Abstract
Background and Aims:
The aim of this study is to assess the effect of atrial natriuretic peptide (ANP) on intestinal ischemia-reperfusion injury in septic shock.
Material and Methods:
A prospective randomized controlled, observer-blinded study was carried out in surgical Intensive Care Unit (ICU), University Hospital. Forty adult patients in septic shock were randomly divided into two groups, control group (Group C) received normal saline and ANP group (Group A) patients received ANP in the form of 1.5 mg vial added to 250 ml solvent in plastic bag (1 ml = 6 micg) given at 2 mcg/kg intravenous bolus over 1 min followed by 0.01 mcg/kg/min for 24 h. The primary outcome measurements were blood marker of intestinal hypoperfusion in form of intestinal fatty acid binding protein (I-FABP), malondialdehyde (MDA), myloperoxidase enzyme activity (MPO), protein carbonyl (PC), and glutathione peroxidase activity (GPA) measured before start of ANP infusion, 6 h, 12 h, and 24 h after start of infusion. The secondary outcome measurements were the duration of noradrenaline infusion, duration of ICU stay, hospital mortality rate, and complications related to ANP.
Results:
In comparison with Group C, Group A showed a significant decrease (P < 0.05) in serum level of MPO, MDA, PC, and I-FABP, with a significant increase (P < 0.05) in serum level of GPA, 6 h, 12 h, and 24 h after the start of ANP infusion. There was significant decrease (P < 0.05) in mean duration of noradrenaline infusion, the length of ICU stay and mortality rate in Group A in comparison with Group C. In Group A, seven patients had mean arterial blood pressure < 65 mmHg but respond to volume resuscitation, three patients serum sodium was 125–130 mmol/L.
Conclusion:
In cases of septic shock, concomitant administration of ANP with noradrenaline may have a protective effect against intestinal injury through a decrease in the level of intestinal hypoperfusion owing to its anti-inflammatory and antioxidant effect.
Key words: Atrial natriuretic peptide, chemical markers, Intensive Care Unit, ischemia-reperfusion injury
Introduction
Severe trauma and major surgery frequently result in the development of inflammatory complications, including systemic inflammatory response syndrome, sepsis, and septic shock with subsequent splanchnic hypoperfusion. Intestinal cell damage developed within 1 h after shock induction.[1]
Tissue injury after ischemia is primarily caused by reduced oxygen supply and the injury may be worsened during reperfusion phase via formation of reactive oxygen radicals which can promote cell destruction and bowel necrosis through peroxidation of membrane lipid with subsequent increase in the intestinal mucosal level of malondialdehyde (MDA).[2] In addition, membrane protein oxidation induces early increase in mucosal protein carbonyl (PC) level owing to formation of reactive chlorinating species capable of oxidizing protein. Moreover, the oxidative stress inhibit the endogenous antioxidant system which includes glutathione peroxidase and superoxide dismutase.[3]
Intestinal fatty acid binding protein (I-FABP), a cytosolic protein distributes in the small bowel mucosa from the duodenum to the distal segment of the ileum, and its serum level elevates rapidly at the early stages of small bowel ischemia. Hence, it is considered as an early biomarker for intestinal disease, ischemia, and damage.[4]
Ischemia stimulates localized infiltration of activated neutrophils which is manifested by significant increase in the mucosal level of myloperoxidase enzyme activity (MPO).[5]
Research in this field has identified key molecular and signaling players that could modulate the tissue injury during this disease process. However, further elucidation of the molecular mechanisms should provide the rationale to identify much needed novel therapeutic options to ameliorate organ damage.[6]
Atrial natriuretic peptide (ANP) besides its role in regulating volume homeostasis, has a protective effect during ischemia-reperfusion injury (IRI) in many tissues. Recent research documented anti-inflammatory, antifibrotic, and antimitogenic actions for NPs. Based on the multiple protective effects of NPs, it was approved for use in the United States in 2001 by the Food and Drug Administration for acute decompensated heart failure. A previous study demonstrated protective effect of NP in IRI in heart, lung, brain, and liver owing to the extracardiac expression for NP receptors.[7] Hence, in this work, we attempted to evaluate the role of ANP in attenuating intestinal injury in septic shock.
Material and Methods
This is a prospective observer-blinded study carried out in surgical Intensive Care Unit (ICU) from March 2013 to April 2014 on 40 patients in septic shock after approval by Hospital Ethical Committee. The CONSORT 2010 statement was followed in reporting this study.
Inclusion criteria
Patients in septic shock according to the American college of chest physicians/society of critical care medicine definitions,[8] which include strongly suspected infection or positive blood culture plus persistent hypotension despite adequate fluid resuscitation requiring vasopressors to maintain mean arterial blood pressure (MAP) ≥65 mmHg, and lactate ≥2 mmol/L
Patients were treated with antibiotics according to blood culture and sensitivity test
Patients on vasopressor noradrenaline intravenous (IV) infusion (0.05–0.2 micg/kg/min)
MAP ≥65 mmHg.
Exclusion criteria
Patients on noradrenaline dose >0.2 micg/kg/min
Patients with electrolyte imbalance
Patients with one or more organ failure
Patients with renal dysfunction
Patients with valvular stenosis, restrictive or obstructive cardiomyopathy, constrictive pericarditis, and pericardial tamponade.
Randomization was performed through a computer-generated, random number list. The random number list was generated using the QuickCalcs (GraphPad Software Inc., La Jolla, CA, USA). The group assignment numbers were sealed in an envelope and kept by the study supervisor. After admission to ICU, a written informed consent was obtained from the nearest relatives. Patients were randomly divided into two groups of twenty patients each.
Group (C): Control group received normal saline.
Group (E): patients received ANP (Natrecor Scios Inc., Titusville, NJ 08560, Austria)) supplied from Sigma company in form of 1.5 mg vial added to 250 ml plastic IV bag (1 ml = 6 mg) given at 2 mcg/kg IV bolus over 1 min followed by 0.01 mcg/kg/min for 24 h (the dose approved for acute decompensated heart failure).[7]
Patients were monitored for mean arterial pressure (MAP) (mmHg), heart rate (HR) (b/min), central venous pressure (CVP) (cmH2O), temperature, urine output (UOP), total fluid balance and routine investigation (complete blood count, arterial blood gases, serum electrolytes, liver, and kidney function).
Measurements
The primary outcome measurements were blood marker of intestinal hypoperfusion in the form of the following laboratory data which was measured before the start of ANP infusion, 6 h, 12 h and 24 h after the start of infusion. Blood sample (5 ml) was centrifuged and serum was used to measure:
I-FABP as a marker of intestinal ischemia using (ELISA) measured by spectrophotometer at wavelength 450 nm[9]
MDA measured by spectrophotometer at wavelength 535 nm[10]
PC level measured by spectrophotometer at wavelength 366 nm[11]
MPO measured by spectrophotometer at wavelength 655 nm[12]
Glutathione peroxidase activity (GPA) (lipid peroxide scavenger) was expressed as nanomols of reduced nicotinamide adenine dinucleotide phosphate oxidized to NADH measured by spectrophotometer at wavelength 340 nm.[13]
The secondary outcome measurements were the duration of noradrenaline infusion (days), duration of ICU stay, hospital mortality rate and complications related to ANP such as hypotension, headache, nausea, and back pain.(if developed during time of the study, the drug infusion was stopped, and complication was recorded).
Statistical analysis
Sample size of forty patients was calculated for 90% power, α =0.05, β =0.1, and anticipated effect size = 0.40 using sample size software (G * Power Version 3.00.10, Germany). Analytic statistics was performed on IBM compatible computer using SPSS 11.5 (SPSS Inc., Chicago, IL, USA) under Windows XP operating system. All results presented in the form of mean ± standard deviation. Data compared using unpaired Student's t-test; P < 0.05 was considered as statistically significant.
Results
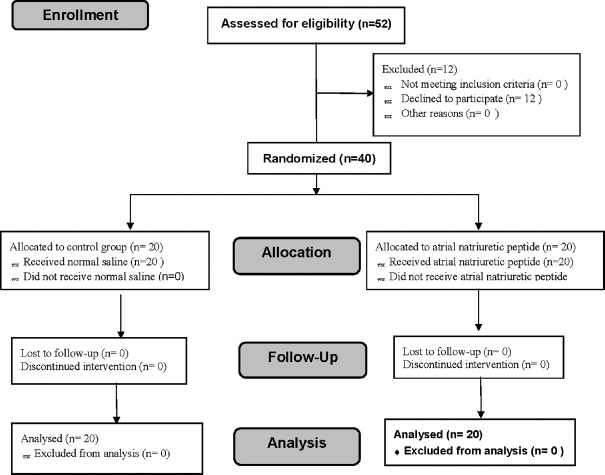
Figure 1 shows the flow diagram for this study, in which 52 patients were assessed for eligibility and 40 adult patients were included in the study. The results of 40 adult patients were analyzed.
Figure 1.
The trial flow diagram, including enrollment, intervention allocation, and analysis
There was no significant difference between both groups as regard patients characteristics and disease severity [Table 1].
Table 1.
Patients characteristics
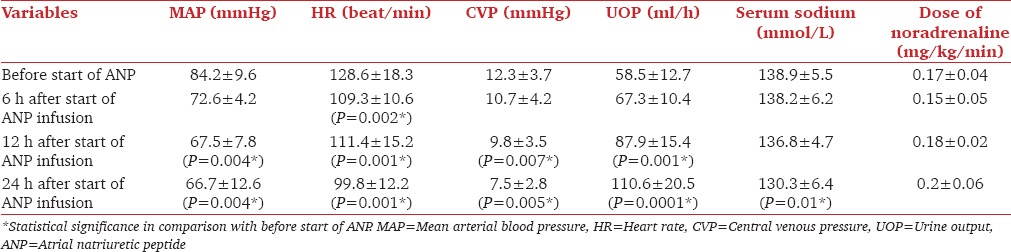
In comparison with Group C, Group A showed a significant decrease (P < 0.05) in serum level of MPO, MDA, PC, and I-FABP, with a significant increase (P < 0.05) in serum level of GPA 6 h, 12 h and 24 h after the start of ANP infusion [Table 2]. There was significant decrease (P < 0.05) in mean duration of noradrenaline infusion, the length of ICU stay and hospital mortality rate in group A in comparison with Group C [Table 3]. In Group A, after 12 h of ANP infusion, there was significant decrease (P < 0.05) in MAP, CVP, HR, and serum sodium with a significant increase (P < 0.05) in UOP [Table 4]. Seven patients had MAP <65 mmHg but respond to volume resuscitation with insignificant change in noradrenaline dose, three patients serum sodium was 125–130 mmol/L.
Table 2.
Primary outcomes variables
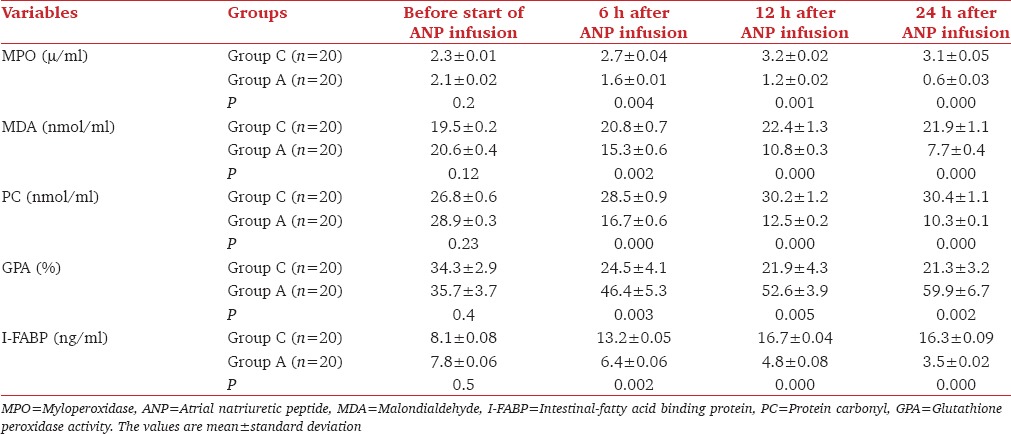
Table 3.
Secondary outcome variables
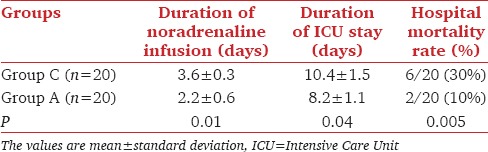
Table 4.
Patients data during atrial natriuretic infusion
Discussion
Tissue perfusion following a period of hypoperfusion represents complex series of events during shock. In this study, before administration of ANP, there was a significant increase in serum level of MDA, PC, MPO, and I-FABP with a significant decrease in serum GP activity. These results could be attributed to the generation of reactive oxygen species which are generated when oxygen is reintroduced to xanthine oxidase enzyme activation during ischemia. These potentially toxic reactive oxygen species can promote cell destruction and bowel necrosis through peroxidation of membrane lipid and protein manifested in this study by a significant increase in serum level of MDA and PC.[14]
The intestine is one of the richest sources in the body of xanthine oxidase enzyme that exhibit a gradient of increasing activity which play a major role in the generation of toxic superoxide radicals. Under normal condition xanthine dehydrogenase form is predominant but during ischemia xanthine dehydrogenase will be oxidized into xanthine oxidase which becomes a major source for toxic superoxide radical production once the tissues are reperfused and oxygen is reintroduced.[15]
Moreover, the reactive oxygen species could stimulate localized infiltration of activated neutrophils which is demonstrated in this work by a significant increase in serum level of MPO which in turn is a potential source for further release of reactive oxygen radicals with enhanced secretion of proinflammatory cytokines by mast cells and macrophages. The inflammatory cytokines leading to the formation of reactive chlorinating species capable of oxidizing protein with a subsequent increase in plasma PC level.[16]
This work demonstrated that IV infusion of ANP for 24 h recorded a significant decrease in serum level of MDA, PC, MPO, and IFAP with a significant increase in serum GP activity in comparison with control group.
ANP and its receptors were demonstrated to be expressed in diverse tissues beside the cardiovascular and renal system. Many previous studies recorded significant antioxidant and anti-inflammatory effects of ANP in many tissues.[7]
The initial signaling events responsible for the protective potential of ANP are still unknown. Some studies reported ANP could attenuate activation of nuclear factor kappa B in macrophages. The reduced binding activity of this redox-sensitive transcription factor was accompanied by diminished m RNA expression of tumor necrotizing factor alpha chemokines and cell adhesion molecules thereby reducing leukocytes infiltration manifested in this study by a significant decrease in the serum level of MPO.[5]
FABP is one of the intracellular proteins that plays important role in transportation and metabolism of long-chain fatty acids which is rapidly released into circulation just after small intestinal mucosal injury.[4]
Alexandra et al.[17] stated that ANP could inhibit the activity of inducible NO synthetase (iNOS) in macrophages via destabilization of iNOS mRNA and this protective effects of ANP are mediated by cyclic guanosine monophosphate. Inhibition of NO formation will inhibit the reactive oxygen species and hence inhibit lipid and protein peroxidation manifested in this work by a significant decrease in serum level of MDA and PC.
In addition, this study shows that ANP induces significant increase in serum GP activity. Chujo et al.[18] stated that ANP could stimulate the antioxidant defense system or may induce antioxidant activity by itself.
Aoyama et al.[19] revealed that administration of ANP infusion at the onset of lung reperfusion attenuates reperfusion injury.
Chujo et al. and Moriyama et al.[20,21] found that ANP attenuates ischemia-reperfusion in induced renal failure through inhibition of inflammatory mediators, MPO and neutrophils activation when given as IV infusion for 2 h after reperfusion injury.
Yamada et al.[22] found the protective effect to ANP in hepatic ischemia-reperfusion.
Conclusion
In cases of septic shock, concomitant administration of ANP with noradrenaline may have a protective effect against intestinal injury through a decrease in the level of intestinal hypoperfusion markers owing to its anti-inflammatory and antioxidant effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank the nurses at the surgical ICU of Tanta University Hospital, Tanta; for their assistance in conducting the study.
References
- 1.de Haan JJ, Lubbers T, Derikx JP, Relja B, Henrich D, Greve JW, et al. Rapid development of intestinal cell damage following severe trauma: A prospective observational cohort study. Crit Care. 2009;13:R86. doi: 10.1186/cc7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–8. doi: 10.1097/00000542-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Jamel MJ, Pereira Lde P, Mello NB, Eleuthério EC, Schanaider A. Blood carbonyl protein measurement as a specific oxidative stress biomarker after intestinal reperfusion in rats. Acta Cir Bras. 2010;25:59–62. doi: 10.1590/s0102-86502010000100014. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto K, Kanda T, Bamba T, Funaoka H, Kosugi S, Yajima K, et al. Serum intestinal fatty acid binding protein in patients with small bowel obstruction. Surg Sci. 2013;4:302–7. [Google Scholar]
- 5.Salehi P, Madsen K, Zhu J, Castillo E, Avila J, Lakey JR, et al. Alleviating ischemia-reperfusion injury in small bowel. Am J Transplant. 2004;4:728–37. doi: 10.1111/j.1600-6143.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu KX, Li YS, Huang WQ, Chen SQ, Wang ZX, Liu JX, et al. Immediate postconditioning during reperfusion attenuates intestinal injury. Intensive Care Med. 2009;35:933–42. doi: 10.1007/s00134-009-1428-1. [DOI] [PubMed] [Google Scholar]
- 7.Shatsky M. Nesiritide (natrecor) for acute decompensated heart failure. Am Fam Physician. 2006;73:687–8. [PubMed] [Google Scholar]
- 8.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: Time for change. Lancet. 2013;381:774–5. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funaoka H, Kanda T, Fujii H. Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal diseases. Rinsho Byori. 2010;58:162–8. [PubMed] [Google Scholar]
- 10.Tüközkan N, Erdamar H, Seven I. Measurement of total malondialdehyde in plasma and tissues by high-performance liquid chromatography and thiobarbituric acid assay. Fırat Tıp Derg. 2006;11:88–92. [Google Scholar]
- 11.Robinson CE, Keshavarzian A, Pasco DS, Frommel TO, Winship DH, Holmes EW. Determination of protein carbonyl groups by immunoblotting. Anal Biochem. 1999;266:48–57. doi: 10.1006/abio.1998.2932. [DOI] [PubMed] [Google Scholar]
- 12.Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic Biol Med. 2000;29:403–9. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 13.Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vollmar B, Menger MD. Intestinal ischemia/reperfusion: Microcirculatory pathology and functional consequences. Langenbecks Arch Surg. 2011;396:13–29. doi: 10.1007/s00423-010-0727-x. [DOI] [PubMed] [Google Scholar]
- 15.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251(4 Pt 1):G567–74. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 16.Guan Y, Worrell RT, Pritts TA, Montrose MH. Intestinal ischemia-reperfusion injury: Reversible and irreversible damage imaged in vivo. Am J Physiol Gastrointest Liver Physiol. 2009;297:G187–96. doi: 10.1152/ajpgi.90595.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandra K, Stefanic K, Tobias G, Angelika M. Vasoprotective action of ANP. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:11–21. doi: 10.2174/1568016052773333. [DOI] [PubMed] [Google Scholar]
- 18.Chujo K, Ueki M, Asaga T, Taie S. Atrial natriuretic peptide attenuates ischemia/reperfusion-induced renal injury by reducing neutrophil activation in rats. Tohoku J Exp Med. 2008;215:257–66. doi: 10.1620/tjem.215.257. [DOI] [PubMed] [Google Scholar]
- 19.Aoyama A, Chen F, Fujinaga T, Sato A, Tsuruyama T, Zhang J, et al. Post-ischemic infusion of atrial natriuretic peptide attenuates warm ischemia-reperfusion injury in rat lung. J Heart Lung Transplant. 2009;28:628–34. doi: 10.1016/j.healun.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Chujo K, Ueno M, Asaga T, Sakamoto H, Shirakami G, Ueki M. Atrial natriuretic peptide enhances recovery from ischemia/reperfusion-induced renal injury in rats. J Biosci Bioeng. 2010;109:526–30. doi: 10.1016/j.jbiosc.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Moriyama T, Kanmura Y, Lindahl SG. Atrial natriuretic peptide attenuation of renal ischemia-reperfusion injury after major surgery. J Surg Res. 2016;201:213–8. doi: 10.1016/j.jss.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 22.Yamada T, Kotake Y, Nagata H, Takeda J. Atrial natriuretic peptide reduces hepatic ischemia-reperfusion injury in rabbits. J Anesth. 2013;27:901–8. doi: 10.1007/s00540-013-1643-3. [DOI] [PubMed] [Google Scholar]