Abstract
Pulmonary vascular medial hypertrophy caused by excessive pulmonary artery smooth muscle cell (PASMC) proliferation is a major cause for the elevated pulmonary vascular resistance in patients with idiopathic pulmonary arterial hypertension (IPAH). Increased Ca2+ influx is an important stimulus for PASMC proliferation. Transient receptor potential (TRP) channel genes encode Ca2+ channels that are responsible for Ca2+ entry during cell proliferation. Normal human PASMC expressed multiple canonical TRP (TRPC) isoforms; TRPC6 was highly expressed and TRPC3 was minimally expressed. The protein expression of TRPC6 in normal PASMC closely correlated with the expression of Ki67, suggesting that TRPC6 expression is involved in the transition of PASMC from quiescent phase to mitosis. In lung tissues and PASMC from IPAH patients, the mRNA and protein expression of TRPC3 and -6 were much higher than in those from normotensive or secondary pulmonary hypertension patients. Inhibition of TRPC6 expression with TRPC6 small interfering RNA markedly attenuated IPAH-PASMC proliferation. These results demonstrate that expression of TRPC channels correlates with the progression of the cell cycle in PASMC. TRPC channel overexpression may be partially responsible for the increased PASMC proliferation and pulmonary vascular medial hypertrophy in IPAH patients.
Idiopathic pulmonary arterial hypertension (IPAH) is a fatal disease that causes right heart failure and death. The elevated pulmonary vascular resistance (PVR) and arterial pressure in IPAH patients result mainly from pulmonary vasoconstriction, vascular remodeling, and in situ thrombosis (1). A central aspect of pulmonary vascular remodeling is medial hypertrophy caused by sustained pulmonary vasoconstriction (2–4), excessive pulmonary artery smooth muscle cell (PASMC) proliferation (5), and inhibited PASMC apoptosis (6, 7), resulting in a narrowed vascular lumen and increased PVR. Although its etiology remains unclear, elevated levels of circulating mitogens, dysfunction or down-regulation of receptors and ion channels, upregulation of transporters, and heightened activity of elastases and glycoproteins have been implicated in IPAH (5, 6, 8–20).
A rise in cytoplasmic Ca2+ concentration ([Ca2+]cyt) can activate signal transduction proteins and transcription factors essential for cell proliferation (21–25). IPAH-PASMC show elevated resting [Ca2+]cyt and enhanced [Ca2+]cyt after mitogenic stimulation (16, 22), whereas removal or chelation of extracellular Ca2+ significantly inhibit serum and growth factor-induced PASMC growth (26, 27). These observations indicate that enhanced Ca2+ influx and elevated [Ca2+]cyt are requisites for normal PASMC growth, whereas an excessive increase in Ca2+ entry and the subsequent sustained increase in [Ca2+]cyt may be critical stimuli for IPAH-PASMC overgrowth. Previously, we showed that increased Ca2+ influx during PASMC proliferation was due largely to increased capacitative Ca2+ entry (CCE) (26–27). CCE is essential for maintaining a high level of [Ca2+]cyt and for refilling intracellular Ca2+ stores [i.e., sarcoplasmic reticulum (SR)] (28, 29).
Transient receptor potential (TRP) channel genes may encode subunits that form receptor-(ROC) and store-(SOC) operated Ca2+ channels in many cell types, including PASMC and pulmonary artery endothelial cells (PAEC) (28, 30–34). Ca2+ entry through ROC and SOC increases [Ca2+]cyt, allowing for phosphorylation of signal transduction proteins and transcription factors (23, 24, 35–38), that are essential for the progression of the cell cycle (21). High levels of [Ca2+]cyt and sufficient levels of Ca2+ in the SR are required for vascular smooth muscle cell proliferation (22, 25, 39). Because they regulate SR and cytoplasmic Ca2+, CCE and SOC may play significant roles in regulating cell proliferation (28, 29). This study tested the hypothesis that canonical TRP (TRPC) channels are involved in human PASMC proliferation and that their overexpression is responsible for PASMC hyperplasia in IPAH.
Materials and Methods
Subjects. The clinical and hemodynamic characteristics of the lung transplant tissue donors are shown in Table 1. The diagnosis of IPAH was established clinically in three patients on the basis of the criteria used in the National Institutes of Health Registry on IPAH, and confirmed histopathologically. Eight subjects had secondary pulmonary hypertension (SPH) resulting from chronic thromboembolic pulmonary hypertension, lymphangioleiomyomatosis, chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis. Three subjects had COPD without pulmonary hypertension (Table 1). Use of human pulmonary tissues for these experiments was approved by an Institutional Review Board at the University of California at San Diego.
Table 1. Demographic and hemodynamic data of the patients.
| Age | Sex/race | Diagnosis | mPAP, mmHg | PAOP, mmHg | CO, liter/min | PVR*, mmHg·liter-1·min-1 |
|---|---|---|---|---|---|---|
| 57 | F/AA | IPAH | 50 | 14 | 4.2 | 8.6 |
| 57 | F/C | IPAH | 66 | 7 | 4.9 | 12.0 |
| 32 | M/C | IPAH | 53 | 11 | 2.9 | 14.5 |
| 49 ± 8 | 56.3 ± 4.9 | 10.7 ± 2.0 | 4.0 ± 0.6 | 11.7 ± 1.7 | ||
| 62 | F/C | CTEPH | 33 | 8 | 3.5 | 7.1 |
| 45 | F/C | CTEPH | 43 | 4 | 6.1 | 6.4 |
| 62 | F/C | CTEPH | 36 | 7 | 3.5 | 8.3 |
| 44 | F/C | CTEPH | 44 | 8 | 3.9 | 9.2 |
| 56 | M/C | COPD | 43 | NA | 4.6 | - |
| 60 | M/C | COPD | 33 | 15 | NA | NA |
| 69 | M/C | IPF | 26 | NA | 6.5 | - |
| 34 | F/C | LAM | 37 | 14 | 5.5 | 4.2 |
| 54 ± 4 | 36.9 ± 2.2 | 9.3 ± 1.7 | 4.8 ± 0.5 | 7.0 ± 0.9 | ||
| 48 | M/C | COPD | 23 | 18 | 4.6 | 1.1 |
| 52 | F/C | COPD | 25 | 16 | NA | NA |
| 56 | F/AA | COPD | 16 | 6 | 3.6 | 2.8 |
| 52 ± 2 | 21.3 ± 2.7 | 13.3 ± 3.7 | 4.1 ± 0.5 | 1.9 ± 0.8 |
mPAP, mean pulmonary arterial pressure; PAOP, pulmonary arterial occlusion pressure (wedge pressure); CO, cardiac output; F, female; M, male; AA, African-American; C, Caucasian; CTEPH, chronic thromboembolic pulmonary hypertension; IPF, idiopathic pulmonary fibrosis; LAM, lymphangioleiomyomatosis; COPD, chronic obstructive pulmonary disease; NA, data not available (not measured or cannot be estimated) for these patients. 1 mm Hg = 133 Pa.
Calculated PVR based on the measured mPAP, PAOP, and CO.
Cell Preparation and Culture. PASMC from transplant patients were isolated and prepared for culture as described (16). Cells were cultured in 5% CO2 in air at 37°C in smooth muscle growth medium (SMGM, which contains 5% FBS and growth factors). Human PASMC from normal subjects (NPH) were purchased from Cambrex, cultured at 37°C in SMGM, and used at the fourth to sixth passage. There were no significant changes in cell morphology, TRPC expression, or proliferation rate at passages 4–6. The purity of PASMC in primary cultures was confirmed by the specific mAb raised against smooth muscle α-actin (16).
RNA Isolation and RT-PCR. Total RNA was isolated from lung tissues or PASMC by using TRIzol reagent (Invitrogen). RNA was used to synthesize first-strand cDNA by using the Super-ScriptJ First-Strand Synthesis System (Invitrogen). PCR was performed by a GeneAmp PCR System (Perkin–Elmer) using the Platinum PCR Supermix (Invitrogen). The sense and antisense primers were specifically designed from the coding regions of TRPC genes and GAPDH (refs. 26 and 27; see Table 2, which is published as supporting information on the PNAS web site). The net intensity values of the cDNA bands from electrophoretically separated PCR products were measured and normalized to the net intensity values of the GAPDH mRNA signals; ratios are expressed in arbitrary units.
Cell Cycle Phase Determination and Flow Cytometry. Immunofluorescent double staining of incorporated BrdUrd and cell cycle phase distribution were used to detect cell proliferation (26, 27). Growth-arrested PASMC were incubated at 37°C in SMGM for 24 h, with BrdUrd added to PASMC for 2 h before cells were trypsinized, fixed, and permeabilized. To detect incorporated BrdUrd, cells were treated with DNase, then incubated with FITC-conjugated BrdUrd Ab (BD Pharmingen) or FITC-conjugated mouse IgG. After being washed, PASMC were cultured with 7-amino-actinomycin and resuspended, and proliferation was analyzed by flow cytometry. Permeabilized PASMC also were stained with FITC-conjugated Ki67 mAb, phycoerythrin (PE)-conjugated BrdUrd mAb and rabbit TRPC6 polyclonal Ab (Alomone Labs, Jerusalem). After washing, the cells were resuspended and stained with Cy5-conjugated secondary Ab. Control cells were labeled with FITC- and PE-conjugated mouse and Cy5-conjugated secondary rabbit IgG.
Western Blot Analysis. Lysed lung tissues or PASMC were sonicated and centrifuged to pellet cell debris. Immunoreactive proteins from the whole-cell protein extracts were separated and detected as described (27). Polyclonal Abs against TRPC3, TRPC6 (Alomone Labs), and α-actin (Sigma) were used as primary Abs. Horseradish peroxidase-conjugated anti-rabbit or -mouse IgG was used as secondary Ab. The bound Ab was detected by using a chemiluminescence detection system (Amersham Pharmacia). Band intensity was normalized to α-actin controls and is expressed in arbitrary units.
Immunofluorescence Staining. PASMC were fixed in 4% paraformaldehyde in PBS, permeabilized, and blocked in PBS containing saponin, fish gelatin, and BSA. Cells were exposed to unlabeled TRPC6 rabbit Ab and FITC-conjugated Ki67 mAb. After washing, rhodamine- or FITC-conjugated goat anti-rabbit IgG was used to detect TRPC6. The coverslips were visualized by using a Zeiss microscope. To differentiate nonspecific binding of Abs, isotype-matched control mouse and goat Abs were applied to cells and incubated under the same conditions. The staining intensity of the TRPC6 Ab was quantified by using quantity one imaging analysis software (Bio-Rad).
Design and Transfection of Small Interfering RNA (siRNA) for Human TRPC6. TRPC6 siRNA and scrambled control sequences were prepared by the SilencerJ siRNA Construction kit (Ambion). Four 19-nt sequences were selected from the ORF sequence of human TRPC6 (GenBank accession no. AJ271066). A scrambled control oligonucleotide was also generated that bore no significant homology to any mammalian gene sequence. Suppression of TRPC6 gene expression by the siRNA oligonucleotides was first evaluated in COS-7 cells. Two (siRNA-1 and -2) of the siRNA sequences suppressed TRPC6 protein and mRNA levels, with siRNA-2 being most effective. Based on these results, we constructed DNA-based vectors (Ambion) that expressed TRPC6 siRNA-2 (AAGGTCTTTATGCAATTGCTG). A scrambled siRNA vector served as a nonsilencing control.
Cell DNA Synthesis. [3H]Thymidine incorporation was used to evaluate DNA synthesis and cell proliferation. PASMC were growth-arrested by exposure to smooth muscle basal medium (SMBM, no serum and growth factors) for 24 h. Cells were then incubated in SMGM for 48 h, with 1 μCi of [3H]thymidine added to the cells for the last 16 h (1 Ci = 37 GBq). Incorporation of radioactivity into trichloroacetic acid-insoluble material was measured by a liquid scintillation counter.
Statistical Analysis. Data are expressed as means ± SEM. Statistical analyses were performed by using unpaired Student's t test or one-way ANOVA and Fisher's protected least significant difference tests where appropriate. Differences were considered to be significant when P < 0.05.
Results
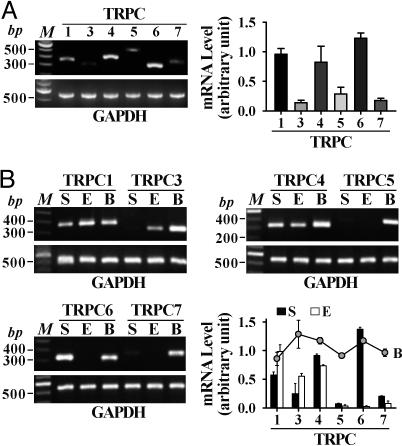
mRNA Expression of TRPC Isoforms. All TRPC mRNA transcripts were detected in NPH lung tissues; the mRNA expression levels of TRPC1, -4, and -6 exceeded those of TRPC3, -5, and -7 (Fig. 1A). NPH-PASMC highly expressed TRPC1, -4, and -6, whereas PAEC highly expressed TRPC1, -3, and -4 (Fig. 1B). PASMC also expressed low levels of TRPC5 and -7. These data suggest that, although TRPC1 and -4 are ubiquitously expressed, TRPC3 and -6 appear to be PAEC- and PASMC-dominant TRPC isoforms, respectively, in normal subjects.
Fig. 1.
The mRNA expression of TRPC channels in NPH lung tissue and PASMC. (A) Representative and summarized (n = 4) mRNA products for TRPC genes and GAPDH in NPH human lung. (B) Representative and summarized (n = 3) data showing TRPC gene mRNA products in human brain tissue (B), PASMC (S), and PAEC (E). M, 100-bp DNA ladder.
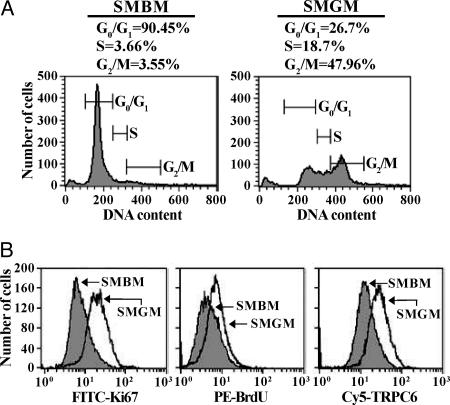
Correlation of Cell Proliferation with TRPC6 Expression. Sixty-seven percent of the proliferating PASMC were in the S and G2/M phases and 27% were in the G0/G1 phases. In growth-arrested PASMC, 7% of the cells were in the S and G2/M phases and 90% of the cells were in the G0/G1 phases (Fig. 2A). Expression of Ki67 (Fig. 2B), a nuclear protein expressed during the S and G2/M phases (40), and BrdUrd incorporation (Fig. 2B), which occurs in the S phase of the cell cycle, were both significantly higher in proliferating PASMC. TRPC6 protein expression also was enhanced in proliferating PASMC (Fig. 2B).
Fig. 2.
Cell cycle analysis of growth-arrested and proliferating PASMC and correlation of TRPC6 expression with PASMC proliferation. (A) Flow cytometry graphs (representative of three trials) of cell cycle analysis for growth-arrested (SMBM) and proliferating (SMGM) PASMC. Number of cells is plotted as a function of DNA content. (B) Graphs showing cell number as a function of Ki67 expression, BrdUrd incorporation, or TRPC6 expression in SMBM- and SMGM-treated PASMC.
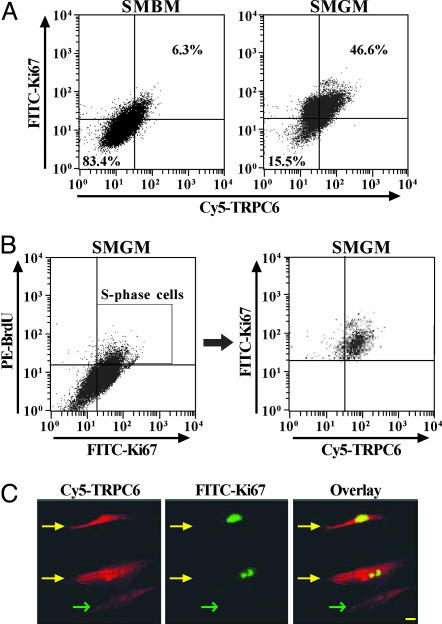
To examine whether TRPC6 expression correlates with PASMC proliferation, we compared the distribution of TRPC6 with Ki67 distribution and BrdUrd incorporation in growth-arrested and proliferating PASMC. Only 6% of the growth-arrested cells were stained with both Ki67 and TRPC6, compared to 47% of the proliferating PASMC (Fig. 3A). Furthermore, a majority of S-phase cells (double-labeled by BrdUrd and Ki67) also expressed TRPC6 (Fig. 3B), indicating that its expression is elevated during DNA synthesis. Immunocytochemistry experiments also showed a link between TRPC6 and Ki67 expression. The Ki67-positive cells had higher level of TRPC6 than the Ki67-negative cells (Fig. 3C). These results indicate that the protein expression of TRPC6 in normal PASMC positively correlates with the incorporation and expression of markers of DNA synthesis and mitosis (40, 41). The data also directed us to speculate that TRPC6 expression is important for the transition from quiescence to DNA synthesis in proliferating PASMC.
Fig. 3.
Correlation of Ki67 expression and BrdUrd incorporation with TRPC6 expression. (A) Dot-plot analysis (representative of three trials) of TRPC6 expression vs. Ki67 expression in growth-arrested and proliferating PASMC. Upper right quadrants represent cells in the S phase. (B) Three-color dot-plot analysis of TRPC6 distribution vs. BrdUrd incorporation and Ki67 expression in proliferating PASMC (representative of three trials). The box in Left indicates the S-phase cells that are stained by both Ki67 and BrdUrd. (C) Fluorescent images (×40) showing PASMC stained by Ki67 and TRPC6. An overlay of the two fluorescent images identifies PASMC expressing both Ki67 and TRPC6. Yellow and green arrows indicate Ki67-positive and -negative PASMC, respectively. (Scale bar, 20 μm.)
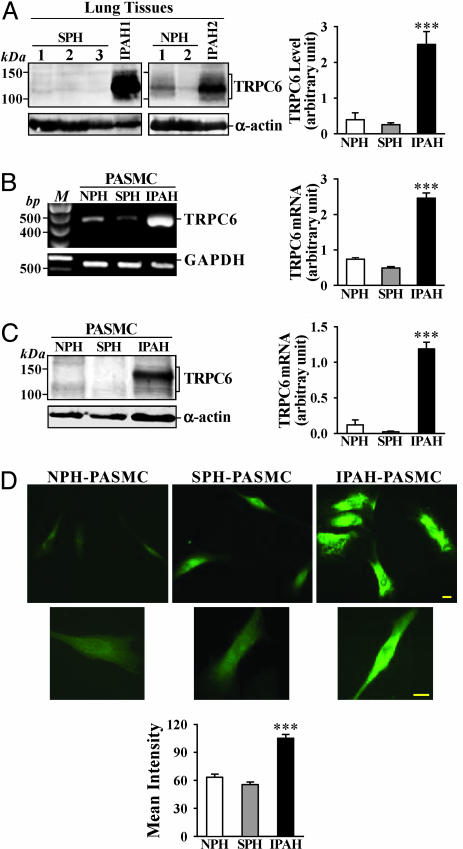
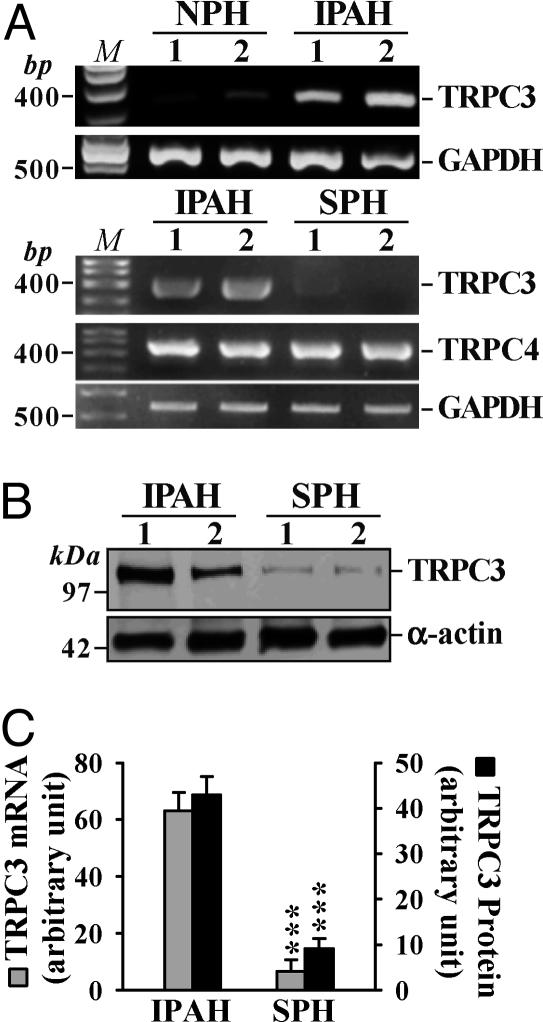
Up-Regulated TRPC Expression in IPAH Lung Tissues and PASMC. To test whether up-regulation of TRPC6 is involved in PASMC hyperplasia in IPAH patients, we compared mRNA and protein levels of TRPC6 in lung tissues and PASMC from NPH, IPAH, and SPH patients (Table 1). In lung tissues from IPAH patients, the protein level of TRPC6 was significantly higher than in NPH and SPH tissues (Fig. 4A). Because the vasculature forms a significant portion of the explanted lung tissues we use experimentally, the high protein level of TRPC6 may be due to up-regulated TRPC6 expression in PASMC. Indeed, the mRNA (Fig. 4B) and protein (Fig. 4C) levels of TRPC6 in IPAH-PASMC were much higher than those in NPH and SPH PASMC. Immunocytochemistry experiments confirmed that TRPC6 expression was significantly enhanced in IPAH-PASMC (Fig. 4D).
Fig. 4.
TRPC6 mRNA and protein expression is increased in pulmonary tissues from IPAH patients. (A) Representative and summarized (NPH, n = 3; SPH, n = 8; IPAH, n = 3; normalized to α-actin) TRPC6 protein expression in SPH, NPH, and IPAH lung tissues and their α-actin controls. (B) Representative and summarized (n = 3 for each) data showing TRPC6 mRNA products in isolated NPH-, SPH-, or IPAH-PASMC. M, 100-bp DNA ladder. (C) Representative and summarized (n = 5 for each) TRPC6 protein expression in isolated PASMC from NPH, SPH, and IPAH patients. (D) Representative (Top, ×40; Middle, ×100) and summarized immunofluorescence staining of FITC-conjugated TRPC6 (Bottom) in isolated NPH-(n = 11), SPH (n = 21), and IPAH-(n = 36) PASMC. (Scale bars, 20 μm.) ***, P < 0.001 vs. NPH and/or SPH.
TRPC6 shares significant homology with TRPC3 (42, 43). Furthermore, TRPC3 can form homomeric channels, or can combine with TRPC6 to form heterotetrameric channels activated by second messengers and store depletion (29, 44–48). TRPC3 was minimally expressed in NPH-PASMC (Fig. 1B); TRPC3 mRNA and protein expressions were considerably enhanced in PASMC from IPAH patients compared to SPH-PASMC (Fig. 5). This finding suggests that up-regulated TRPC6 and -3 may increase the numbers of homo- and heterotetrameric channels that can be activated by mitogens and/or store depletion in IPAH-PASMC.
Fig. 5.
Up-regulated TRPC3 expression in IPAH-PASMC. (A) TRPC3 mRNA expression in PASMC from two NPH patients and two IPAH patients. TRPC3 and -4 mRNA expression in PASMC from two IPAH patients and two SPH patients. M, 100-bp DNA ladder. (B) TRPC3 protein expression in the same IPAH and SPH samples as shown in A. (C) Summarized bar graphs (n = 3 for each) depicting normalized TRPC3 mRNA and protein expression. ***, P < 0.001 vs. IPAH.
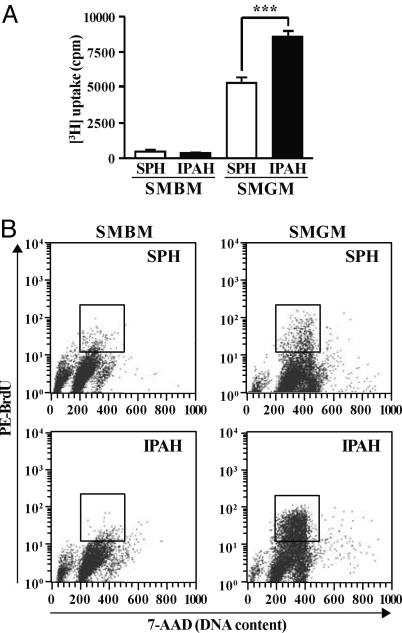
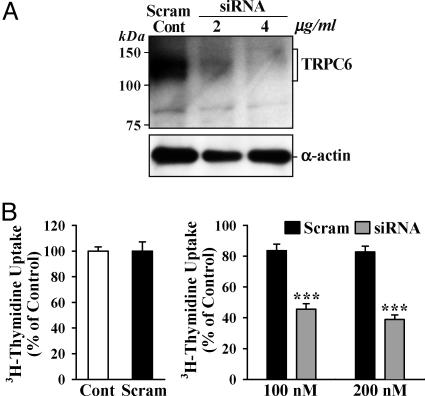
Inhibition of TRPC6 Expression Attenuates IPAH-PASMC Proliferation. [3H]Thymidine incorporation (Fig. 6A) and BrdUrd uptake (Fig. 6B) were much greater in proliferating IPAH-PASMC than in SPH-PASMC, but comparable in growth-arrested cells. This finding indicates that the proliferative response to serum and growth factors is significantly enhanced in IPAH-PASMC. If up-regulated TRPC expression is responsible for IPAH-PASMC overgrowth, inhibition of TRPC expression should attenuate proliferation. Transfection of TRPC6 siRNA into IPAH-PASMC significantly decreased TRPC6 protein expression (Fig. 7A) and [3H]thymidine incorporation in proliferating IPAH-PASMC (Fig. 7B); both were unaffected in IPAH-PASMC transfected with a scrambled oligonucleotide. These results suggest that up-regulated TRPC6 plays an important role in the overgrowth of IPAH-PASMC, and inhibition of TRPC6 can attenuate PASMC hyperplasia in IPAH patients.
Fig. 6.
Increased proliferation of IPAH-PASMC. (A) [3H]Thymidine incorporation in SPH (n = 8) and IPAH (n = 8) PASMC before (12 h, SMBM) and 24 h after the addition of 5% FBS and growth factors (SMGM). ***, P < 0.001 vs. SMGM-SPH. (B) Bivariate distribution of BrdUrd vs. DNA content for SMBM- or SMGM-treated SPH- and IPAH-PASMC. Boxes indicate S phase PASMC. Data are representative of three experiments.
Fig. 7.
TRPC6 siRNA attenuates TRPC6 expression and proliferation in IPAH cells. (A) Effect of two concentrations of TRPC6-siRNA-2 expression vector, and its scrambled control (4 μg/ml), on TRPC6 protein expression. (B) (Left) Normalized [3H]thymidine uptake plotted for cells untreated (Cont) or treated with the scrambled siRNA duplex (Scram, 200 nM). (Right) Cell growth in cells transfected with two concentrations of either the scrambled (n = 8) or TRPC6 (n = 8) siRNA duplexes. Data are normalized to the basal [3H]thymidine uptake in the absence of siRNA (Cont). ***, P < 0.001 vs. Scram.
Discussion
PASMC proliferation is regulated by intracellular Ca2+. Removal of extracellular free Ca2+ or blockade of Ca2+ channels inhibits PASMC proliferation (22, 26, 27, 49). Depletion of SR Ca2+ attenuates smooth muscle cell proliferation (25) and promotes apoptosis (50). In contrast, elevated [Ca2+]cyt may induce PASMC proliferation by initiating and promoting the cell cycle, and by triggering or promoting gene transcription via transcription factor phosphorylation (21, 25, 37).
An important pathway in regulating [Ca2+]cyt in PASMC is Ca2+ entry via ROC and SOC, potentially encoded by TRP genes (22, 26, 28). Cells stimulated by mitogens or growth factors produce diacylglycerol and inositol 1,4,5-trisphosphate (IP3), two important second messengers that increase [Ca2+]cyt by inducing Ca2+ release from the SR or by promoting sarcolemmal Ca2+ influx through ROC and SOC (42, 43). IP3-mediated Ca2+ mobilization transiently depletes SR Ca2+ and triggers CCE via opened SOC (28, 29, 52). Thus, SOC-mediated CCE and Ca2+ entry through ROC concurrently mediate the increases in cytoplasmic and nuclear [Ca2+] required for propagation of the cell cycle and for synthesizing proteins and lipids necessary for mitosis (21, 23, 24, 39). CCE is also responsible for replenishing Ca2+ within the SR, where peptides are sorted to functional proteins by cation (e.g., Ca2+ and Mg2+)-dependent enzymes.
The molecular composition of native ROC and SOC has not been completely elucidated in vascular smooth muscle cells. Functional TRPC channels exist in native cells as homo- or heterotetramers composed of two or more TRPC subunits (29, 42, 43). Studies have shown that overexpression of TRPC genes in mammalian cells leads to the formation of Ca2+ permeable cation channels activated by both ligand–receptor interaction and store depletion (29, 31, 33, 42–47, 51–55).
As in this study, normal human PASMC express relatively high levels of TRPC1, -4, and -6, but express extremely low levels of TRPC3 (22, 56). To understand the role of TRPC6 in normal PASMC proliferation, we compared the protein expression level of TRPC6 in growth-arrested and proliferating PASMC. TRPC6 mRNA and protein expression was significantly increased in proliferating PASMC. In this study, we extended the analysis of the correlation between TRPC6 expression and PASMC proliferation by examining TRPC6 protein levels at the different cell cycle phases. We observed a positive correlation between TRPC6 expression and the S and G2/M phases of the cell cycle, indicating that TRPC6 is involved in the progression of human PASMC proliferation.
PASMC hyperplasia is an important contributor to the pulmonary medial hypertrophy in IPAH patients. By comparing the mRNA and protein levels of TRPC channels in lung tissues and PASMC from NPH, SPH, and IPAH patients, we observed that the expression levels of both TRPC3 and -6 were significantly greater in IPAH patients. These results suggest that up-regulated expression of TRPC3, which is minimally expressed in normal PASMC, and TRPC6 contribute to the enhanced PASMC growth in IPAH patients. Furthermore, inhibition of endogenous TRPC6 expression by using siRNA markedly attenuated IPAH-PASMC proliferation, further suggesting that overexpression of TRPC6 in PASMC may play an important role in the development of pulmonary medial hypertrophy. It is unclear why up-regulation of TRPC3 and TRPC6 occur in IPAH-PASMC but not in SPH-PASMC, although both IPAH and SPH patients have excessive pulmonary medial hypertrophy. It is possible that medial hypertrophy or enhanced PASMC proliferation result from different mechanisms SPH and IPAH patients.
In unpublished observations, we also noted that TRPC1 expression, although increased in proliferating PASMC, was only minimally enhanced in IPAH-PASMC in comparison to NPH- and SPH-PASMC. Similarly, TRPC4 levels remained relatively unchanged in IPAH-PASMC relative to the levels in SPH-PASMC (Fig. 5A). However, we cannot discount the possibility that these subunits are components of the native SOC in IPAH-PASMC.
Whether TRPC3 and TRPC6 form ROC or SOC is still debatable. Data from different cell types and studies involving overexpression of specific TRP proteins have yielded conflicting results when attempting to correlate specific TRP isoforms with SOC properties (29). This could be due to the heterotetrameric structure of native TRP channels. In PASMC, inhibition of TRPC1 or TRPC6 with antisense oligonucleotides specifically targeting TRPC1 or TRPC6 genes both attenuated CCE-mediated increases in [Ca2+]cyt (22, 26). In rat aorta, antisense inhibition of TRPC6 also markedly inhibited the α1-adrenoreceptor-mediated increase in [Ca2+]cyt (53). Therefore, which TRPC isoform(s) form ROC or SOC may depend on (i) cell type (e.g., TRPC6 may be involved in forming SOC in PASMC, but ROC in aortic smooth muscle cells), (ii) composition (or ratio) of different TRP subunits in the heterotetram (e.g., a channel made up of one TRPC3 and three TRPC6 may be a ROC and a channel made of one TRPC1, two TRPC3, and one TRPC6 may be a SOC), (iii) heteromultimeric assembly of functional channels (e.g., a ROC-TRPC6 may form heterologous SOC with SOC-TRPCs like TRPC3 and TRPC7) (44, 45, 47, 48, 51, 52), and (iv) localization or compartmentalization (e.g., ligand–receptor interaction and store depletion can activate a TRPC1-dominant channel in caveolae) (30). In addition, human TRP genes often show tissue-specific expression patterns and likely encode both ROC and SOC (29). The up-regulation of TRPC3 and -6 in IPAH-PASMC could increase the numbers of (i) TRPC3 and -6 homotetramers, (ii) TRPC3 and -6 (as well as TRPC1 and -7) heterotetramers, and ultimately (iii) both ROC and SOC. The consequent increase in [Ca2+]cyt could then cause sustained vasoconstriction and excessive PASMC growth, contributing to the pulmonary vascular remodeling. Blockade of SOC by Ni2+ has been reported to inhibit PASMC proliferation (22, 26, 27) and attenuate pulmonary vascular remodeling (57).
Previouly, we demonstrated that the mRNA expression of voltage-gated K+ (KV) channels and the amplitude of KV currents were decreased, whereas membrane potential was depolarized and [Ca2+]cyt was elevated, in IPAH-PASMC compared to SPH-PASMC (15, 16). The increased [Ca2+]cyt in IPAH-PASMC was believed to be partially caused by opening of voltage-dependent Ca2+ channels (VDCC), thereby stimulating PASMC contraction and proliferation (15, 16, 58). These results suggested an important role for down-regulated/dysfunctional KV channels in the development of pulmonary vasoconstriction and vascular remodeling (15, 16). However, clinical trials showed that ≈20–25% of IPAH patients responded to conventional VDCC blockers (i.e., nifedipine and diltiazem) (59). This finding suggested that the increased [Ca2+]cyt in IPAH-PASMC results from Ca2+ influx via VDCC and other Ca2+-permeable channels. The current study indicates that up-regulated TRPC channel activity also serves as an important pathway for elevating [Ca2+]cyt, triggering pulmonary vasoconstriction, and stimulating PASMC proliferation in IPAH patients.
Pulmonary vasoconstriction and vascular smooth muscle hypertrophy both contribute to the elevated PVR in IPAH patients. Medial hypertrophy caused by PASMC hyperplasia and hypertrophy in distal vessels accounts for some of the pulmonary vascular remodeling in IPAH patients (3, 5, 6). Identifying the factors involved in promoting PASMC proliferation could assist in the development of effective therapeutic approaches for patients with IPAH. Our results suggest that overexpression of TRPC3 and -6 channels may be another pathogenic mechanism involved in the increased PASMC proliferation, and may contribute to the development of pulmonary medial hypertrophy in IPAH patients. Development of drugs specifically targeting TRPC gene expression and TRPC channel function may enhance therapeutic efficiency for IPAH patients.
Supplementary Material
Acknowledgments
We thank Dr. S. Zhang and Ms. A. Nicholson for technical assistance. This work was supported by National Institutes of Health Grants HL64945, HL54043, HL66012, HL69758, and HL66941. Y.Y. is supported by a fellowship from the Pulmonary Hypertension Association.
Abbreviations: IPAH, idiopathic pulmonary arterial hypertension; PVR, pulmonary vascular resistance; PASMC, pulmonary artery smooth muscle cell(s); CCE, capacitative Ca2+ entry; SR, sarcoplasmic reticulum; TRP, transient receptor potential; TRPC, canonical TRP; SOC, store-operated Ca2+ channel; ROC, receptor-operated Ca2+ channel; PAEC, pulmonary artery endothelial cell(s); SPH, secondary pulmonary hypertension; SMGM, smooth muscle growth medium; NPH, normal human PASMC; siRNA, small interfering RNA; SMBM, smooth muscle basal medium.
References
- 1.Runo, J. R. & Loyd, J. E. (2003) Lancet 361, 1533–1544. [DOI] [PubMed] [Google Scholar]
- 2.Fishman, A. P. (1998) Chest 114, 242S–247S. [DOI] [PubMed] [Google Scholar]
- 3.Wagenvoort, C. A. (1960) Circulation 22, 535–546. [DOI] [PubMed] [Google Scholar]
- 4.Wood, P. (1958) Br. Heart J. 20, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenmark, K. R. & Mecham, R. P. (1997) Annu. Rev. Physiol. 59, 89–144. [DOI] [PubMed] [Google Scholar]
- 6.Cowan, K. N., Jones, P. L. & Rabinovitch, M. (2000) J. Clin. Invest. 105, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, S., Fantozzi, I., Tigno, D. D., Yi, E. S., Platoshyn, O., Thistlethwaite, P. A., Kriett, J. M., Yung, G., Rubin, L. J. & Yuan, J. X.-J. (2003) Am. J. Physiol. 285, L740–L754. [DOI] [PubMed] [Google Scholar]
- 8.Berg, J. T., Breen, E. C., Fu, Z., Mathieu-Costello, O. & West, J. B. (1998) Am. J. Respir. Crit. Care Med. 158, 1920–1928. [DOI] [PubMed] [Google Scholar]
- 9.Cacoub, P., Dorent, R., Nataf, P. & Carayon, A. (1993) N. Engl. J. Med. 329, 1967–1968. [DOI] [PubMed] [Google Scholar]
- 10.Giaid, A., Yanagisawa, M., Langleben, D., Michel, R. P., Shennib, H., Kimura, S., Masaki, T., Duguid, W. P. & Steward, D. J. (1993) N. Engl. J. Med. 328, 1732–1739. [DOI] [PubMed] [Google Scholar]
- 11.Kourembanas, S., Marsden, P. A., McQuillan, L. P. & Faller, D. V. (1991) J. Clin. Invest. 88, 1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson, C., Stewart, S., Upton, P. D., Machado, R., Thomson, J. R., Trembath, R. C. & Morrell, N. W. (2002) Circulation 105, 1672–1678. [DOI] [PubMed] [Google Scholar]
- 13.Du, L., Sullivan, C. C., Chu, D., Cho, A. J., Kido, M., Wolf, P. L., Yuan, J. X.-J., Deutsch, R., Jamieson, S. W. & Thistlethwaite, P. A. (2003) N. Engl. J. Med. 348, 500–509. [DOI] [PubMed] [Google Scholar]
- 14.Lane, K. B., Machado, R. D., Pauciulo, M. W., Thomson, J. R., Phillips, J., III, Loyd, J. E., Nichols, W. C. & Trembath, R. C. (2000) Nat. Genet. 26, 81–84. [DOI] [PubMed] [Google Scholar]
- 15.Yuan, X.-J., Wang, J., Juhaszova, M, Gaine, S. P. & Rubin, L. J. (1998) Lancet 351, 726–727. [DOI] [PubMed] [Google Scholar]
- 16.Yuan, J. X.-J., Aldinger, A. M., Juhaszova, M., Wang, J., Conte, J. V., Jr., Gaine, S. P., Orens, J. B. & Rubin, L. J. (1998) Circulation 98, 1400–1406. [DOI] [PubMed] [Google Scholar]
- 17.Eddahibi, S., Humbert, M., Fadel, E., Raffestin, B., Darmon, M., Capron, F., Simonneau, G., Dartevelle, P., Hamon, M. & Adnot, S. (2001) J. Clin. Invest. 108, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Launay, J. M., Herve, P., Peoc'h, K., Tournois, C., Callebert, J., Nebigil, C. G., Etienne, N., Drouet, L., Humbert, M., Simonneau, G., et al. (2002) Nat. Med. 8, 1129–1135. [DOI] [PubMed] [Google Scholar]
- 19.Cowan, K. N., Heilbut, A., Humpl, T., Lam, C., Ito, S. & Rabinovitch, M. (2000) Nat. Med. 6, 698–702. [DOI] [PubMed] [Google Scholar]
- 20.Jones, P. L., Crack, J. & Rabinovitch, M. (1997) J. Cell Biol. 139, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge, M. J. (1995) BioEssays 17, 491–500. [DOI] [PubMed] [Google Scholar]
- 22.Golovina, V. A., Platoshyn, O., Bailey, C. L., Wang, J., Limsuwan, A., Sweeney, M., Rubin, L. J. & Yuan, J. X.-J. (2001) Am. J. Physiol. 280, H746–H755. [DOI] [PubMed] [Google Scholar]
- 23.Hardingham, G. E., Chawla, S., Johnson, C. M. & Bading, H. (1997) Nature 385, 260–265. [DOI] [PubMed] [Google Scholar]
- 24.Hardingham, G. E., Chawla, S., Cruzalegui, F. H. & Bading, H. (1999) Neuron 22, 789–798. [DOI] [PubMed] [Google Scholar]
- 25.Short, A. D., Bian, J., Ghosh, T. K., Waldron, R. T., Rybak, S. L. & Gill, D. L. (1993) Proc. Natl. Acad. Sci. USA 90, 4986–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeney, M., Yu, Y., Platoshyn, O., Zhang, S., McDaniel, S. S. & Yuan, J. X.-J. (2002) Am. J. Physiol. 283, L144–L155. [DOI] [PubMed] [Google Scholar]
- 27.Yu, Y., Sweeney, M., Zhang, S., Platoshyn, O., Landsberg, J., Rothman, A. & Yuan, J. X.-J. (2003) Am. J. Physiol. 284, C316–C330. [DOI] [PubMed] [Google Scholar]
- 28.Birnbaumer, L., Zhu, X., Jiang, M., Boulay, G., Peyton, M., Vannier, B., Brown, D., Platano, D., Sadeghi, H., Stefani, E., et al. (1996) Proc. Natl. Acad. Sci. USA 93, 15195–15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatachalam, K., van Rossum, D. B., Patterson, R. L., Ma, H. T. & Gill, D. L. (2002) Nat. Cell Biol. 4, E263–E272. [DOI] [PubMed] [Google Scholar]
- 30.Bergdahl, A., Gomez, M. F., Dreja, K., Xu, S.-Z., Adner, M., Beech, D. J., Broman, J., Hellstrand, P. & Sward, K. (2003) Circ. Res. 93, 839–847. [DOI] [PubMed] [Google Scholar]
- 31.Boulay, G., Zhu, X., Peyton, M., Jiang, M., Hurst, R., Stefani, E. & Birnbaumer, L. (1997) J. Biol. Chem. 272, 29672–29680. [DOI] [PubMed] [Google Scholar]
- 32.Brough, G. H., Wu, S., Cioffi, D., Moore, T. M., Li, M., Dean, N. & Stevens, T. (2001) FASEB J. 15, 1727–1738. [PubMed] [Google Scholar]
- 33.Ng, L. C. & Gurney, A. M. (2001) Circ. Res. 89, 923–929. [DOI] [PubMed] [Google Scholar]
- 34.Xu, S.-Z. & Beech, D. J. (2001) Circ. Res. 88, 84–87. [DOI] [PubMed] [Google Scholar]
- 35.Husain, M., Bein, K., Jiang, L., Alper, S. L., Simons, M. & Rosenberg, R. D. (1997) Circ. Res. 80, 617–626. [DOI] [PubMed] [Google Scholar]
- 36.Morgan, J. I. & Curran, T. (1989) Ann. N.Y. Acad. Sci. 568, 283–290. [DOI] [PubMed] [Google Scholar]
- 37.Sheng, M., McFadden, G. & Greenberg, M. E. (1990) Neuron 4, 571–582. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson, A. S., Cartin, L., Wellman, T. L., Dick, M. H., Nelson, M. T. & Lounsbury, K. M. (2001) Exp. Cell Res. 263, 118–130. [DOI] [PubMed] [Google Scholar]
- 39.Somlyo, A. P. & Somlyo, A. V. (1994) Nature 372, 231–236. [DOI] [PubMed] [Google Scholar]
- 40.Kuga, T., Kobayashi, S., Hirakawa, Y., Kanaide, H. & Takeshita, A. (1996) Circ. Res. 79, 14–19. [DOI] [PubMed] [Google Scholar]
- 41.Hirst, S. J., Twort, C. H. & Lee, T. H. (2000) Am. J. Respir. Cell. Mol. Biol. 23, 335–344. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T. & Schultz, G. (1999) Nature 397, 259–263. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann, T., Schaefer, M., Schultz, G. & Gudermann, T. (2002) Proc. Natl. Acad. Sci. USA 99, 7461–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiselyov, K., Mignery, G. A., Zhu, M. X. & Muallem, S. (1999) Mol. Cell 4, 423–429. [DOI] [PubMed] [Google Scholar]
- 45.Kiselyov, K., Xu, X., Mozhayeva, G., Kuo, T., Pessah, I., Mignery, G., Zhu, X., Birnbaumer, L. & Muallem, S. (1998) Nature 396, 478–482. [DOI] [PubMed] [Google Scholar]
- 46.Trebak, M., Bird, G. St. J., McKay, R. R. & Putney, J. W., Jr. (2002) J. Biol. Chem. 277, 21617–21623. [DOI] [PubMed] [Google Scholar]
- 47.Vazquez, G., Lievremont, J.-P., Bird, G. St. J. & Putney, J. W., Jr. (2001) Proc. Natl. Acad. Sci. USA 98, 11777–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, X., Barnigg, G. & Villereal, M. L. (2000) Am. J. Physiol. 278, C526–C536. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed, A., Kobayashi, S., Shikasho, T., Nishimura, J. & Kanaide, H. (1998) Eur. J. Pharmacol. 344, 323–331. [DOI] [PubMed] [Google Scholar]
- 50.Husain, M., Jiang, L., See, V., Bein, K., Simons, M., Alper, S. L. & Rosenberg, R. D. (1997) Am. J. Physiol. 272, C1947–C1959. [DOI] [PubMed] [Google Scholar]
- 51.Ma, H. T., Patterson, R. L., Van Rossum, D. B., Birnbaumer, L., Mikoshiba, K. & Gill, D. L. (2000) Science 287, 1647–1651. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, X., Jiang, M., Peyton, M., Boulay, G., Hurst, R., Stefani, E. & Birnbaumer, L. (1996) Cell 85, 661–671. [DOI] [PubMed] [Google Scholar]
- 53.Inoue, R., Okada, T., Onoue, H., Hara, Y., Shimizu, S., Naitoh, S., Ito, Y. & Mori, Y. (2001) Circ. Res. 88, 325–332. [DOI] [PubMed] [Google Scholar]
- 54.Philipp, S., Cavalie, A., Freichel, M., Wissenbach, U., Zimmer, S., Trost, C., Marquart, A., Murakami, M. & Flockerzi, V. (1996) EMBO J. 15, 6166–6171. [PMC free article] [PubMed] [Google Scholar]
- 55.Zitt, C., Zobel, A., Obukhov, A. G., Harteneck, C., Kalkbrenner, F., Luckhoff, A. & Schultz, G. (1996) Neuron 16, 1189–1196. [DOI] [PubMed] [Google Scholar]
- 56.Walker, R. L., Hume, J. R. & Horowitz, B. (2001) Am. J. Physiol. 280, C1184–C1192. [DOI] [PubMed] [Google Scholar]
- 57.Christou, H., Morita, T., Hsieh, C.-M., Koike, H., Arkonac, B., Perrella, M. A. & Kourembanas, S. (2000) Circ. Res. 86, 1224–1229. [DOI] [PubMed] [Google Scholar]
- 58.Platoshyn, O., Golovina, V. A., Bailey, C. L., Limsuwan, A., Krick, S., Juhaszova, M., Seiden, J. E., Rubin, L. J. & Yuan, J. X.-J. (2000) Am. J. Physiol. 279, C1540–C1549. [DOI] [PubMed] [Google Scholar]
- 59.Rich, S., Kaufmann, E. & Levy, P. S. (1992) N. Engl. J. Med. 327, 76–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.