSK9 is bound to the LDL-C receptor, the internalized receptor is more likely to be degraded by hepatocyte lysosomes. With lower PCSK9 activity, LDL receptors are more likely to be recycled to the cell surface, where they serve to clear plasma LDL-C [Figure 1].[47]
Therefore, genetic studies of patients with gain-or-loss of function mutations of PCSK9 led to interest in drugs that could inhibit PCSK9 activity. The US Food and Drug Administration (FDA) expressed concern that these new drugs could drive cholesterol so low that patients would suffer metabolic and/or neurocognitive effects. After extensive testing, two new agents were approved by the FDA in mid-2015 for certain cases of hyperlipidemia. The approved monoclonal antibodies are alirocumab (Praluent®) and evolocumab (Repatha®). A third agent (bococizumab) is in phase-III trials.
Evidence suggests that these new agents can lower LDL-C levels significantly. Alirocumab was approved as an add-on to diet and statins for adults with heterozygous familial hypercholesterolemia (FHC) or for additional lowering of LDL-C in patients with clinical atherosclerotic cardiovascular disease. Evolocumab was approved with similar indications but also includes an indication for homozygous FHC.
Clinical studies have shown a >60% reduction in LDL-C when PCSK9 inhibitors were added to standard therapy compared to standard treatment alone.[48,49] There was no increase in adverse events, treatment versus standard group, for injection site reactions, neurocognitive events, and liver function test changes. Alirocumab was associated with increased myalgias versus placebo, 5.4 versus 2.9%, P = 0.006. Simultaneously, major adverse cardiac events have been reduced significantly in the patients treated with PCSK9 inhibitors.
For instance, Sabatine et al. showed that the rate of cardiovascular events at 1 year was reduced significantly in patients receiving evolocumab plus standard therapy versus those receiving standard therapy alone, 2.18% versus 0.95%, P = 0.003.[50] The adjudicated cardiovascular events studied included transient ischemic attack, stroke, unstable angina, heart failure, MI, coronary revascularization, and death.
Alirocumab must be injected every 2 weeks, and evolocumab can be injected either every 2 weeks or once per month. The PCSK9 inhibitors carry substantially higher costs (approximately $1,400 USD per month) than standard therapy. The evidence available at this time does not correlate long-term cardiovascular outcomes data although several longitudinal studies are underway (e.g., FOURIER study of Evolocumab, NCT01764633, scheduled for completion in 2018). However, several early lines of evidence suggest that these drugs will improve long-term outcomes in patients with hyperlipidemia. First, the PCSK9 inhibitors have been shown to effectively reduce the levels of LDL-C cholesterol; second, they ultimately have the same mechanism of action of traditional statins (increase hepatocyte LDL receptor activity); and third, patients with genetic loss of function of PCSK9 activity have lower lifetime risk of cardiovascular disease.[46] These new agents seem to be reasonably safe; however, they are not without potential adverse events, such as increased visceral adiposity secondary to decreased free fatty acid clearance, glucose intolerance, and insulin resistance (reported in patients with PCSK9 loss of function mutation).[49]
Implications of Proprotein Convertase Subtilisin/kexin Type 9 Inhibitors for Perioperative Care
There is no data yet to guide the perioperative clinician regarding the perioperative effects of PCSK9 inhibitors. It is reasonable to speculate that these drugs will improve perioperative outcomes, similar to the statins, and they should be continued when possible around the time of surgery. However, because the current formulation of PCSK9 inhibitors are injected subcutaneously on a monthly or every-other-week basis, the anesthesiologist will have little control over perioperative dosing of these drugs. Like the statins, it is appropriate to remind surgical colleagues regarding administration of PCSKP inhibitors in the immediate perioperative period. At a minimum, anesthesiologists should be aware of this new class of medications as more information becomes available.
Summary
While PCSK9 inhibitors offer hope of effective therapy for patients with familial hyperlipidemia or intolerance of statins, several important questions remain. Long-term (phase-III) cardiovascular studies are ongoing, and the chronic effects of dramatic lowering of LDL-C levels via PCSK9 inhibitors are unknown. The economic implications of this new class of drugs are substantial, and patient compliance with an injectable medication that produces no outward physical benefit (e.g., pain reduction, improved joint function) is in question. While currently unknown, the perioperative effects of new LDL-C-lowering drugs are likely to be similar to the older agents.
Financial support and sponsorship
Nil.
Conflicts of interest
Steven G. Avey is Vice-President for Specialty Pharmacy, MedImpact, a pharmacy benefits management company.
References
- 1.Sando KR, Knight M. Nonstatin therapies for management of dyslipidemia: A review. Clin Ther. 2015;37:2153–79. doi: 10.1016/j.clinthera.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Robinson JG. Management of familial hypercholesterolemia: A review of the recommendations from the national lipid association expert panel on familial hypercholesterolemia. J Manag Care Pharm. 2013;19:139–49. doi: 10.18553/jmcp.2013.19.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stancu C, Sima A. Statins: Mechanism of action and effects. J Cell Mol Med. 2001;5:378–87. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 5.de Waal BA, Buise MP, van Zundert AA. Perioperative statin therapy in patients at high risk for cardiovascular morbidity undergoing surgery: A review. Br J Anaesth. 2015;114:44–52. doi: 10.1093/bja/aeu295. [DOI] [PubMed] [Google Scholar]
- 6.European Association for Cardiovascular Prevention & Rehabilitation. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, et al. ESC/EAS guidelines for the management of dyslipidaemias: The task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 7.Last AR, Ference JD, Falleroni J. Pharmacologic treatment of hyperlipidemia. Am Fam Physician. 2011;84:551–8. [PubMed] [Google Scholar]
- 8.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 9.McKenney JM, Jones PH, Adamczyk MA, Cain VA, Bryzinski BS, Blasetto JW. STELLAR Study Group. Comparison of the efficacy of rosuvastatin versus atorvastatin, simvastatin, and pravastatin in achieving lipid goals: Results from the STELLAR trial. Curr Med Res Opin. 2003;19:689–98. doi: 10.1185/030079903125002405. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan CJ, Gotto AM., Jr Update on statins: 2003. Circulation. 2004;110:886–92. doi: 10.1161/01.CIR.0000139312.10076.BA. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 12.Golomb BA, Evans MA. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients – the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 14.Pohjola-Sintonen S, Julkunen H. Muscle-related adverse effects of statins. Duodecim. 2014;130:1622–7. [PubMed] [Google Scholar]
- 15.Brookhart MA, Patrick AR, Schneeweiss S, Avorn J, Dormuth C, Shrank W, et al. Physician follow-up and provider continuity are associated with long-term medication adherence: A study of the dynamics of statin use. Arch Intern Med. 2007;167:847–52. doi: 10.1001/archinte.167.8.847. [DOI] [PubMed] [Google Scholar]
- 16.Korhonen MJ, Helin-Salmivaara A, Huupponen R. Dynamics of long-term statin therapy. Eur J Clin Pharmacol. 2011;67:925–31. doi: 10.1007/s00228-011-1019-2. [DOI] [PubMed] [Google Scholar]
- 17.Backes JM, Venero CV, Gibson CA, Ruisinger JF, Howard PA, Thompson PD, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother. 2008;42:341–6. doi: 10.1345/aph.1K604. [DOI] [PubMed] [Google Scholar]
- 18.Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol. 2008;101:1747–8. doi: 10.1016/j.amjcard.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 19.Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep. 2010;12:322–30. doi: 10.1007/s11883-010-0120-9. [DOI] [PubMed] [Google Scholar]
- 20.Pirillo A, Catapano AL. Statin intolerance: Diagnosis and remedies. Curr Cardiol Rep. 2015;17:27. doi: 10.1007/s11886-015-0582-z. [DOI] [PubMed] [Google Scholar]
- 21.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–53. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 22.Birtcher K. When compliance is an issue-how to enhance statin adherence and address adverse effects. Curr Atheroscler Rep. 2015;17:471. doi: 10.1007/s11883-014-0471-8. [DOI] [PubMed] [Google Scholar]
- 23.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19:638–45. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: A meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–8. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 25.Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): An internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6:208–15. doi: 10.1016/j.jacl.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Parris ES, Lawrence DB, Mohn LA, Long LB. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28:595–9. doi: 10.2337/diacare.28.3.595. [DOI] [PubMed] [Google Scholar]
- 27.Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153–84. doi: 10.1097/00000542-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Mangano DT, Goldman L. Preoperative assessment of patients with known or suspected coronary disease. N Engl J Med. 1995;333:1750–6. doi: 10.1056/NEJM199512283332607. [DOI] [PubMed] [Google Scholar]
- 29.Skrlin S, Hou V. A review of perioperative statin therapy for noncardiac surgery. Semin Cardiothorac Vasc Anesth. 2010;14:283–90. doi: 10.1177/1089253210386387. [DOI] [PubMed] [Google Scholar]
- 30.Dunkelgrun M, Boersma E, Schouten O, Koopman-van Gemert AW, van Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate-risk patients undergoing noncardiovascular surgery: A randomized controlled trial (DECREASE-IV) Ann Surg. 2009;249:921–6. doi: 10.1097/SLA.0b013e3181a77d00. [DOI] [PubMed] [Google Scholar]
- 31.Durazzo AE, Machado FS, Ikeoka DT, De Bernoche C, Monachini MC, Puech-Leão P, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: A randomized trial. J Vasc Surg. 2004;39:967–75. doi: 10.1016/j.jvs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Schouten O, Boersma E, Hoeks SE, Benner R, van Urk H, van Sambeek MR, et al. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361:980–9. doi: 10.1056/NEJMoa0808207. [DOI] [PubMed] [Google Scholar]
- 33.Huffmyer JL, Mauermann WJ, Thiele RH, Ma JZ, Nemergut EC. Preoperative statin administration is associated with lower mortality and decreased need for postoperative hemodialysis in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23:468–73. doi: 10.1053/j.jvca.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Welten GM, Chonchol M, Hoeks SE, Schouten O, Dunkelgrün M, van Gestel YR, et al. Statin therapy is associated with improved outcomes in vascular surgery patients with renal impairment. Am Heart J. 2007;154:954–61. doi: 10.1016/j.ahj.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 35.Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291:2092–9. doi: 10.1001/jama.291.17.2092. [DOI] [PubMed] [Google Scholar]
- 36.Amar D, Zhang H, Heerdt PM, Park B, Fleisher M, Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. 2005;128:3421–7. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 37.Young-Xu Y, Jabbour S, Goldberg R, Blatt CM, Graboys T, Bilchik B, et al. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003;92:1379–83. doi: 10.1016/j.amjcard.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 38.Tabata M, Khalpey Z, Pirundini PA, Byrne ML, Cohn LH, Rawn JD. Renoprotective effect of preoperative statins in coronary artery bypass grafting. Am J Cardiol. 2007;100:442–4. doi: 10.1016/j.amjcard.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 39.Kor DJ, Brown MJ, Iscimen R, Brown DR, Whalen FX, Roy TK, et al. Perioperative statin therapy and renal outcomes after major vascular surgery: A propensity-based analysis. J Cardiothorac Vasc Anesth. 2008;22:210–6. doi: 10.1053/j.jvca.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: Executive summary: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971–96. doi: 10.1161/CIRCULATIONAHA.107.185700. [DOI] [PubMed] [Google Scholar]
- 41.Le Manach Y, Godet G, Coriat P, Martinon C, Bertrand M, Fléron MH, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007;104:1326–33. doi: 10.1213/01.ane.0000263029.72643.10. [DOI] [PubMed] [Google Scholar]
- 42.Cubillo EI, 4th, Rosenfeld DM, Hagstrom SG, Hu FL, Demenkoff JH, Cheng MR, et al. Patient understanding of the importance of statin use in the perioperative period. J Cardiothorac Vasc Anesth. 2015;29:670–7. doi: 10.1053/j.jvca.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Turan A, Mendoza ML, Gupta S, You J, Gottlieb A, Chu W, et al. Consequences of succinylcholine administration to patients using statins. Anesthesiology. 2011;115:28–35. doi: 10.1097/ALN.0b013e31822079fa. [DOI] [PubMed] [Google Scholar]
- 44.Heyer EJ, Mergeche JL, Bruce SS, Ward JT, Stern Y, Anastasian ZH, et al. Statins reduce neurologic injury in asymptomatic carotid endarterectomy patients. Stroke. 2013;44:1150–2. doi: 10.1161/STROKEAHA.111.000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 46.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 47.Page MM, Watts GF. Evolocumab in the treatment of dyslipidemia: Pre-clinical and clinical pharmacology. Expert Opin Drug Metab Toxicol. 2015;11:1505–15. doi: 10.1517/17425255.2015.1073712. [DOI] [PubMed] [Google Scholar]
- 48.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 49.Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol. 2015;65:2638–51. doi: 10.1016/j.jacc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Sabatine MS, Wasserman SM, Stein EA. PCSK9 inhibitors and cardiovascular events. N Engl J Med. 2015;373:774–5. doi: 10.1056/NEJMc1508222. [DOI] [PubMed] [Google Scholar]


