Abstract
Background and Aim:
Preservative free tramadol has been used as an adjuvant to intrathecal bupivacaine. However, the effect of the addition of tramadol on intrathecal isobaric ropivacaine has never been studied.
Material and Methods:
This prospective, randomized, double-blind study was conducted in 50 adult male American Society of Anesthesiologists grade I or II patients, aged 18–60 years, being operated for unilateral femur fractures. An epidural catheter was inserted in L2-L3 interspace and subarachnoid block was given in L3-L4 space. The patients were randomized to receive 0.5 mL normal saline (group R) or 0.5 mL (25 mg) preservative free tramadol (group RT) with 2.5 mL of 0.75% intrathecal ropivacaine. Hemodynamic parameters, sensory level, motor block, sedation and side-effects were recorded. Statistical analysis was done using Student's t-test, Chi-square test, Fischer's exact test and repeated measures ANOVA.
Results:
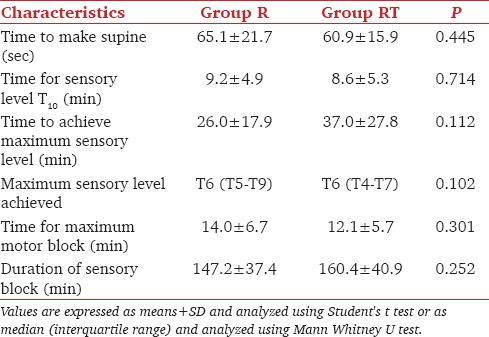
The time of sensory block onset was 9.2 ± 4.9 min and 8.6 ± 5.3 min (P = 0.714) in group R and group RT, respectively. The motor block onset was also comparable in both the groups (P = 0.112). The duration of sensory block was 147.2 ± 37.4 min in group R and 160.4 ± 40.9 min in group RT (P = 0.252). The median maximum block height achieved in both the groups was T6 and the time to achieve the maximum block was also comparable statistically (P = 0.301).
Conclusion:
The addition of intrathecal tramadol 25 mg to the isobaric ropivacaine does not alter the block characteristics produced by intrathecal ropivacaine alone.
Key words: Anesthesia, spinal, tramadol, ropivacaine
Introduction
Tramadol, a centrally acting opioid, has been used as an additive to epidural and intrathecal local anesthetics as it is not associated with as much respiratory depression as other opioids.[1,2] Preservative free tramadol when used as an adjuvant to intrathecal bupivacaine for transurethral resection of the prostate (TURP)[3] did not decrease the postoperative morphine requirements. However, in another study, addition of tramadol to intrathecal bupivacaine significantly prolonged the duration of analgesia.[4] Therefore, the efficacy of intrathecal tramadol as an adjuvant to local anesthetics remains controversial and needs further investigation.
Ropivacaine is safer as compared with bupivacaine as it is less cardiotoxic and neurotoxic.[5,6]
We hypothesized that addition of tramadol would improve the block characteristics of intrathecal isobaric ropivacaine in adult patients undergoing major orthopedic procedures of the femur. The primary outcome measure of the study was duration of sensory block.
Material and Methods
This prospective, randomized, double-blind study was carried out after institutional ethics committee approval and written informed consent of the participating subjects from November 2011 to February 2013. Male patients with femur fractures, American Society of Anesthesiologists (ASA) class I or II and 18–60 years of age were recruited. Patients with a known hypersensitivity to any of the drugs used, bilateral lower limb fractures, contraindications to regional anesthesia, height <150 cm or >180 cm and emergency surgery were excluded.
After attaching standard monitors, intravenous (IV) access was secured with 18G cannula and baseline systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) were recorded. Preloading with lactated Ringer's solution 10 mL/kg was done. An epidural catheter was inserted to provide postoperative analgesia. Epidural space was located in sitting position in L2-L3 vertebral interspace with loss of resistance technique via midline approach and epidural catheter was threaded 4 cm inside the space. No test dose or saline flush was given via the epidural catheter. This was followed by subarachnoid block (SAB) in L3-L4 intervertebral space using 25 G Quincke's needle and study solution was given according to the group allocated after randomization by draw of lots.
Group R patients received 2.5 mL isobaric ropivacaine 0.75% with 0.5 mL normal saline while group RT patients received 2.5 mL isobaric ropivacaine 0.75% with 0.5 mL (25 mg) preservative free tramadol.
Study drug was prepared by an anesthesiologist not involved in block assessment during or after the study. The investigator assessing the block characteristics and other variables was blind to the group allocation. Patients were made supine thereafter. Time of completion of subarachnoid injection was noted (T0). SBP, DBP and HR were recorded at T0 and every 5 min thereafter till 30 min. Sensory and motor block levels were assessed every 2 min for the first 10 min and then every 5 min for next 20 min. Sensory level was assessed subsequently every 15 min till the patient complained of pain or till the block level receded to T12, whichever was earlier. Rescue analgesia was given via the epidural catheter with 0.375% ropivacaine 6 mL. This was considered as the end point of study for sensory blockade. Failure of block was defined as nonachievement of sensory level of at least T10 within 20 min of administration of intrathecal drug. Sensory block was assessed with a pin prick using a sterile 24 G needle in the midline and motor block was assessed according to Bromage score. Sedation was assessed according to Ramsay's sedation score.
Hypotension was defined as 25% decrease in SBP[6] from the baseline and was treated with fluids and vasopressors (mephentermine 6 mg IV) if required. Bradycardia was defined as HR <50 bpm and was treated with 0.6 mg atropine IV.[7] Respiratory depression was defined as respiratory rate <8 bpm. Any episode of nausea, vomiting, shivering or pruritus intra-operatively or postoperatively for the first 24 h was recorded.
Based on the results of a pilot study conducted with 10 patients in each group, a sample size of 21 patients per group was calculated to detect a difference of 30 min of duration of block between the two groups at 5% level of significance and 80% power. Considering the possibility of failure of block in some cases, 25 subjects were included in each group. Statistical analysis was done using SPSS version 17 (SPSS Inc., Chicago, Illinois, USA). Student's t-test was used to compare demographic data and block characteristics. Qualitative data were analyzed using Chi-square test and Fischer's exact test. Repeated measures ANOVA test was used to compare hemodynamic variables.
Results
Patient characteristics [Table 1] and baseline HR, SBP and DBP [Figures 1 and 2] were comparable in the two groups.
Table 1.
Patient characteristics
Figure 1.
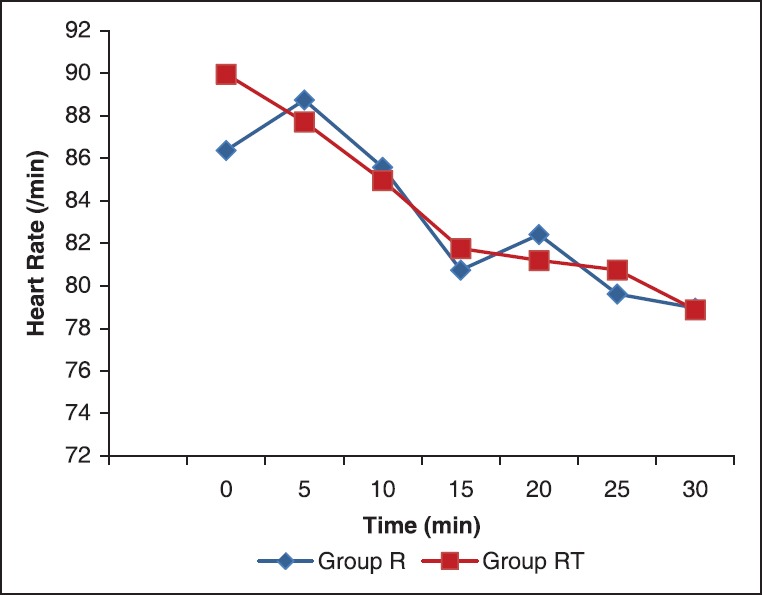
The mean heart rate at different time intervals
Figure 2.
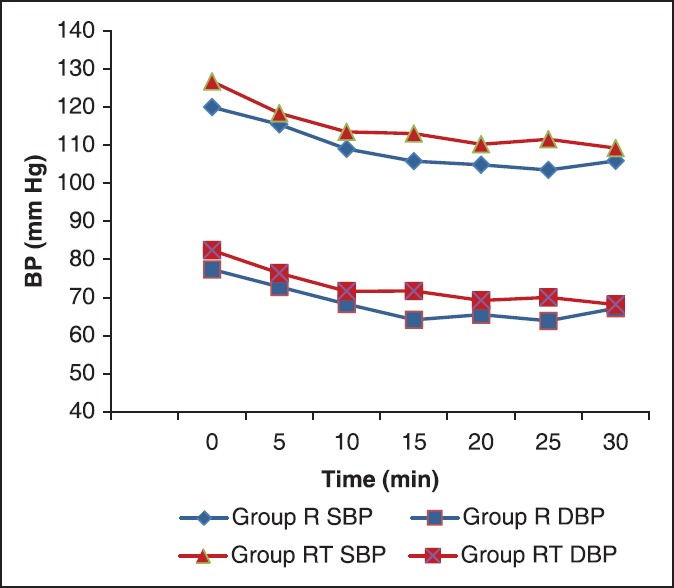
The mean systolic and diastolic blood pressure at different time intervals (SBP = Systolic blood pressure, DBP = Diastolic blood pressure)
There was statistically significant fall in HR in both the groups after administration of intrathecal drug [Figure 1]. However, HR between two groups were statistically similar (P = 0.920). Bradycardia was seen in only one patient in group R and in none of the patients in group RT.
There was statistically significant fall in SBP and DBP within both the groups after administration of intrathecal drug [Figure 2] while SBP and DBP values were comparable between the two groups (P = 0.763 and P = 0.318, respectively). Hypotension occurred in nine patients in group R and 10 patients in group RT.
The sensory and motor block characteristics were statistically comparable in the two groups [Table 2].
Table 2.
Sensory and motor block characteristics
The incidence of intra-operative side-effects was comparable in the two groups. Intra-operatively, nausea was seen in one patient (4%) in group R and in six patients (24%) in group RT (P = 0.098). One patient had an episode of vomiting (4%) in group R as compared to 4 (16%) patients in group RT (P = 0.349). Shivering was observed in 3 (12%) patients in each group. Sedation scores were comparable in both the groups (P = 0.985). Respiratory depression was not observed in any of the patients in either of the two groups.
Discussion
Opioids have been used traditionally as adjuvants to local anesthetics in central neuraxial blocks to provide better anesthesia, improved quality of block without prolongation of motor blockade and to reduce the dose of local anesthetic agent being used. Lipophilic opioids like morphine, fentanyl and sufentanil have been used effectively for this purpose.[7,8] However, they are associated with the development of complications such as respiratory depression, nausea, vomiting, pruritus and delayed voiding.[8] Tramadol is a safer opioid which is known to have less respiratory depression.[2] Various doses of preservative free tramadol have been used alone or as an additive to bupivacaine in the subarachnoid space and have been found to be safe.[3,4]
In the present study, a dose of 2.5 mL intrathecal ropivacaine (18.75 mg) was used for SAB as a similar dose has been previously shown to produce effective and safe anesthesia for lower limb surgeries like total hip arthroplasty.[9]
The addition of 25 mg tramadol did not result in prolongation of the duration of action of intrathecal ropivacaine in the present study. There are contradictory reports in the literature on the efficacy of intrathecal tramadol.[3,4] When added to intrathecal bupivacaine, it prolonged the duration of analgesia in women undergoing gynecological[4] or obstetric procedures[10] but did not affect the postoperative morphine requirements or the time to first analgesic requirement in patients undergoing TURP.[3] Various theories have been proposed to explain the efficacy as well as the inefficacy of tramadol as an adjuvant in subarachnoid block. The failure to prolong the duration of analgesia may be because of its lesser affinity for µ receptors,[11] high lipophilicity leading to rapid diffusion out of subarachnoid space[3] and anti-analgesic effects at low doses.[12]
Mechanism of action of tramadol is inhibition of re-uptake of both central and peripheral monoaminergic neurotransmitters (5-hydroxytryptamine and noradrenaline).[13] It also has a local anesthetic like effect, that is blocking of action potential following subcutaneous administration[14] and at the peripheral nerves.[15] However, another study, where tramadol was used as an adjunct to psoas compartment block with levobupivacaine 0.5%, failed to prove a clinical local anesthetic or peripheral analgesic effect.[16] Recently, Brummet and Williams have even recommended against the use of perineural tramadol.[17]
None of the patients in the present study developed respiratory depression and the incidence of sedation was also comparable in the two groups. However, the incidence of nausea and vomiting was higher in the tramadol group than the control group (24% vs. 4% for nausea and 16% vs. 4% for vomiting, respectively). This difference, although clinically relevant, could not achieve statistical significance. This could be because our sample size was not powered enough to study these side-effects. Scott et al have also pointed out that there may be a higher incidence of side-effects such as nausea and vomiting with the use of tramadol.[11]
To conclude, the addition of tramadol 25 mg to intrathecal 18.75 mg isobaric ropivacaine did not improve the sensory or motor block characteristics. Further trials with increased dose of intrathecal tramadol cannot be recommended in view of clinically significant higher incidence of nausea and vomiting with 25 mg intrathecal tramadol.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hurley RW, Wu CL. Acute Postoperative pain. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Miller's Anesthesia. 7th ed. USA: Churchill Livingston Elsevier; 2010. pp. 2757–82. [Google Scholar]
- 2.Mohta M, Kumari N, Tyagi A, Sethi AK, Agarwal D, Singh M. Tramadol for prevention of postanaesthetic shivering: A randomised double-blind comparison with pethidine. Anaesthesia. 2009;64:141–6. doi: 10.1111/j.1365-2044.2008.05711.x. [DOI] [PubMed] [Google Scholar]
- 3.Alhashemi JA, Kaki AM. Effect of intrathecal tramadol administration on postoperative pain after transurethral resection of prostate. Br J Anaesth. 2003;91:536–40. doi: 10.1093/bja/aeg213. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty S, Chakrabarti J, Bhattacharya D. Intrathecal tramadol added to bupivacaine as spinal anesthetic increases analgesic effect of the spinal blockade after major gynecological surgeries. Indian J Pharmacol. 2008;40:180–2. doi: 10.4103/0253-7613.43166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berde CB, Strichartz GR. Local Anesthetics. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL, editors. Miller's Anesthesia. 7th ed. USA: Churchill Livingston Elsevier; 2010. pp. 913–40. [Google Scholar]
- 6.Boztug N, Bigat Z, Karsli B, Saykal N, Ertok E. Comparison of ropivacaine and bupivacaine for intrathecal anesthesia during outpatient arthroscopic surgery. J Clin Anesth. 2006;18:521–5. doi: 10.1016/j.jclinane.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Hamber EA, Viscomi CM. Intrathecal lipophilic opioids as adjuncts to surgical spinal anesthesia. Reg Anesth Pain Med. 1999;24:255–63. doi: 10.1016/s1098-7339(99)90139-6. [DOI] [PubMed] [Google Scholar]
- 8.Chinachoti T, Nilrat P, Samarnpiboonphol P. Nausea, vomiting and pruritus induced by intrathecal morphine. J Med Assoc Thai. 2013;96:589–94. [PubMed] [Google Scholar]
- 9.McNamee DA, Parks L, McClelland AM, Scott S, Milligan KR, Ahlén K, et al. Intrathecal ropivacaine for total hip arthroplasty: Double-blind comparative study with isobaric 7.5 mg ml(-1) and 10 mg ml(-1) solutions. Br J Anaesth. 2001;87:743–7. doi: 10.1093/bja/87.5.743. [DOI] [PubMed] [Google Scholar]
- 10.Subedi A1, Biswas BK, Tripathi M, Bhattarai BK, Pokharel K. Analgesic effects of intrathecal tramadol in patients undergoing caesarean section: a randomised, double-blind study. Int J Obstet Anesth. 2013;22:316–21. doi: 10.1016/j.ijoa.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Scott LJ, Perry CM. Tramadol: A review of its use in perioperative pain. Drugs. 2000;60:139–76. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Wilder-Smith CH, Wilder-Smith OH, Farschtschian M, Naji P. Preoperative adjuvant epidural tramadol: the effect of different doses on postoperative analgesia and pain processing. Acta Anaesthesiol Scand. 1998;42:299–305. doi: 10.1111/j.1399-6576.1998.tb04920.x. [DOI] [PubMed] [Google Scholar]
- 13.Shipton EA. Tramadol — present and future. Anaesth Intensive Care. 2000;28:363–74. doi: 10.1177/0310057X0002800403. [DOI] [PubMed] [Google Scholar]
- 14.Behdad S, Sekhavat L, Ayatollahi V, Meshkat F, Mortazavi A. Comparison of postoperative analgesic effect of tramadol and bupivacaine subcutaneous infiltration in patients undergoing cesarean section. Acta Clin Croat. 2013;52:93–7. [PubMed] [Google Scholar]
- 15.Sousa AM, Ashmawi HA, Costa LS, Posso IP, Slullitel A. Percutaneous sciatic nerve block with tramadol induces analgesia and motor blockade in two animal pain models. Braz J Med Biol Res. 2012;45:147–52. doi: 10.1590/S0100-879X2011007500164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannion S, O'Callaghan S, Murphy DB, Shorten GD. Tramadol as adjunct to psoas compartment block with levobupivacaine 0.5%: A randomized double-blinded study. Br J Anaesth. 2005;94:352–6. doi: 10.1093/bja/aei057. [DOI] [PubMed] [Google Scholar]
- 17.Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin. 2011;49:104–16. doi: 10.1097/AIA.0b013e31820e4a49. [DOI] [PMC free article] [PubMed] [Google Scholar]


