Abstract
Context:
The audiological features and cochlear morphology of individuals with noise-induced hearing loss (NIHL) are well characterized. However, the molecular processes in the cochlea are not well understood.
Aims:
To explore the role of the endoplasmic reticulum stress (ERS) response in the guinea pig model of cochlear damage induced by exposure to intense noise.
Settings and Design:
A pilot case–control study.
Subjects and Methods:
Forty-eight guinea pigs were divided into four equal groups. At 1, 4, or 14 days (d) post-exposure, the auditory brainstem responses (ABRs) were tested before sacrificing the subjects. The expression levels of the binding immunoglobulin protein/glucose-regulated protein 78 (BiP/GRP78) and C/EBP-homologous protein/growth arrest and DNA damage-inducible gene 153 (CHOP/Gadd153) proteins were evaluated using immunohistochemistry and Western blotting. The number of cochlear hair cells with altered nuclei was counted using confocal fluorescence microscopy.
Statistical analysis used:
One-way analysis of variance (ANOVA) and the least squares difference (LSD) test.
Results:
The outer hair cells (OHCs) showed changes of apoptosis, necrosis, and loss after noise exposure. In the 1- and 4-d groups, more apoptotic cells were found than necrotic cells (P < 0.01). The level of BiP/GRP78 was significantly higher in all three experimental groups compared to the control group (P < 0.01). The level of CHOP/Gadd153 was increased at 1 d post-exposure, achieving a peak that was maintained until 4 d, after which it returned to baseline levels by 14 d post-exposure.
Conclusions:
ERS response was activated by inducing the expression of BiP/GRP78 to lessen the extent of the resulting cellular damage and activating the CHOP/Gadd153 pathway to eliminate the most severely damaged cells.
Keywords: Cochlea, endoplasmic reticulum stress, noise-induced hearing loss
INTRODUCTION
In the majority of Asian countries, noise-induced hearing loss (NIHL), a major form of acute sensorineural hearing loss, is the most prevalent occupational disease.[1] The large number of affected laborers and the limited preventive programs resulting from the state of the developing economies have caused NIHL to be a serious health problem in these countries.
The audiological features and cochlear morphology of individuals with NIHL are well characterized. Continuous and intense noise stimulation leads to a temporary or even permanent shift in the hearing threshold due to the damage or loss of inner and outer cochlear hair cells. Noise initially injures the organ of Corti outer hair cells (OHCs) and later damages the inner hair cells (IHCs), the supporting cells, the stria vascularis, or even the spiral cochlear ganglion cells.[2] Recent studies have demonstrated that after exposure to intense noise, two types of cochlear cell death occur, namely, apoptosis and necrosis. Apoptosis has been found to be the primary cell death pathway leading to the rapid expansion of hair cell lesions after exposure to intense noise.[3]
However, the molecular processes in the cochlea that drive the development of NIHL are not well understood. Notably, endoplasmic reticulum stress (ERS) was recently found to be a new cause of apoptosis.[4] ERS can be caused by an increase in the content of endoplasmic reticulum unfolded protein (ERUP) or by an imbalanced calcium status induced by harmful stimuli such as hypoxia, a disturbance of calcium metabolism, free radicals, and drugs. To reduce the level of protein synthesis and promote the correct folding of proteins, the unfolded-protein response (UPR) and the endoplasmic reticulum overload response are activated in stressed cells, although apoptosis-inducing molecules are also activated by high levels of stress.[5]
The molecular chaperone binding immunoglobulin protein/glucose-regulated protein 78 (BiP/GRP78) and the ERS-specific transcription factor C/EBP-homologous protein/growth arrest and DNA damage-inducible gene 153 (CHOP/Gadd153) are two major indicators of ERS.[6,7] In the absence of ERS, BiP/GRP78 is maintained in a non-active state and binds to PKR-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1), while CHOP exists as a heterodimer with other members of the C/EBP (CCAAT/enhancer binding protein) family and is expressed at a negligible level. In the presence of ERS, the accumulation of ERUP in the endoplasmic reticulum causes BiP/GRP78 to dissociate from the three aforementioned proteins and to unfold.
These dissociated proteins are activated and begin to induce the UPR, which protects against cellular damage caused by ERS and restores cellular functioning. In contrast, the activation of IRE1, PERK, and ATF6 promotes the production of a large amount of CHOP, which in turn accelerates apoptosis.
Our previous study showed that ERS was also involved in the pathological process of noise-induced cochlear cell apoptosis.[8] The aims of the present study were to clarify the mechanisms by which ERS induced by exposure to intense noise results in cochlear damage by evaluating the expression of relevant factors, namely, the BiP/GRP78 and CHOP/Gadd153 proteins, and to obtain experimental evidence for their positive intervention in NIHL.
SUBJECTS AND METHODS
Subjects
Forty-eight healthy male guinea pigs weighing 400–450 g were supplied by the Wuhan University Center for Animal Experiment. The guinea pigs were selected according to the following criteria: a sensitive auricular reflex, intact tympanic membranes, no otitis media, and no history of noise or drug exposure. A computational random-number generator was served to generate a sequence of numbers to divide the subjects into groups or select sections and cells. The guinea pigs were randomly divided into two main groups: the control (n=12) group, which was not exposed to noise, and the experimental group (n=36), which was further divided into three subgroups with different periods of recovery after the noise exposure, namely, 1, 4, or 14 days (d) (n=12 each). All the guinea pigs were adequately fed for 1 week in a sound-protected room. All of the procedures for the use and care of the animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC protocol S53002002).
Noise exposure protocol
The stimulus of 4-kHz narrow-band noise (cutoff frequencies: 2840–5680 Hz, slopes: ±12 dB/decade) was generated using 4kHzNBN-316HzBW-50 mV software (a gift of the Institute of Otolaryngology, Chinese PLA General Hospital, China) and was amplified and delivered through speakers (YD115-80A, China) located in the four corners of a bilayered acoustic chamber (internal cell dimensions: 80 cm × 80 cm × 85 cm, SB-5530, TOPS, China). The level of sound exposure was measured using a HS6280 sound-level meter (Hongshen, China) and was confirmed to be 120 dB at multiple positions in the chamber. The experimental groups were placed individually in rearing cages without restraint on their activity and were then exposed to noise in the acoustic chamber for 4 h. No intervention was given to the control group. Routine feeding was provided to all of the subjects.
Auditory brainstem response test
The control group and experimental groups (pre- and post-exposure to noise) were anesthetized by intraperitoneal injections of 10% chloral hydrate (30 mg/kg) and were then subjected to auditory brainstem response (ABR) testing in an electrostatically shielded room. The parameters of the ABR test were as follows: a Nicolet compass system was used; the stimulus was click stimuli; the repetition rate was 20 beats/s; the scan cycle was 10 ms; the filter bandwidth was 100–3000 Hz; and the signal was superimposed 1240 times. The lowest sound level, at which the response peaks of the III wave were clearly present, was defined as the standard hearing threshold.
Surface preparation of the cochlea and Hoechst 33342 staining
The animals were rapidly decapitated under general anesthesia, and the bilateral otocysts were segregated. The bony capsule was partially dissected away using finely pointed micro-hooks. Approximately 0.5 mL of ice-cold 4% paraformaldehyde-phosphate buffer, pH 7.4 was injected through the round and oval windows after having made a small hole in the apex of the cochlea. The cochlea was immersed in the fixative for 2 h and stored in phosphate buffer. The membranous parts were microdissected from the modiolus. The basal, middle, and apical turns were separately collected and cut into smaller segments, with each segment containing both the basilar membrane with the organ of Corti and the lateral wall. Randomly, for each guinea pig one side of cochlea was selected for Hoechst 33342 staining. After overnight decalcification of the microdissected cochlea in 10% EDTA–2Na (pH 7.4) at 4°C, individual turns were then stained using Hoechst 33342 dye (Thermo Scientific, USA) for 10 min at ambient temperature and were then washed three times using 10 mmol/L phosphate buffered saline (PBS). The stained sections of surface were mounted in sequential order on glass slides using 50% glycerol. The number of nuclei and changes in the nuclei were observed using a confocal fluorescence microscope (Leica, Germany). The changes that represented the most obvious types of damage were evaluated throughout the entire cochlear surface to quantify the percentage of apoptotic, necrotic, and lost OHCs. As revealed by Hoechst 33342 staining, nuclear condensation and/or nuclear fragmentation were considered to be signs of apoptosis, and nuclear swelling was considered a sign of necrosis. The lost OHCs presented as gaps in the normal arrangement of hair cells.
Immunohistochemistry
As mentioned above, the other sides of the microdissected segments of cochlea were embedded in paraffin according to general procedure. The embedded segments were cut in a transverse plane during sectioning. Sections with a thickness of 5 μm were stored at room temperature (RT) before staining. After dewaxing and rehydration, endogenous peroxidase was blocked by incubation with 3%H2O2 at 37°C, 10min. Then, the antigens were retrieved by microwave heating (Panasonic, JPN) of the tissues for 3 min, 900 W. Streptavidin-peroxidase immunohistochemistry was performed according to the kit manufacturer's instructions. The sections were incubated with a solution containing a goat polyclonal antibody directed against BiP/GRP78, a mouse monoclonal antibody directed against CHOP/Gadd153 (Santa Cruz Biotech, USA), 10% normal goat serum, and 1% bovine serum albumin at 4°C overnight. Sections verified to be positively stained were used as the positive control, and sections incubated with PBS instead of the primary antibodies served as negative controls. Positive staining was observed as brown particles distributed throughout the nucleus, cytoplasm, and/or membrane. The average staining intensity of the organ of Corti, the spiral ganglion, and the cochlear lateral wall was measured using an HPIAS-100 high-resolution pathological imaging system (Huahai, China). Four hundred cells in each group were randomly selected for statistical analysis.
Western blot analysis
The total cochlear proteins were extracted by homogenizing the cochleae in a lysis buffer and then centrifuging the lysates at 10,000 × g for 10 min at 4°C. The proteins were separated by sodium dodecyl sulfate polyacrylamide-gel electrophoresis (SDS-PAGE) and were transferred to polyvinylidine difluoride (PVDF) membranes (Millipore, USA). The membranes were blocked using 5% skim milk in Tris Buffered Saline with Tween® 20 (TBST). After 2 h at RT, the membranes were washed using TBST and were incubated overnight at 4°C with the following primary antibodies: goat polyclonal antibodies directed against BiP/GRP78 (1:400, Santa Cruz Biotech, USA), a mouse monoclonal antibody directed against CHOP/Gadd153 (1:400, Santa Cruz Biotech, USA), and a mouse monoclonal antibody directed against β-actin (1:400, Sigma, USA). After being rinsed with TBST, horseradish peroxidase (HRP)-conjugated rabbit-anti-goat or rabbit-anti-mouse immunoglobulin G (IgG) (1:5000, Abcam, UK) was added for 1 h, and the reactive bands were visualized using enhanced chemiluminescence. Densitometric analysis of the bands was performed using Kodak Digital Science ID software (Kodak, USA).
Statistical analyses
The statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) software 19.0 (IBM, USA). The data are presented as the mean values ± sd. The significance of the differences between two groups was determined using the t test (threshold shift of ABR testing, based on number of animals), and the multi-group comparisons were conducted using one-way analysis of variance (ANOVA) and the least squares difference (LSD) test (OHC damage, based on randomly selected cells; immunohistochemical and Western blot analyses of BiP/GRP78 and CHOP/Gadd153 expressions, based on number of selected sections). Statistical significance was attributed to P values < 0.01.
RESULTS
Auditory brainstem r testing: Persistent threshold shift caused by exposure to intense noise
Typical plots detailing the ABR results and graphs showing the shift of average values are presented in Figure 1. Before noise exposure, the average ABR threshold values of the animals in the 1-, 4-, and 14-d experimental groups and in the control group were 32.23 ± 3.54, 36.23 ± 4.11, 35.23 ± 2.86, and 34.00 ± 5.28 dB, respectively. In the experimental groups, these values significantly shifted after noise exposure to 92.00 ± 3.13, 65.50 ± 6.15, and 64.00 ± 6.47 dB, respectively, and significant differences were found by comparing these values with that in the control group (P < 0.01). Significant differences were found between the values for the 1-and 4-d groups (P < 0.01), as well as between the values for the 1-and 14-d groups (P<0.01), but not between the values for the 4-and 14-d groups (P > 0.01). These results demonstrated that the noise stimulus used in this study led to a relatively stable and ptable and persistent shift of the guinea pigs and there was no recovery during observation period.
Figure 1.
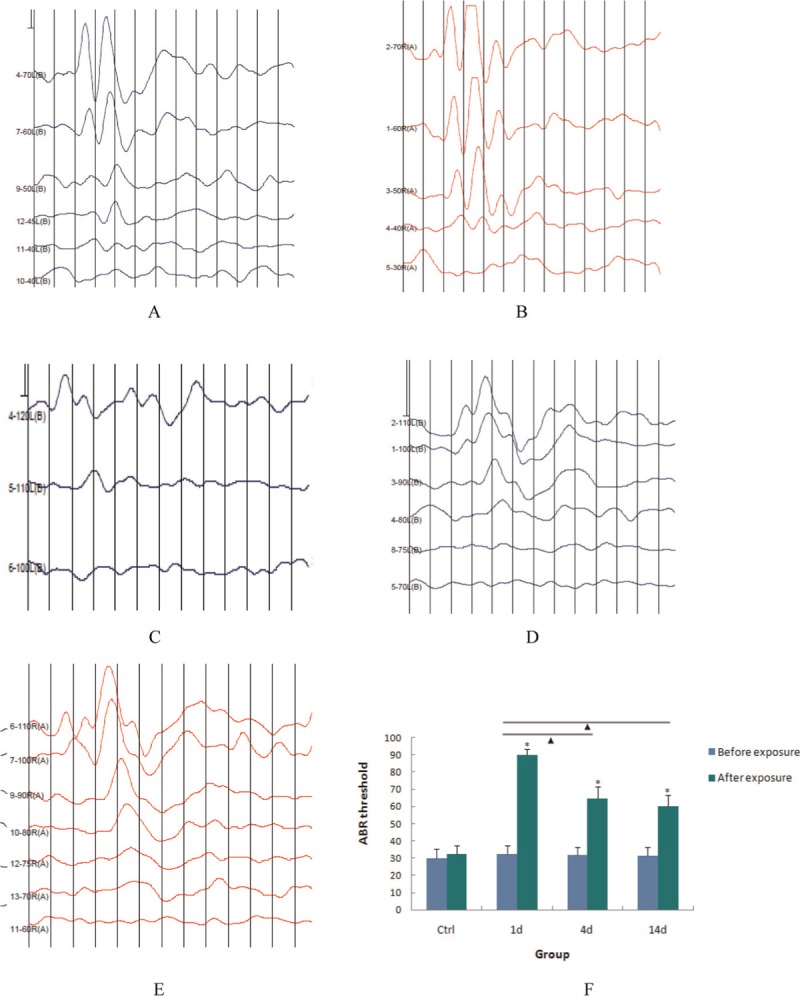
Shift of ABR threshold caused by noise exposure. (a–e) Typical ABR plots presented in the control group, experimental group before noise exposure, 1-, 4-, and 14-d post-exposure groups (blue curve: left ear; red curve: right ear.) (f) Average of ABR threshold was calculated and shift of these values was analyzed by one-way ANOVA. *P < 0.01, values compared with control group; NP < 0.01, values compared among experimental groups
Hoechst 33342 staining: Pathological changes occurred after noise exposure
As shown in Figure 2a, when viewed under a confocal fluorescence microscope, the nuclei of the cochlear hair cells in the control group exhibited pale-blue fluorescent Hoechst 33342 staining, and the nuclei were of a consistent size. One row of IHCs and three rows of neatly arranged OHCs were observed in the control animals. After intense noise exposure, the experimental groups exhibited disorganized OHCs and three types of pathological changes in both the OHCs and IHCs [Figure 2b–d], namely, (1) nuclear swelling, (2) karyopyknosis, and (3) nuclear loss, which were evident from the Hoechst 33342 staining patterns. These nuclear changes indicated the necrosis, apoptosis, or loss of cells, respectively. These pathological changes were noted most frequently in the first row of OHCs, followed by the second and then the third row of OHCs. Less nuclear damage was observed in the IHCs. The morphological changes in the HC nuclei were observed throughout the entire organ of Corti but were particularly evident in the region between the basal turn and the initial portion of the second turn (9–14 mm from the apex of the cochlea in a region that was approximately 6 mm long).
Figure 2.

Pathological changes revealed by Hoechst 33342 staining (×400). (a) Control and (b–d) 1, 4, and 14 days after noise exposure, respectively. ↑ = Nuclear swelling: increased nuclear volume and reduced staining (as shown in e), fx1 = karyopyknosis: shrunken nucleus and enhanced staining (as shown in f), q = nuclear loss: original position of nucleus replaced by a blank area (as shown in g). (h) Quantitative analyses of OHC damage. The percentages of three pathological changes in OHC at different times were analyzed by using the one-way ANOVA. *P < 0.01, between percentage of apoptotic and necrotic cells
Quantitative analyses of OHC damage: The severity of damage was dependent on the length of the period following noise exposure
The region of the basilar membrane with the most severe noise damage, as described above, was quantitatively analyzed by determining the percentage of apoptotic, necrotic, and absent OHCs. After noise exposure, the overall trend observed was the notable increase in the percentage of lost OHCs but the slight decrease in the percentage of necrotic and apoptotic OHCs. When comparing the number of apoptotic and necrotic cells at different times after noise exposure, the average percentage of apoptotic cells was found to be significantly higher than that of the necrotic cells (15.20 ± 1.20% vs. 5.23 ± 0.54% in the 1-d group, 13.31 ± 0.39% vs. 4.99 ± 0.72% in the 4-d group, P < 0.01, Figure 2h). The overall percentages of apoptotic, necrotic, and absent OHCs in the 14-d group were significantly increased compared with those in the control group, but the difference between the percentages of apoptotic and necrotic cells was not significant (3.20 ± 0.35 vs. 4.23 ± 0.65%, P > 0.01, Figure 2h).
Immunohistochemical analysis of BiP/GRP78 and CHOP/Gadd153 expression: Positive staining of inner ear cells
At 1, 4, or 14 d after noise exposure, brown-stained BiP/GRP78-immunopositive particles distributed throughout the organ of Corti, the spiral ganglion, and the cytoplasm/cytomembrane of lateral wall cells, respectively, were observed by light microscopy [Figure 3a]. Weakly positive staining was observed in the control group [Figure 3c]. Using image analysis, the mean intensities of staining of the organ of Corti, spiral ganglion, and lateral wall cells in each group were determined. As shown in Table 1, significant differences in the staining intensities relative to those in the control group were found in the experimental groups (P<0.01). However, the differences in the staining intensities between the three experimental groups were not significant (P > 0.01).
Figure 3.

Microscopic analysis of the distribution of BiP/GRP78 and CHOP/Gadd153 in immunohistochemically stained cochleae (SP, ×400). (a-1–a-3) Cells that were positively stained for BiP/GRP were located in the organ of Corti, the lateral wall, and the spiral ganglion, respectively, in the 1d group (positive expression: immuoreactivity is brown versus the blue hematoxylin stain, indicated by ↑). (b-1–b-3) Cells that were positively stained for CHOP/Gadd153 were located in the same regions as those shown in (a), in the 1d group (positive expression: immunoreactivity is tan or medium-brown particles versus hematoxylin-stain, indicated by ↑). (c-1–c-3) Negative control incubated with PBS instead of the primary antibodies (organ of Corti, the lateral wall, and the spiral ganglion, respectively)
Table 1.
Mean intensity of BiP/GRP78 immunohistochemical staining in different regions of the cochlea
| Group | Organ of Corti (mean ± SD) | Spiral ganglion (mean ± SD) | Lateral wall cells (mean ± SD) | |
|---|---|---|---|---|
| BiP/GRP78 | Control | 0.201 ± 0.038 | 0.428 ± 0.113 | 0.287 ± 0.081 |
| 1 d | 0.406 ± 0.066a,b | 0.952 ± 0.287a,b | 0.734 ± 0.189a,b | |
| 4 d | 0.388 ± 0.045a,b | 0.884 ± 0.183a,b | 0.668 ± 0.182a,b | |
| 14 d | 0.380 ± 0.041a,b | 0.876 ± 0.199a,b | 0.662 ± 0.186a,b |
One-way ANOVA test results: aP < 0.01, bP > 0.01
Tan or medium-brown particles were visible in the organ of Corti, spiral ganglion, and nuclei of the lateral wall cells following CHOP/Gadd153 staining in the experimental groups [Figure 3b]; however, the corresponding cells and nuclei in the control group were not positively stained. As shown in Table 2, the mean intensities of CHOP/Gadd153 staining in each of the experimental groups were significantly higher than those in the control group (P < 0.01). Significant differences were found between the values for the 1-and 14-d groups and those for the 4-and 14-d groups but not between the values for the 1-and 4-d groups (P > 0.01).
Table 2.
Mean intensity of CHOP/Gadd153 immunohistochemical staining in different regions of the cochlea
| Group | Organ of Corti (mean ± SD) | Spiral ganglion (mean ± SD) | Lateral wall cells (mean ± SD) | |
|---|---|---|---|---|
| CHOP/Gadd153 | Control | 0.2845 ± 0.051 | 0.327 ± 0.058 | 0.335 ± 0.052 |
| 1 d | 0.595 ± 0.084a | 0.904 ± 0.075a | 0.856 ± 0.077 | |
| 4 d | 0.521 ± 0.138b | 0.884 ± 0.183a,b | 0.821 ± 0.118a | |
| 14 d | 0.314 ± 0.383a,b | 0.858 ± 0.098a | 0.548 ± 0.054a,b |
One-way ANOVA test results: aP < 0.01, bP > 0.01
BiP/GRP78 and CHOP/Gadd153 Western blot analysis: Higher levels of expression in the experimental groups
The results of the Western blot analysis examining the levels of BiP/GRP78 and CHOP/Gadd153 protein are shown in Figure 4. The experimental groups showed a significantly increased level of BiP/GRP78 protein and maintained a higher level of this protein compared with the control group (P < 0.01). No significant differences in the level of this protein were discovered between the experimental groups (P > 0.01).
Figure 4.
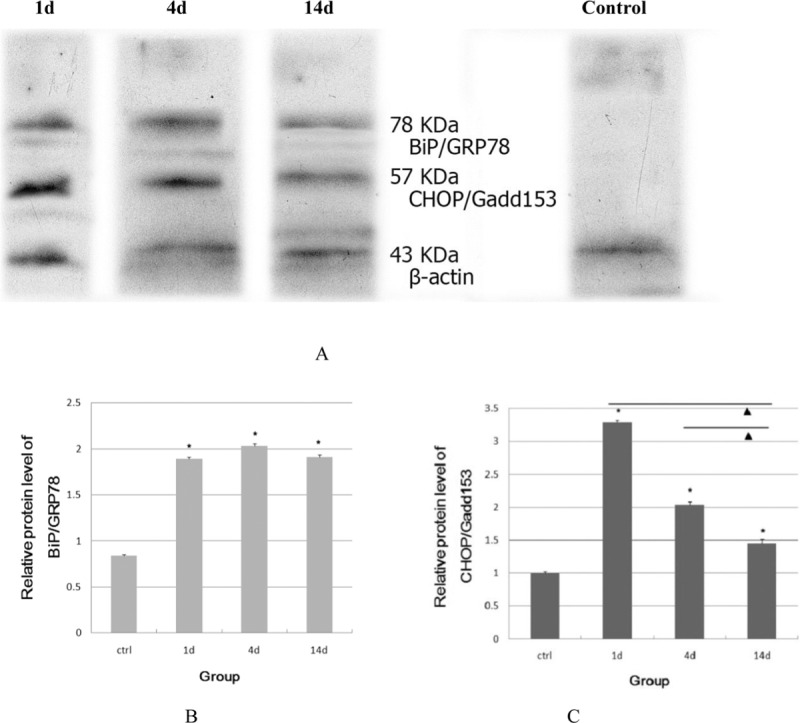
Expression of BiP/GRP78 and CHOP/Gadd153 proteins evaluated by Western blot analysis (a). Relative protein level of BiP/GRP 78 and CHOP/Gadd153 was analyzed by using one-way ANOVA (b, c). *Comparison with control group, P < 0.01. NComparison among the experimental groups, P < 0.01. β-Actin was used as the internal control and no significant difference of this protein was found among these groups
The level of CHOP/Gadd153 expression increased shortly after noise exposure and achieved a peak at 1 d after noise exposure, which was maintained until 4 d post-exposure, but the level of CHOP/Gadd153 expression was decreased at 14 d post-exposure. Using image analysis, significant differences in the levels of CHOP/Gadd153 expression were found between the experimental and control groups, the 1-and 14-d groups, and the 4-and 14-d groups (P < 0.01) but not between the 1-and 4-d groups (P > 0.01).
DISCUSSION
Our results showed that the noise stimulus conditions chosen for this study caused a relatively stable and persistent shift in the hearing threshold of the guinea pigs. A recent study revealed that necrosis and apoptosis occurred simultaneously in the cochlea after intense noise exposure.[9] Moreover, the observation of nuclear swelling, karyopyknosis, and nuclear loss, the three types of pathological changes demonstrated by Hoechst 33342 staining, confirmed that this noise stimulus led to the apoptosis and necrosis of OHCs. The quantitative analysis of damaged OHCs indicated that 1, 4, and 14 d after noise exposure, the trend over time was a significant increase in the loss of OHCs and a decrease in the quantity of necrotic and apoptotic cells. Comparing the quantity of apoptotic and necrotic cells at different post-exposure periods revealed that the percentage of apoptotic cells in the 1-and 4-d groups was significantly greater than the percentage of necrotic cells, whereas in the 14-d group, no significant difference between the percentages of these two types of dying cells was found. Thus, apoptosis occurred mainly during the early stage of cochlear pathogenesis and was induced by exposure to intense noise in the guinea pigs, and this process continued until at least 14 d after noise exposure. These phenomena may be due to secondary damage caused by the production of a large amount of free radicals following intense noise exposure. Free radicals can damage DNA, RNA, proteins and lipids, leading to further apoptosis and necrosis of hair cells.[10,11]
The significant increase in the total number of damaged OHCs (including those with the three types of damage) at 14 d after noise exposure did not prevent the recovery of hearing in the guinea pigs. During the prolonged period after the exposure to the noise stimulus, a considerable number of the cochlear cells that had been less severely damaged gradually recovered through the use of various cellular protective mechanisms, and, therefore, hearing was progressively restored. The cells with irreversible pathological changes (necrosis, apoptosis, and loss) that we considered in our statistical analyses comprised only a fraction of the damaged cells; therefore, the relationship observed between the intense noise-induced apoptotic process and hearing loss was plausible.
ERS-initiated apoptosis was recently found to be a new cell death pathway, but the underlying signal transduction mechanism has not yet been entirely clarified. To date, at least three pathways are known to initiate apoptosis, which occurs in the following manner: (1) activation of caspase-12, a unique member of the caspase family that is located in the endoplasmic reticulum (ER), (2) activation of the c-Jun N-terminal kinases (JNK) signaling pathway, and (3) transcription and expression of CHOP/Gadd153.[12] Both BiP/GRP78 and CHOP/Gadd153 function as ER chaperones during the stress response. However, these two ER chaperones play conflicting roles. BiP/GRP78 exhibits combined activity as an ER transmembrane protein with enzymatic activity to play a protective role, whereas CHOP/Gadd153 suppresses the expression of Grp78/BiP and the anti-apoptotic protein Bcl-2, exhausts the glutathione content, and induces the formation of oxygen radicals to initiate the apoptotic process, which finally leads to cell death.[13]
It is known that noise can cause ERS and ERS-induced apoptosis of cochlear cells.[2] In a study of noise-induced apoptosis in the inner ear, Nicotera et al.[14] investigated the level of expression of caspase-8, caspase-9, and caspase-3 and the level of cytochrome C activity in the chinchilla cochlea after noise exposure and concluded that this type of apoptosis involved multiple pathways, including the death-receptor pathway and the mitochondrial pathway. In addition, our previous study demonstrated that activation of caspase-12 and the JNK signaling pathway was involved in the noise-induced apoptosis of cochlear cells.[8] If the above-mentioned hypothesis regarding the signaling pathways involved in ERS-initiated apoptosis is also applicable to noise-induced ERS, one would expect that CHOP/Gadd153 and BiP/GRP78 also regulate this apoptotic process.
In our study, the level of BiP/GRP78 expression in each group that was exposed to intense noise was significantly higher than that in the control group, which suggests that a high level of BiP/GRP78 had a protective effect on the noise-damaged cochlear cells and that this effect lasted 14 days. This may be related to the continuation of the effects of noise-induced damage and the enlargement of the lessened region that occurred during this period. Moreover, the secondary damage stimulated the cochlea to sustain the elevated level of BiP/GRP78 expression to prevent a persistent ERS response, ultimately leading to the restoration of normal functioning in the cells that were not seriously damaged. Comparison of the extent of hearing loss in the experimental groups showed a tendency for it to be decreased at 4 and 14 d following noise exposure, indirectly demonstrating that the function of the hair cells had been restored due to the achievement of calcium homeostasis and a stable internal environment in the ER via BiP/GRP78 regulation. Our immunohistochemical results demonstrated positive BiP/GRP78 staining in the hair cells, the lateral wall cells, and the spiral ganglion cells, indicating that BiP/GRP78 exerted a protective effect not only in hair cells, but also in all of the inner ear cells. Notably, the protective effect of the upregulated BiP/GRP78 expression is not absolute because the ERS response triggers both survival signaling and proapoptotic signaling. In the case of severely damaged cells, the main function of the ERS is to eliminate them via apoptosis.[15]
In the noise exposure groups, CHOP/Gadd153 was detected in the nuclei of cells in the organ of Corti, the stria vascularis, and the spiral ganglion but was not detected in the control group. As the Western blotting results showed, the level of CHOP/Gadd153 expression was increased at 1 d after intense noise exposure and maintained at 4 d post-exposure but was decreased significantly at 14 d post-exposure. Thus, after noise exposure, CHOP/Gadd153 may eliminate the severely damaged cochlear cells by inducing their apoptosis.
In conclusion, the present study strongly suggests that the up-regulated expression of the chaperone BiP/GRP78 leads to the correct folding of protein, reducing the extent of cochlear damage. Simultaneously, the CHOP/Gadd153 pathway was activated to eliminate the severely damaged cochlear cells by initiating their apoptosis. We hypothesize that these two mechanisms could partially drive the endogenous protective process utilized in the inner ear. Understanding that these mechanisms are involved in this process may lead to novel ideas and research aimed at the prevention and treatment of NIHL.
Financial support and sponsorship
Wuhan University of Science and Technology Research Grant (53002002).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fuente A, Hickson L. Noise-induced hearing loss in Asia. Int J Audiol. 2011;50(Suppl 1):S3–10. doi: 10.3109/14992027.2010.540584. [DOI] [PubMed] [Google Scholar]
- 2.Sliwinska-Kowalska M, Davis A. Noise-induced hearing loss. Noise Health. 2012;14:274–80. doi: 10.4103/1463-1741.104893. [DOI] [PubMed] [Google Scholar]
- 3.Hu BH, Guo W, Wang PY, Henderson D, Jiang SC. Intense noise-induced apoptosis in hair cells of guinea pig cochleae. Acta Otolaryngol. 2000;120:19–24. [PubMed] [Google Scholar]
- 4.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, et al. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–42. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 5.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5:a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, et al. Coupling endoplasmic reticulum stress to the cell death program: Role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–8. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 8.Xue QH, Chen J, Gong SS, He J, Xie J, Chen XL. Role of caspase 12 in apoptosis of cochlea induced by intense noise in guinea pigs. Chin J Otorhinolaryngol Head Neck Surg. 2009;44:154–9. [PubMed] [Google Scholar]
- 9.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–70. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samson J, Wiktorek-Smagur A, Politanski P, Rajkowska E, Pawlaczyk-Luszczynska M, Dudarewicz A, et al. Noise-induced time-dependent changes in oxidative stress in the mouse cochlea and attenuation by d-methionine. Neuroscience. 2008;152:146–50. doi: 10.1016/j.neuroscience.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Yang WP, Henderson D, Hu BH, Nicotera TM. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res. 2004;196:69–76. doi: 10.1016/j.heares.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Zhang M, Yin H. Signaling pathways involved in endoplasmic reticulum stress-induced neuronal apoptosis. Int J Neurosci. 2013;123:155–62. doi: 10.3109/00207454.2012.746974. [DOI] [PubMed] [Google Scholar]
- 13.Tajiri S, Yano S, Morioka M, Kuratsu J, Mori M, Gotoh T. CHOP is involved in neuronal apoptosis induced by neurotrophic factor deprivation. FEBS Lett. 2006;580:3462–8. doi: 10.1016/j.febslet.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Nicotera TM, Hu BH, Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol. 2003;4:466–77. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jäger R, Bertrand MJ, Gorman AM, Vandenabeele P, Samali A. The unfolded protein response at the crossroads of cellular life and death during endoplasmic reticulum stress. Biol Cell. 2012;104:259–70. doi: 10.1111/boc.201100055. [DOI] [PubMed] [Google Scholar]


