Abstract
Noise exposure might be a risk factor for ischemic heart disease (IHD). Unlike residential exposure, however, evidence for occupational noise is limited. Given that high-quality quantitative synthesis of existing data is highly warranted for occupational safety and policy, we aimed at conducting a systematic review and meta-analysis of the risks of IHD morbidity and mortality because of occupational noise exposure. We carried out a systematic search in MEDLINE, EMBASE, and on the Internet since April 2, 2015, in English, Spanish, Russian, and Bulgarian. A quality-scoring checklist was developed a priori to assess different sources of methodological bias. A qualitative data synthesis was performed. Conservative assumptions were applied when appropriate. A meta-analysis was not feasible because of unresolvable methodological discrepancies between the studies. On the basis of five studies, there was some evidence to suggest higher risk of IHD among workers exposed to objectively assessed noise >75–80 dB for <20 years (supported by one high, one moderate, and one low quality study, opposed by one high and one moderate quality study). Three moderate and two low quality studies out of six found self-rated exposure to be associated with higher risk of IHD, and only one moderate quality study found no effect. Out of four studies, a higher mortality risk was suggested by one moderate quality study relying on self-rated exposure and one of high-quality study using objective exposure. Sensitivity analyses showed that at higher exposures and in some vulnerable subgroups, such as women, the adverse effects were considerably stronger. Despite methodological discrepancies and limitations of the included studies, occupational noise appeared to be a risk factor for IHD morbidity. Results suggested higher risk for IHD mortality only among vulnerable subgroups. Workers exposed to high occupational noise should be considered at higher overall risk of IHD.
Keywords: Coronary heart disease, gender differences, myocardial infarction, noise exposure, work environment
INTRODUCTION
Cardiovascular diseases (CVDs) are the most important cause of mortality worldwide (31% in 2012), with coronary heart disease (CHD) alone being accountable for 7.4 million deaths.[1] CHD or ischemic heart disease (IHD) implies “obstruction to blood flow due to [atherosclerotic] plaques in the coronary arteries or, much less frequently, to other obstructing mechanisms”; one of the manifestations of CHD, myocardial infarction (MI), is defined by “myocardial cell necrosis due to significant and sustained ischaemia”.[2]
In addition to individual factors, noise pollution is regarded as an environmental factor able to raise the risk for IHD. Noise may contribute to some well-researched risk factors for CVD–increased blood pressure, dyslipidemia, inflammatory processes, promotion of blood-clotting factors, and changes in heart rate variability.[3] It acts as a general stressor for the hypothalamic–pituitary–adrenal axis, increasing cortisol and catecholamine levels, disrupting normal sleep patterns, leading to vasoconstriction and vascular impairment;[4,5,6] it has deleterious effects on glucose and lipid metabolism and insulin sensitivity.[7] Atherosclerotic changes can be enhanced by noise-related overproduction of cortisol, increased blood cholesterol and triglycerides, and generation of proinflammatory agents and raised interleukin-6 because of higher oxidative stress.[3] Noise may also decrease arterial compliance and lead to vascular hypertonicity and dysregulate the balance between the sympathetic and parasympathetic nervous system, which is associated with higher risk of adverse cardiovascular events.[3] Animal models have even shown that chronic noise exposure reduces male testosterone levels, which, on the other hand, might further impair their cardiovascular system.[8]
Another factor to consider in understanding noise-induced stress is the duration of exposure. For example, Gan et al.[9] found dose–response relationship between the cumulative exposure to occupational noise and blood lipids and glucose concentrations. In the long run, workers may consciously inhibit the noise stress by isolating it from the prefrontal cortex (the so called “emotional flight”), but this comes at the expense of an increased “allostatic load” for the autonomic nervous system.[3] Owing to this lack of physiological habituation, the pathological changes, taken place in the neuroendocrine and autonomic nervous system, may persist even after the noise stress is discontinued.[3] According to Recio et al.,[3] “noise may […] act at the chronic level increasing the allostatic load, and at the acute level triggering cardiovascular […] events”. This complex interplay can be viewed as a biological explanation of how noise might raise the risk for CVDs and IHD, in particular.
The World Health Organization estimated an annual loss of 60,000 disability-adjusted life-years because of traffic noise-attributed IHD.[10] However, both primary and synthetic research has mostly focused on residential exposure.[11] Conversely, according to the Fifth European Survey on Working Conditions 2012, nearly 30% of all European workers are exposed to so loud noise that they would have to raise their voice to talk to other people during at least one-quarter of the time at work, and, even more strikingly, the proportion of these workers has actually increased since 1991,[12] exceeding the safety thresholds. According to the Directive 2003/10/EC, the exposure limit value for the daily noise exposure levels at work is LEX,8h=87 dB(A), and the lower and upper exposure action values are LEX,8h=80 dB(A) and LEX,8h=85 dB(A), respectively.[13] This fact, coupled with the alarming prevalence of IHD, calls for a quantitative risk estimate linking exposure and outcome, to advance occupational noise policy. In the past, quantitative reviews have identified important links between occupational noise and CVDs, but they provide little evidence of IHD. For example, van Kempen et al.[14] estimated a pooled risk for hypertension of 1.14 (95% confidence interval, CI: 1.01, 1.29) per 5 dB increase in occupational noise. More recently, Tomei et al.[15] found that workers exposed to high noise (92.2 ± 6.5 dB(A)) had significant increase in blood pressure, heart rate, prevalence of hypertension, and electrocardiogram abnormalities. As far as we are aware, the only meta-analysis that quantified the risk for IHD was done by Ha et al.[16], who reported a relative risk (RR) of 1.06 (95% CI: 0.95, 1.18) among workers exposed to >70–85 dB. That meta-analysis, however, was based just on four studies and did not apply stringent criteria for their synthesis, which would have been mandatory, given their methodological discrepancies.
Given the importance of quantifying the link between occupational noise and IHD and the very limited and unreliable previous synthetic research outlined earlier, we aimed at conducting a systematic review and meta-analysis of the risks for IHD morbidity and mortality because of occupational noise exposure.
METHODS
Search strategy and eligibility criteria
Initially, we developed and agreed on a review protocol and data extraction forms. The research question was: “What is the risk for IHD morbidity and mortality among workers exposed to high occupational noise levels in comparison to those exposed to low levels or not exposed?” Both authors carried out the searches independently. Discrepancies were resolved through consensus. The meta-analysis of observational studies in epidemiology statement was followed while reporting the results.[17]
We searched MEDLINE and EMBASE (PubMed and ScienceDirect, respectively, with filters relevant to journal articles, work environment, cardiovascular health, and public health) through April 2, 2015, with combinations of the following free-term keywords: “occupational noise”, “industrial noise”, “work noise”, “ischemic heart disease”, “myocardial infarction”, and “coronary heart disease”. The search string did not contain International Classification of Diseases (ICD) codes to identify relevant studies regardless of whether they used objective or doctor-diagnosed outcome or subtype of IHD. These searches were complemented with a general Internet search (Google). They were limited to English, Spanish, Russian, and Bulgarian languages. Articles were sequentially screened on title, abstract, and full-text levels and evaluated for relevance. We included primary epidemiological studies reporting risk estimates linking occupational noise and IHD/MI, or raw data that could be used to compute risk estimates. If several studies were based on the same population or dataset, we used the one with highest quality and most thorough reporting − for example, facing the choice between Willich et al.[18] and Kersten and Backé,[19] we included the latter because the authors used objective noise assessment for the effect sizes of interest; another issue to consider was that Babisch et al.[20] scrutinized Willich et al.[18] for mistreating their environmental noise data by choosing inadequate reference category; this argument was made for residential exposure, but we could not determine whether it also affected occupational exposure data. The reference lists of all included studies and previous reviews on occupational risks for IHD/MI and cardiovascular effects of noise were also hand searched. Experts in noise research and cardiovascular health were contacted with a request to search their personal archives for unpublished material; authors were asked to provide their articles that we did not have access to the full texts of or to mine their datasets for additional information, if it was not reported. Overall, we were able to retrieve data from 15 studies (11 for IHD morbidity and four for IHD mortality as an outcome). The agreement on these studies was assessed using Krippendorff's alpha coefficient[21] and it was high (>0.76) for the different selection criteria. Figure 1 presents a flow chart of the search protocol. (For the list of excluded studies and reasons for exclusion, see Appendix.)
Figure 1.
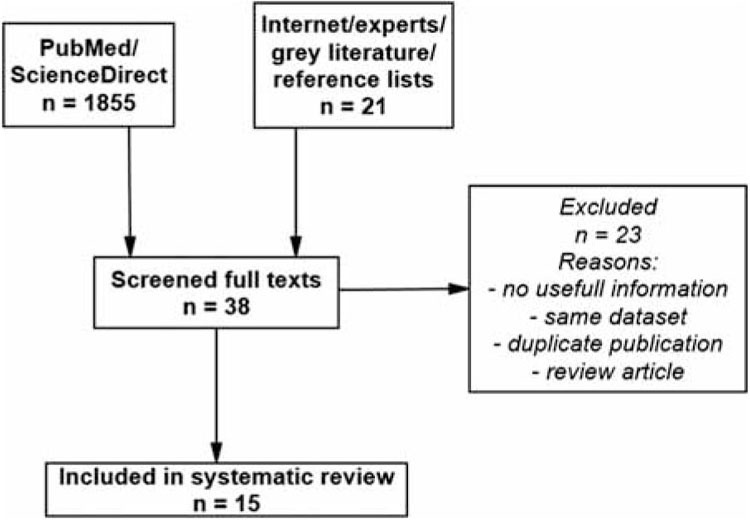
Flow chart of the study selection process gr1
Data extraction and quality assessment
The selected outcome indicator was IHD (ICD-10: I20–I25 and ICD-9/ICD-8: 410–414). Some studies reported risks for MI, in which case the risk for MI was considered as a proxy for the risk of IHD.[2] With respect to different noise exposure groups, for our main analysis, we extracted effect sizes associated with comparable groups and durations of exposure. For Virkkunen et al.,[22] we used the risk estimate for “continuous” noise exposure, and for Gopinath et al.,[23] we gave preference to the comparison “unable to hear speech” versus “none” as opposed to “>5 years of exposure” versus “0 years”. For Davies et al.[24], we included the estimate from their model, which used an internal low-exposure group as controls. Combined results from the two sites studied by McNamee et al.[25] were used. Information on the study by Bellach et al.[26] was obtained from other publications because of technological and language restrictions.[27,28,29,30]
Self-rated exposure was transformed to corresponding decibels for presentation purposes and to facilitate interpretation. If studies measured noise exposure with a question on workers’ need to raise their voice (vocal effort), we assumed this to represent a threshold of 66 dB, unless otherwise specified by the authors (ISO 9921/1 cited by Willich et al.[18] and Lazarus[31]). When relevant data was reported only in graphs, we extracted these values using GetData Graph Digitizer v. 2.26 (http://www.getdata-graph-digitizer.com/). Owing to the fact that some studies took into consideration participants’ hearing status when estimating their exposure, we extracted the risk estimates reported in the subgroup without hearing protection from the study of Davies et al.,[24] to ensure comparability with the other studies.
Some articles reported combined risk for men and women, others reported separate analyses, and some analyzed male-only samples. On the basis of prevalence of those reporting risks for males alone and the arguments of Babisch[32] (ie, CVDs are more common in men, male blue-collar workers are predominantly working in high-noise places, if necessary, risks might be generalized to both the genders), when we faced a choice between risk estimates for males and females, we gave priority to the estimates for males.
We report results for IHD morbidity and mortality separately. In the subsections Sensitivity analyses, we also discuss changes in some effects, if different subgroups were included in the synthesis, for example, women instead of men or an alternative exposure threshold.
To quantify differences between studies and their limitations, we developed a predefined list of safeguards against methodological bias, which was used to rate each study's quality. For the purposes of this systematic review, we modified a previously checklist[7] (see Supplementary Table S1). Study information was double coded and stored in a Microsoft Excel (Version 2010) spreadsheet. Studies were classified as of high, moderate, or low quality according to the observed empirical percentile distribution of the quality scores: ≥31, 24.5–31, and <24.5, respectively. The thresholds of 31 and 24.5 corresponded to 73.81 and 58.33% of the maximum possible quality score, respectively. The scoring protocol was subjective, but it was refined through expert discussions. Each quality element was rated according to its relative importance and contribution to the overall score.
Table S1.
Methodological quality checklist
| 1. Design |
|---|
| a. Cohort (4) |
| b. Case-control (3) |
| c. Cross-sectional (2) |
| d. Using aggregated data (1) |
| 2. Timeframe |
| a. Reported (0.5) |
| b. Unknown (0) |
| 3. Country where the study was carried out |
| a. With good working and living conditions AND/OR high socio-economic standard (2) |
| b. Difficult conditions AND/OR lower socio-economic standard (1.5) |
| c. Very difficult conditions AND/OR very low socioeconomic standard (1) |
| d. Unknown (0) |
| 4. Mode of selection |
| a. Random AND/OR considerable part OR the whole target population (3) |
| b. Non-random OR unknown (0) |
| 5. Response rate |
| a. ≥80% OR, if not reported, considerable part of the target population (3) |
| b. 60–80% (1) |
| c. <60% OR unknown (0) |
| 6. Final sample size |
| a. Completely satisfactory AND/OR justified by power analysis OR total population (3) |
| b. Somewhat satisfactory AND no statistical justification (1) |
| c. Not satisfactory AND no statistical justification (0) |
| 7. Participants |
| a. Clearly described (1) |
| b. Ambiguous description OR unknown (0) |
| 8. Representativeness of the sample |
| a. Representative of the population/whole population (3) |
| b. Somewhat representative (1) |
| c. Not representative/unknown (0) |
| 9. Definition and assessment of ischemic heart disease/myocardial infarction |
| a. Official WHO diagnostic criteria/official registry/database/records/physician-diagnosed (3) |
| b. Self-reported (1) |
| 10. Assessment of noise exposure |
| a. Long term personal dosimetry/at-site measurement (3) |
| b. Job-exposure matrix/hygienists assessment (2) |
| c. Self-report noisiness/vocal effort (1) |
| d. Unknown method (0) |
| 11. Biological plausibility for ischemic heart disease/myocardial infarction given the overall noise exposure and the compared noise exposure categories |
| a. Plausible (1.5) |
| b. Speculative (0.5) |
| c. Unplausible/unknown (0) |
| 12. Adjustments for personal covariates |
| a. All/almost all of the relevant covariates (age, smoking, dyslipidemia, abdominal obesity, diabetes, history of hypertension, psychosocial factors, unhealthy diet, regular |
| alcohol consumption, irregular physical activity) (5) |
| b. Some of the covariates (2) |
| c. No adjustments OR none of the above included (0) |
| 13. Adjustments for environmental covariates |
| a. Other occupational AND residential risks (eg, air pollution, chemicals, noise, shift work, etc) (4) |
| b. Other occupation OR residential risks (2) |
| c. No adjustments (0) |
| 14. Effect size calculation for meta-analysis |
| a. No transformations AND no data imputation OR transformations not creating bias (3) |
| b. Transformations creating bias OR data imputation (1) |
| c. Transformations creating bias AND data imputation (0) |
| 15. Additional transformations/imputation to the data AND/OR other source of bias associated with data extraction/interpretation |
| a. None (3) |
| b. Creating minor bias (1) |
| c. Major source of bias (0) |
Data analysis
A quantitative meta-analysis under the quality-effects model[33,34] was initially planned in the review protocol. Heterogeneity was to be assessed using the χ2 test and quantified across studies by the I2 statistic[35] − I2 < 30% (mild), I2=31–50% (moderate), and I2 > 50% (high).[36] Sensitivity analyses were to explore the impact of different study characteristics on the summary effect by excluding studies and pooling the remaining ones. Funnel plot under the fixed-effects model was to be used to check for asymmetry.[37] However, because of the unresolvable differences in effect sizes and, more importantly, exposure metrics across studies, we undertook a qualitative method of data synthesis to answer the review question.
RESULTS
IHD morbidity
Eleven studies contained data linking occupational noise to IHD [Table 1]. Four were designed as cross-sectional, or the nature of the relationship we were interested in was treated as such, six were case–control or nested case–control studies, and one was cohort study. Studies were conducted in high-income countries, with the exception of a small Bulgarian study by Dimitrova and Karaslavova[39] investigating the link between air pollution levels and hospital admissions because of MI. Other small studies were those of Jovanovic et al.[41] and Thériault et al.,[42] based on several hundred participants. Most samples were drawn from community surveys, whereas Jovanovic et al.[41] and Thériault et al.[42] specifically surveyed blue-collar workers, and Dimitrova and Karaslavova[39] included hospitalized patients and controls who underwent prophylactic examinations. Five articles reported summary risks for both the genders and four analyzed male-only samples; for Kersten and Backé[19] and Dimitrova and Karaslavova,[39] we extracted the effect sizes for males.
Table 1.
Characteristics of Studies Included in Systematic Review Under Comparable Scenarios
| Study | Design | Population | Outcome | Compared noise exposure categories | Duration of exposure | Definition/assessment of outcome | Assessment of noise | Analysis and effect size | Adjustments | Sex | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischemic heart disease morbidity subgroup | |||||||||||
| Kersten and Backé[19] | Case control (1998–2001) | 1527 male cases and 1527 men controls (20–69 years); German study | MI morbidity | 62–84 vs 46–61 dB | Preceding 10 years | Hospital records | Work history + validation (ISO 1999) | Conditional logistic regression (sex, age, and hospital match); OR = 0.89 (0.74, 1.06) | Employment status, shift work, work >40 h/week, <12 years at school | M | H |
| Selander et al.[38] | Case control (1992–1994) | 1252 cases and 1798 controls (45–70 years); Stockholm Heart Epidemiology Program | MI morbidity | >75 vs <75 dB | >1 year | Hospital records | Job exposure matrix | Logistic regression; OR = 1.17 (0.98, 1.41) | Age, sex, hospital catchment area, physical inactivity, smoking, air pollution, socioeconomic position | M +F | H |
| Gan et al.[9] | Cross-sectional (1999–2004) | n=6307 (≥20 years), 416 long term (24 cases) vs 5071 never exposed; National Health and Nutrition Examination Survey; United States | CHD (IHD) morbidity | >66 vs. <66 dB (speak in raised voice) | 1.6–18.8 years | Self-report | Self-report (vocal effort) | Logistic regression; OR = 2.04 (1.16, 3.58) | Age, sex, race/ethnicity, BMI, education, physical activity, smoking, diabetes, income, alcohol, waist circumference, total cholesterol | M +F | Mo |
| Gopinath et al.[23] | Cross-sectional (1997–1999) | n=2796 (>50 years), 245 unable to hear speech vs 1909 none exposure; Blue Mountains Eye Study, Australia | MI morbidity | Unable hear speech vs none | >1 year | Self-report | Self-report (perceived noisiness and hearing) | Logistic regression; OR = 1.19 (0.66, 2.16) | Age, sex, occupational prestige, BMI, mean BP, physical activity, total dietary fat, self-reported poor health for morbidity | M +F | Mo |
| Dimitrova and Karaslavova[39] | Case control (2004–2005) | 253 hospitalized cases and 1677 controls (<65 years), men (202/1452); Bulgaria | MI morbidity | Working under excess production noise vs no excess production noise | Unknown | Clinical evaluation in hospital | Self-report (perceived noisiness) | Logistic regression; OR = 0.680 (0.379, 1.220) | None | M | Mo |
| Fujino et al.[40] | Cross-sectional (1988–1990) | 14,568 male workers (40–59 years); Japan collaborative cohort | MI morbidity | Perceived extreme noise vs none | Lifetime exposure at work | Self-report | Self-report (perceived noisiness) | 2×2 table; OR = 1.58 (1.15, 2.16) | None | M | L |
| Virkkunen et al.[22] | Cohort (1982–1999) | 6005 Finnish industrially employed men (40–56 years); Helsinki heart study | IHD morbidity | >80 vs <80 dB (continuous noise only) | 18 years follow-up (occupation data assessed at one-point in time; duration of exposure unknown) | Hospital register; ICD-8/9: 410–414 | Job exposure matrix | Cox’s proportional hazards regression; HR = 1.27 (1.13, 1.44) | Age, SBP, total cholesterol, smoking, BMI | M | Mo |
| Ising et al.[27] | Case control (not specified) | 395 cases and 2148 controls (31–65 years), exposed cases/controls: 71/397, unexposed: 149/1221; Berlin traffic noise study | MI morbidity | Electric lawn-mower (70–88 dB) vs refrigerator + typewriter (<67 dB) | Not specified | Hospital records; ICD-9: 410 | Self-report (common noise sources) + validation | Logistic regression; OR = 1.4 (1.03, 1.97) | Age, smoking, BMI, social class, education, marital status, shift work, housing area | M | Mo |
| Jovanovic et al.[41] | Case control | 180 metal industry workers (48.9 ± 10.3 years) and 90 control workers (49.1 ± 11.9 years) | CHD (IHD) morbidity | >80 vs. <80 dB | Mean exposure for cases = 19.24 ± 7.6 and 19.8 ± 8.4 for controls | Clinical examination | Objective but unknown method | 2×2 table: 10 cases in exposed group and 1 in control; OR = 5.24 (0.66, 41.56) | Groups statistically not different on age, duration of exposure, sex, obesity, smoking, hereditary predisposition to cardiovascular diseases | M +F | L |
| Bellach et al.[26] | Cross-sectional | n=1002 (40–65 years); Germany | MI morbidity | Perceived noise vs no noise | 11 years of follow-up; exposure duration unknown | Self-report | Self-report | Unknown, possibly logistic regression; OR = 2.78 (1.01, 7.63) | Age, sex, BMI, smoking, alcohol, social status, work conditions, physical activity, neuropsychic disorders | M +F | L |
| Thériault et al.[42] | Case-control (1975–1984) | 306 cases (52.2 ± 6.6 years at diagnosis) and 575 controls (aluminum smelter workers) | IHD morbidity | Median level of exposure vs not exposed; weighted by years of exposure | Lifetime exposure weighting | Medical records, clinical evaluation | Work history + hygienists assessment | Conditional logistic regression (birth date, hiring date, and length of service-match); OR = 0.93 (0.56, 1.54) | High BP, hyperglycemia, hypercholesterolemia | M | Mo |
| Ischemic heart disease mortality subgroup | |||||||||||
| Suadicani et al.[43] | Cohort (1970–1986) | 2998 men (53–75 years); Copenhagen male study | IHD mortality | >66 vs <66 dB (speak in raised voice) | >5 years | Official registry; ICD-8: 410–412 | Self-report (vocal effort) | Cox’s proportional hazards regression; HR = 0.97 (0.71, 1.33) | Age, physical activity, smoking, alcohol, type 2 diabetes/glycosuria, hypertension, blood lipids, social class | M | Mo |
| McNamee et al.[25] | Case-control (1950–1998) | 1101 case–control pairs nuclear power workers (<75 years), 583 exposed, 65% cases; two sites in England | IHD mortality | >85 vs. <85 dB | 10–19.9 years | Official registry; ICD-9: 410–414 | Work history (hygienists assessment); adjusted for hearing protection | Conditional logistic regression (age and year of starting work-match); OR = 0.95 (0.70, 1.30) | BP, BMI, smoking, height, duration of employment | M | H |
| Davies et al.[24] | Cohort (1950–1995) | n=27,464; blue-collar workers from 14 lumber mills in British Columbia (without hearing protection = 8668, 517 cases total, 57 exposed cases) | MI mortality | >85 dB vs <3 years exposure | 10–19 years | Canadian mortality database; ICD-9: 410–410.9 | Prediction regression (work history + dosimetry) | Poisson regression; RR = 1.1 (0.84, 1.49) | Age, calendar year, South Asian ethnicity | M | H |
| Virtanen Notkola[44] | Cohort (follow-up: 1981–1994) | (25–64), 8378 MI deaths, 7.4% exposed cases; Finnish longitudinal census file | MI mortality | High exposure (upper quartile) vs unexposed | 14 years follow-up | Official register; ICD-9: 410 | Job exposure matrix | Poisson regression; rate ratio = 1.10 (0.99, 1.22) | None | M | Mo |
As regards the outcome, four defined it as IHD/CHD; for the remaining, it was MI. Four studies, which did not define the outcome on the basis of official records or clinical examinations, relied on self-reported diagnosis. Six studies determined noise exposure through self-reported noisiness or vocal effort. The remaining relied on job-exposure matrices (JEMs) or assessment by industrial hygienists. Selander et al.[38] used a JEM derived from measurements performed in Sweden during the period 1970–2004; it included 320 occupations and 35 different measurements per occupation. Virkkunen et al.,[22] used the Finnish JEM, covering occupational exposures since 1945 in Finland, based on the assessment by some 20 experts from the Finnish Institute of Occupational Health. Thériault et al.[42] collected detailed occupational histories and classified participants’ exposure based on “measurements made by hygienists in the 1970–1975 period (when available), or by extrapolation from measurements made for jobs with similar conditions”. Kersten and Backé[19] estimated daily exposure levels for 10 years, retrospectively, “from information about workplaces and machines with the help of catalog specifications”. The method for objective noise assessment was not reported in the article by Jovanovic et al.[41] Kersten and Backé[19] carried out validation measurements on 146 workplaces; Ising et al.[27] also validated their perceived noisiness measure in a subsample of 80 men.
Most studies analyzed their data with unconditional or conditional logistic regression, matching cases and controls. The only cohort study reported results from the Cox's proportional hazards model.[22] For Fujino et al.[40] and Jovanovic et al.,[41] we calculated odds ratio (OR) from raw data; Dimitrova and Karaslavova[39] reported no adjustments; the others adjusted their regression models for most of the relevant personal confounders and/or did some kind of case–control matching. Kersten and Backé[19] reduced the number of covariates reported by a previous study on the same dataset[18] through directed acyclic graph. Environmental covariates were not controlled for in most of the models. Virkkunen et al.,[22] Kersten and Backé,[19] and Selander et al.[38] received the highest quality scores in this subgroup. (See Supplementary Table S2 for individual quality scores.
Table S2.
Quality scores of included studies for different methodological elements
| Study | Design | Timeframe | Country | Selection | Response rate | Sample size | Participants | Representatives | Definition of IHD/MI | Assessment of noise | Biological plausibility | Personal covariates | Environmental covariates | Effect size calculation | Additional transformations | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gan et al. (2011) | 2 | 0.5 | 2 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 1.5 | 5 | 0 | 3 | 1 (assigned noise levels) | 28 (moderate) |
| Ising et al. (1997) | 3 | 0 | 2 | 3 | 1 | 3 | 1 | 3 | 3 | 1 | 0.5 | 2 | 2 | 3 | 1(assigned noise levels) | 28.5 (moderate) |
| Selander et al. (2013) | 3 | 0.5 | 2 | 3 | 1 | 3 | 1 | 3 | 3 | 2 | 0.5 (duration of exposure unknown) | 2 | 2 | 3 | 3 | 32 (high) |
| Kersten and Backé (2015) | 3 | 0.5 (obtained from another study) | 2 | 3 | 3 | 3 | 1 | 3 | 3 | 2.5 (validation measurements) | 1.5 | 2 (used directed acyclic graph) | 2 (used directed acyclic graph) | 3 | 3 | 35.5 (high) |
| Virkkunen et al. (2005) | 4 | 0.5 | 2 | 3 | 0 | 3 | 1 | 3 | 3 | 2 | 0.5 (duration of exposure unknown) | 2 | 0 | 1 (HR combined with OR) | 3 | 28 (moderate) |
| Bellach et al. (1995) | 2 | 0 | 2 | 3 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 0 (cited in other sources) | 0 (primary study unavailable) | 16 (low) |
| McNamee et al. (2006) | 3 | 0.5 | 2 | 3 | 3 | 3 | 1 | 3 | 3 | 2 | 1.5 | 2 | 0 | 1 (OR combined with HR/RR/Rate ratio) | 3 | 31 (high) |
| Davies et al. (2005) | 4 | 0.5 | 2 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 1.5 | 2 | 0 | 3 | 3 | 35 (high) |
| Suadicani et al. (2012) | 4 | 0.5 | 2 | 3 | 1 | 3 | 1 | 1 | 3 | 1 | 1.5 | 5 | 0 | 3 | 1 (assigned noise levels) | 30 (moderate) |
| Virtanen and Notkola (2002) | 4 | 0.5 | 2 | 3 | 3 | 3 | 1 | 3 | 3 | 2 | 1.5 | 0 | 0 | 3 | 0 (unassigned noise levels) | 29 (moderate) |
| Gopinath et al. (2011) | 2 | 0.5 | 2 | 3 | 1 | 3 | 1 | 3 | 1 | 1 | 1.5 | 5 | 0 | 3 | 0 (unassigned noise levels) | 27 (moderate) |
| Fujino et al. (2007) | 2 | 0.5 | 2 | 3 | 3 | 3 | 1 | 3 | 1 | 1 | 1.5 | 0 | 0 | 1 (computed by us, rounding error) | 0 (unassigned noise levels) | 22 (low) |
| Jovanovic et al. (1997) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1.5 | 1 (compared groups, but not matched groups) | 0 | 1 (computed by us, rounding error, standard continuity correction of 0.5 added to each cell) | 3 | 11.5 (low) |
| Theriault et al. (1988) | 3 | 0.5 | 0 | 3 | 3 | 3 | 1 | 3 | 3 | 2 | 1.5 | 2 | 0 | 3 | 1 (assigned noise levels) | 29 (moderate) |
| Dimitrova and Karaslavova (2008) | 3 | 0.5 | 1.5 | 3 | 3 | 3 | 0 | 3 | 3 | 1 | 1.5 | 0 | 0 | 3 | 0 (unassigned noise levels) | 24.5 (moderate) |
Overall, studies relying on objective noise assessment (JEM/assessment by industrial hygienists) were somewhat discordant.[19,22,38,41] Three of those found elevated risk for IHD, but it was statistically significant only in the study by Virkkunen et al.[22] In the study by Selander et al.,[38] the OR for the comparison >75 dB versus <75 dB was marginally significant. Jovanovic et al.[41] analyzed very small sample and we had to calculate OR from raw data, which might explain the wide CI of the estimate. Their objective method for exposure assessment was not reported, which further lowered the overall quality score. Thériault et al.[42] found no effect at all. In contrast, self-rated noise exposure was consistently linked to higher risk for IHD, with only two (out of six) studies reporting nonsignificantly higher OR[23] or no effect.[36]
Sensitivity analyses
Using a higher noise threshold (95–124 vs 46–61 dB), Kersten and Backé[19] found significant OR to be 2.18 (95% CI: 1.17, 4.05) among the 54 participants (cases/controls: 39/15) with very high exposure. Comparing the self-rated exposure categories “pneumatic drill (≈100 dB)” (cases/controls: 87/172) versus “refrigerator + typewriter (≈<67 dB)” (149/1221), Ising et al.[27] more than doubled the observed OR. Gopinath et al.[23] actually found higher OR in the lower exposure category “tolerable” noise (n=623) (OR = 1.40, 95% CI: 0.91, 2.14), probably because of the higher number of participants in it, as opposed to the category “unable to hear speech” (n=245). Of note, the OR in Gan et al.[9] was lower (OR = 1.59, 95% CI: 0.73, 3.49) for MI (12 cases exposed for 1.6–18.8 years), than it was for CHD.
Women seemed to be more adversely affected by occupational noise than men. Kersten and Backé[19] studied 353 female cases and 706 controls and found OR to be 1.24 (95% CI: 0.91, 1.69) and OR to be 1.22 (95% CI: 0.34, 4.32) among women exposed to 62–84 and 85–94 dB, respectively, whereas among men, the OR in these categories was below 1.00, indicating no effect. In the study by Dimitrova and Karaslavova,[39] there was no elevated risk among men, whereas they got OR = 4.01 (95% CI: 0.84, 19.11) among women (n=57 and 716, respectively). Gan et al.,[9] on the contrary, reported statistically significantly higher OR for CHD only among men (currently exposed vs never exposed), and not women.
IHD mortality
Only four studies were included in this subgroup. They originated from countries with high socioeconomic standard. Only one was a matched case–control study, whereas the remaining were based on large cohorts [Table 1]. Davies et al.[24] analyzed the largest sample size (nearly 30,000 lumber mill workers), but we only considered estimates from a subgroup analysis without hearing protection. These data referred to male participants only. IHD and MI were defined and assessed according to objective medical criteria and ICD codes. Noise exposure was also derived via methods independent of participants’ perception, except for Suadicani et al.[43] who used self-reported vocal effort.
In most of the studies, RRs, hazard ratios (HRs), and rate ratios were derived from regression models for longitudinal designs, whereas McNamee et al.[25] reported OR from conditional logistic regression. Covariate adjustments were acceptable. Davies et al.[24] was the study with highest quality score in this subgroup. (See Supplementary Table S2.)
Results were insufficient to draw valid conclusions. The only study that used objective exposure assessment and found elevated risk of MI was that of Davies et al.,[24] but it failed statistical significance. The authors reported RR=1.1 (0.84, 1.49) for >85 dB for 10–19 years, whereas McNamee et al.[25] found no effect (OR = 0.95, 0.70, 1.30) under the same exposure conditions. Only one of the two studies relying on self-rated exposure reported higher risk.[44]
Sensitivity analyses
A significant RR (=1.51; 95% CI: 1.08, 2.11) was reported by Davies et al.[24] among people without hearing protection and exposed to >95 dB for >19 years (n=37 cases) versus those exposed for <3 years (n=354 cases). When they considered exposure during participants’ working years, when they were presumably still exposed to noise, the authors observed the highest RRs in the study (RR=4.0, 95% CI: 1.8, 9.3). The HR for IHD mortality in the study of Suadicani et al.[43] was elevated only among participants from the lower social class (599 exposed, 8.4% cases vs 853 controls, 7.0% cases) (HR=1.13, 95% CI: 0.75, 1.68). Finally, McNamee et al.[25] recruited workers from two sites in England (n=583, exposed >10 years; n=186, >20 years) and actually found higher risk (OR = 1.21, 95% CI: 0.80, 1.83) only at site B (exposed cases and controls: 59 and 53%, respectively) for exposure to >85 dB for 10–19.9 years. Noteworthy, at this site B, the predictive validity of their noise exposure data had been confirmed, whereas at site A, the predictive validity was poor.
DISCUSSION
Key results
There is some evidence to suggest higher risk for IHD among workers exposed to objectively measured noise >75–80 dB, which was supported by one high, one moderate, and one low quality study, and opposed by one high and one moderate quality studies, which found no effect. The elevated risks, however, were not statistically significant (except for Virkkunen et al.[22]). If a higher threshold was used in Kersten and Backé[19] to contrast exposure groups, the OR rose above 1.00 and became significant, strengthening the evidence in this subgroup of studies. Three moderate and two low quality studies found self-rated noise exposure to be associated with higher risk for IHD, and only one moderate quality study found no effect. Although inconclusive, results suggest that occupational noise is a risk factor for IHD morbidity. Some studies actually showed considerably higher risk among women.[19,36]
Regarding IHD mortality, only four studies were found. If we compared similar exposure groups across studies, one self-rated study of moderate quality[44] and one of high quality[24] suggested higher risk for IHD, but they were inconclusive. However, three of the studies found elevated risk among some subgroup − for Davies et al.[24], it was workers exposed to higher noise levels or those exposed during their working years; for Suadicani et al.,[43] it was workers from the lower social classes; and for McNamee et al.,[25] elevated risk was found only in one of the recruitment sites where the validity of their noise indicator was higher.
It should be noted that although some risk estimates failed statistical significance, this should not be viewed as evidence of no effect, because in epidemiology statistical significance is sometimes misleading and the 95% CI should be interpreted mainly as an indicator of the precision of the point estimate, and not as a P value.[45,46] Furthermore, treating the risk for MI morbidity as a proxy for the risk for IHD renders the results conservative.
To our knowledge, this was the first thorough systematic review on occupational noise and the risk for IHD, which applied stringent criteria for data synthesis and interpretation. Through an exhaustive literature search and despite our limited resources, we were able to obtain primary studies, which were not previously discussed by narrative reviews or authors of articles in this field. We also developed a methodological quality checklist safeguarding against some sources of bias. In conjunction with the quality effects estimator, it will improve the quality of future meta-analyses.[47]
Limitations
Owing to unresolvable discrepancies across studies (different exposure assessment methods, statistical tests, and designs, including participants with different characteristics and background), a quantitative meta-analysis was not feasible. The reader should also bear in mind that we were unable to retrieve some articles with alleged relevance to the research question or extract necessary data from others. Abstracted information on Bellach et al.[26] was obtained from other publications, and this was reflected in the low-quality weight of the study and its exclusion did not affect the overall effect. Critics might argue that the quality of data from some included studies was poor. Noise assessment in occupational studies is not an easy task and nearly every method is flawed in some way. Perceived exposure might add unique explanatory power to the analyses and is considered a valid exposure metric in some cases.[48,49,50]
Our choice of how to treat gender differences was justified in Methods section and we further presented sensitivity analyses comparing risk among men and women. It was not appropriate to include the risks for males and females from the same study as if they came from different studies, because this raises issues of statistical dependency − although male and female subsamples do not share subjects, being studies by the same investigator introduces bias.[51] Also results should be interpreted with caution because of gender differences in people's general stress-reaction.[52]
The definition of IHD across studies differed. With respect to IHD mortality, MI is usually the cause of fatal IHD and therefore both conditions were used interchangeably: “the incidence of MI in a population can be used as a proxy for estimating the coronary heart disease burden”.[2] Nevertheless, the number of included studies for IHD mortality is low and makes the conclusions weak. For morbidity, however, some of the studies reported effects only for MI and, given that MI is one of the types of IHD, treating MI as a proxy for IHD renders our results conservative.
Future research
The results of this systematic review might be too crude, but they are certainly not without merit. It gives us better idea of the impact of occupational noise on cardiovascular health. Workers exposed to high occupational noise should be considered at higher risk for IHD and routinely screened for other cardiovascular risk factors. According to the precautionary principle, “in cases of serious or irreversible threats to the health of humans or ecosystems, acknowledged scientific uncertainty should not be used as a reason to postpone preventive measures”.[53]
We also highlight the heterogeneity in methodologies of different study, which hinder construction of exposure–response functions. Current evidence is not only scarce in comparison to traffic noise research. Studies on occupational noise need to ascertain adequate exposure assessment through a combination of noise dosimetry, detailed work history, and perceived exposure. Moreover, psychological stress caused by noise, workers’ attitudes toward it, and their individual noise sensitivity have been neglected and might help us to gain a deeper understanding of the issue. Whether and to what extent hearing impairment modifies the cardiovascular effects of noise, the joint and separate effects of continuous and impulse noises, and healthy worker/survivor effects are of general interest. Effects among female workers (including pregnant and postmenopausal) should be more extensively studied. Finally, in addition to hearing protection and physical reduction of noise, organizational, behavioral, and psychosocial interventions might help mitigate the negative reaction to occupational noise. Future research needs to focus on not only exploring noise as a risk factor for CVD, but also the effectiveness of different types of interventions against it.
CONCLUSIONS
Despite the methodological discrepancies and limitations of included studies, occupational noise appeared to be a risk factor for IHD morbidity. Results suggested higher risk for IHD mortality only among vulnerable subgroups and merit caution. Nevertheless, even under our conservative assumptions, we suggest that workers exposed to high occupational noise should be considered at higher risk of IHD and routinely screened for other cardiovascular risk factors. Further evidence is needed to quantify this detrimental effect and understand how this risk can be managed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
APPENDIX:LIST OF STUDIES EXCLUDED AFTER FULL TEXT SCREENING AND REASONS FOR EXCLUSION
Based on the same dataset as already included study:
-
(1)
Willich SN, Wegscheider K, Stallmann M, Keil T. Noise burden and the risk of myocardial infarction. Eur Heart J 2006;27:276-82.
-
(2)
Keil T, Schust M, Stark H, Stallmann M, Babisch W, Wegscheider K, Willich SN. Chronic work noise and myocardial infarction: Results from the NARoMi (Noise and Risk of Myocardial infarction)-Study. In: de Jong RG, Houtgast T, Franssen EAM, Hofman WF, editors. Proceedings of the 8th International Congress on Noise as a Public Health Problem. Schiedam: Foundation ICBEN 2003. 2003. p. 121.
-
(3)
Virkkunen H, Härmä M, Kauppinen T, Tenkanen L. Shift work, occupational noise and physical workload with ensuing development of blood pressure and their joint effect on the risk of coronary heart disease. Scand J Work Environ Health 2007;33:425-34.
-
(4)
Virkkunen H, Härmä M, Kauppinen T, Tenkanen L. The triad of shift work, occupational noise, and physical workload and risk of coronary heart disease. Occup Environ Med 2006;63:378-86.
-
(5)
Koskinen HL, Kauppinen T, Tenkanen L. Dual role of physical workload and occupational noise in the association of the metabolic syndrome with risk of coronary heart disease: Findings from the Helsinki Heart Study. Occup Environ Med 2011;68:666-73.
Double publication of already included material:
1. Ising H, Babisch W, Günther T. Work noise as a risk factor in myocardial infarction. J Clin Basic Cardiol 2007;2:64-8.
Useful information about ischemic heart disease/myocardial infarction and noise could not be extracted:
-
(1)
Olsen O, Kristensen TS. Impact of work environment on cardiovascular diseases in Denmark. J Epidemiol Community Health 2007;45:4-9.
-
(2)
Karnaukh NG, Petrov GA, Dvornichenko GB. Occupationally related morbidity in operators staff workers of metallurgical industry. Ukrainskii Zhurnal z Problem Meditzini Praz 2006;2:3-7.
-
(3)
Hilt B, Qvenild T, Rømyhr O. Morbidity from ischemic heart disease in workers at a stainless steel welding factory. Norsk Epidemiologi 1999;9:21-6.
-
(4)
Izmerov NF, Tikhonova GI, Gorchakova TYu. Working conditions and mortality among men of working age in Russia (experience of Murmansk Region) [in Russian]. Vestn Ross Akad Med Nauk 2007;9:32-6.
-
(5)
Casal EJR. Contaminación acústica: efectos sobre parámetros físicos y psicológicos [in Spanish]. PhD Thesis. Universidad de La Laguna. 1997.
-
(6)
Rivera AL. Intensidad de ruido a la que se exponen los maestros en una escuela superior de la región central de Puerto Rico y su percepción al respeto [in Spanish]. MSc Thesis San Juan: Universidad Metropolitana. 2009.
-
(7)
Sánchez García MJ, Santiago FH. Estudio de las condiciones de trabajo de los conductores de vehículos de carga en Colombia para proponer mejoras en los puestos de trabajo [in Spanish]. Bogota: Pontificia Universidad Javeriana. 2004.
-
(8)
Demina ID, Yukhananova LM, Fedina IN, Serebryakov PV. Evaluation of the influence of production factors on lipid metabolism in industrial workers. Laboratornaia Sluzhba 2012;2:26-9.
-
(9)
Girard SA, Leroux T, Verreault R, Courteau M, Picard M, Turcotte F, Baril J, Richer O. Cardiovascular disease mortality among retired workers chronically exposed to intense occupational noise. Int Arch Occup Environ Health 2015;88:123-30.
-
(10)
Deianov Ch, Vangelova K, Tzvetkov D, Kotzeva K, Stoyneva Zl, Hristov. Occupational risk factors, dyslipoproteinemias and cardiovascular risk [in Bulgarian]. Problemi na Higienata 2000;21:12-8.
-
(11)
Zlateva M, Ivanivich E, Antov G. The cardiovascular system and industrial factors [in Bulgarian]. Sofia: Meditzina i Fizkultura. 1984.
-
(12)
Andersson E, Persson B, Bryngelsson IL, Magnuson A, Torén K, Wingren G, Westberg H. Cohort mortality study of Swedish pulp and paper mill workersnonmalignant diseases. Scand J Work Environ Health 2007;33:470-8.
-
(13)
Bortkiewicz A, Gadzicka E, Siedlecka J, Szyjkowska A, Viebig P, Wranicz JK, et al. Work-related risk factors of myocardial infarction. Int J Occup Med Environ Health 2010;23:255-65.
Review article:
-
(1)
Ha J, Kim SG, Paek D, Park J. The magnitude of mortality from ischemic heart disease attributed to occupational factors in Korea: Attributable fraction estimation using meta-analysis. Saf Health Work 2011;2:70-82.
-
(2)
Telkova IL. Occupational characteristics and cardiovascular diseases: The risk of development and the challenges for prevention. Clinical-epidemiological analysis [in Russian]. Sibirskii Meditsinskii Zhurnal 2012;27:17-26.
-
(3)
Gómez M, Jaramillo JJ, Luna Y, Martínez A, Velásquez MA, Vásquez EM. Industrial noise: Effects on the health of workers exposed [in Spanish]. Rev CES Salud Pública 2012;3:174-83.
-
(4)
Fernandes M, Morata TC. Auditory and extra-auditory effects of occupational exposure to noise and vibration [in Portuguese]. Rev Bras Otorrinolaringol 2002;68:705-13.
REFERENCES
- 1.WHO Fact sheet: Cardiovascular diseases (CVDs) 2015. [Accessed April 3, 2015]. Available at: http://www.who.int/mediacentre/factsheets/en/
- 2.Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, Ngu Blackett K, Lisheng L. Writing group on behalf of the participating experts of the WHO consultation for revision of WHO definition of myocardial infarction. World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40:139–46. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- 3.Recio A, Linares C, Banegas JR, Díaz J. Road traffic noise effects on cardiovascular, respiratory, and metabolic health: An integrative model of biological mechanisms. Environ Res. 2016;146:359–70. doi: 10.1016/j.envres.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Spreng M. Central nervous system activation by noise. Noise Health. 2000;2:49–58. [PubMed] [Google Scholar]
- 5.Ising H, Kruppa B. Health effects caused by noise: evidence in the literature from the past 25 years. Noise Health. 2004;6:5–13. [PubMed] [Google Scholar]
- 6.Westman JC, Walters JR. Noise and stress: A comprehensive approach. Environ Health Perspect. 1981;41:291–309. doi: 10.1289/ehp.8141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzhambov AM. Long-term noise exposure and the risk for type 2 diabetes: A meta-analysis. Noise Health. 2015;17:23–33. doi: 10.4103/1463-1741.149571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzhambov A, Dimitrova D. Chronic noise exposure and testosterone deficiency: Meta-analysis and meta-regression of experimental studies in rodents. Endokrynol Pol. 2015;66:39–46. doi: 10.5603/EP.2015.0007. [DOI] [PubMed] [Google Scholar]
- 9.Gan WQ, Davies HW, Demers PA. Exposure to occupational noise and cardiovascular disease in the United States: The National Health and Nutrition Examination Survey 1999-2004. Occup Environ Med. 2011;68:183–90. doi: 10.1136/oem.2010.055269. [DOI] [PubMed] [Google Scholar]
- 10.WHO Regional Office for Europe. Burden of Disease from Environmental Noise. Quantification of Healthy Life Years Lost in Europe. Copenhagen: WHO Regional Office for Europe; 2011. [Google Scholar]
- 11.Münzel T, Gori T, Babisch W, Basner M. Cardiovascular effects of environmental noise exposure. Eur Heart J. 2014;35:829–36. doi: 10.1093/eurheartj/ehu030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eurofound. Fifth European Working Conditions Survey. Luxembourg: Publications Office of the European Union; 2012. [Google Scholar]
- 13.Directive 2003/10/EC of the European Parliament and of the Council of 6 February 2003 on the minimum health and safety requirements regarding the exposure of workers to the risks arising from physical agents (noise) Off J Eur Commun. 2003;L42((15.02)/38):38–44. [Google Scholar]
- 14.Van Kempen EE, Kruize H, Boshuizen HC, Ameling CB, Staatsen BA, de Hollander AE. The association between noise exposure and blood pressure and ischemic heart disease: A meta-analysis. Environ Health Perspect. 2002;110:307–17. doi: 10.1289/ehp.02110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomei G, Fioravanti M, Cerratti D, Sancini A, Tomao E, Rosati MV, et al. Occupational exposure to noise and the cardiovascular system: A meta-analysis. Sci Total Environ. 2010;408:681–9. doi: 10.1016/j.scitotenv.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 16.Ha J, Kim SG, Paek D, Park J. The magnitude of mortality from ischemic heart disease attributed to occupational factors in Korea: attributable fraction estimation using meta-analysis. Saf Health Work. 2011;2:70–82. doi: 10.5491/SHAW.2011.2.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 18.Willich SN, Wegscheider K, Stallmann M, Keil T. Noise burden and the risk of myocardial infarction. Eur Heart J. 2006;27:276–82. doi: 10.1093/eurheartj/ehi658. [DOI] [PubMed] [Google Scholar]
- 19.Kersten N, Backé E. Occupational noise and myocardial infarction: Considerations on the interrelation of noise with job demands. Noise Health. 2015;17:116–22. doi: 10.4103/1463-1741.153403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babisch W, Beule B, Ising H, Kersten N, Schust M, Wende H. Noise burden and the risk of myocardial infarction: False interpretation of results due to inadequate treatment of data. Eur Heart J. 2006;27:623–4. doi: 10.1093/eurheartj/ehi762. [DOI] [PubMed] [Google Scholar]
- 21.Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Meas. 2007;1:77–89. [Google Scholar]
- 22.Virkkunen H, Kauppinen T, Tenkanen L. Long-term effect of occupational noise on the risk of coronary heart disease. Scand J Work Environ Health. 2005;31:291–9. doi: 10.5271/sjweh.885. [DOI] [PubMed] [Google Scholar]
- 23.Gopinath B, Thiagalingam A, Teber E, Mitchell P. Exposure to workplace noise and the risk of cardiovascular disease events and mortality among older adults. Prev Med. 2011;53:390–4. doi: 10.1016/j.ypmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Davies HW, Teschke K, Kennedy SM, Hodgson MR, Hertzman C, Demers PA. Occupational exposure to noise and mortality from acute myocardial infarction. Epidemiology. 2005;16:25–32. doi: 10.1097/01.ede.0000147121.13399.bf. [DOI] [PubMed] [Google Scholar]
- 25.McNamee R, Burgess G, Dippnall WM, Cherry N. Occupational noise exposure and ischaemic heart disease mortality. Occup Environ Med. 2006;63:813–9. doi: 10.1136/oem.2005.026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellach B, Dortschy R, Muller D, Ziese T. Gesundheitliche Auswirkungen von Larmbelastung − Methodische Betrachtungen zu den Ergebnissen dreier epidemiologischer Studien. Bundesgesundhbl. 1995;38:84–9. [Google Scholar]
- 27.Ising H, Babisch W, Kruppa B, Lindthammer A, Wiens D. Subjective work noise: A major risk factor in myocardial infarction. Soz Praventivmed. 1997;42:216–22. doi: 10.1007/BF02298042. [DOI] [PubMed] [Google Scholar]
- 28.Babisch W. Epidemiological studies of the cardiovascular effects of occupational noise: A critical appraisal. Noise Health. 1998;1:24–39. [PubMed] [Google Scholar]
- 29.Maschke C. Cardiovascular effects of environmental noise: Research in Germany. Noise Health. 2011;13:205–11. doi: 10.4103/1463-1741.80150. [DOI] [PubMed] [Google Scholar]
- 30.Ndrepepa A, Twardella D. Relationship between noise annoyance from road traffic noise and cardiovascular diseases: A meta-analysis. Noise Health. 2011;13:251–9. doi: 10.4103/1463-1741.80163. [DOI] [PubMed] [Google Scholar]
- 31.Lazarus H. Prediction of verbal communication is noise: A review: Part 1. App Acoust. 1986;19:439–64. [Google Scholar]
- 32.Babisch W. Road traffic noise and cardiovascular risk. Noise Health. 2008;10:27–33. doi: 10.4103/1463-1741.39005. [DOI] [PubMed] [Google Scholar]
- 33.Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology. 2008;19:94–100. doi: 10.1097/EDE.0b013e31815c24e7. [DOI] [PubMed] [Google Scholar]
- 34.Doi SA, Thalib L. An alternative quality adjustor for the quality effects model for meta-analysis. Epidemiology. 2009;20:314. doi: 10.1097/EDE.0b013e318196a8d0. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; 2011. [Updated March 2011] [Accessed April 3, 2015]. Available to: www.cochrane-handbook.org .
- 38.Selander J, Bluhm G, Nilsson M, Hallqvist J, Theorell T, Willix P, Pershagen G. Joint effects of job strain and road-traffic and occupational noise on myocardial infarction. Scand J Work Environ Health. 2013;39:195–203. doi: 10.5271/sjweh.3324. [DOI] [PubMed] [Google Scholar]
- 39.Dimitrova T, Karaslavova E. Vibrations in the working environment and risk of acute myocardial infarction [in Bulgarian] Meditzinski Pregled. 2008;44:54–7. [Google Scholar]
- 40.Fujino Y, Iso H, Tamakoshi A JACC study group. A prospective cohort study of perceived noise exposure at work and cerebrovascular diseases among male workers in Japan. J Occup Health. 2007;49:382–8. doi: 10.1539/joh.49.382. [DOI] [PubMed] [Google Scholar]
- 41.Jovanovic J, Popovic V, Milosevic Z, Javanovic M. Cumulative effects of communal and industrial noise on cardiovascular system. Sci J Facta Univ. 1997;4:57–61. [Google Scholar]
- 42.Thériault GP, Tremblay CG, Armstrong BG. Risk of ischemic heart disease among primary aluminum production workers. Am J Ind Med. 1988;13:659–66. doi: 10.1002/ajim.4700130605. [DOI] [PubMed] [Google Scholar]
- 43.Suadicani P, Hein HO, Gyntelberg F. Occupational noise exposure, social class, and risk of ischemic heart disease and all-cause mortality: A 16-year follow-up in the Copenhagen Male Study. Scand J Work Environ Health. 2012;38:19–26. doi: 10.5271/sjweh.3200. [DOI] [PubMed] [Google Scholar]
- 44.Virtanen SV, Notkola V. Socioeconomic inequalities in cardiovascular mortality and the role of work: A register study of Finnish men. Int J Epidemiol. 2002;31:614–21. doi: 10.1093/ije/31.3.614. [DOI] [PubMed] [Google Scholar]
- 45.Poole C. Low P-values or narrow confidence intervals: Which are more durable? Epidemiology. 2001;12:291–4. doi: 10.1097/00001648-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Stang A, Poole C, Kuss O. The ongoing tyranny of statistical significance testing in biomedical research. Eur J Epidemiol. 2010;25:225–30. doi: 10.1007/s10654-010-9440-x. [DOI] [PubMed] [Google Scholar]
- 47.Doi SA. Evidence synthesis for medical decision making and the appropriate use of quality scores. Clin Med Res. 2014;12:40–6. doi: 10.3121/cmr.2013.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlaefer K, Schlehofer B, Schüz J. Validity of self-reported occupational noise exposure. Eur J Epidemiol. 2009;24:469–75. doi: 10.1007/s10654-009-9357-4. [DOI] [PubMed] [Google Scholar]
- 49.Neitzel R, Daniell W, Sheppard L, Davies H, Seixas N. Comparison of perceived and quantitative measures of occupational noise exposure. Ann Occup Hyg. 2009;53:41–54. doi: 10.1093/annhyg/men071. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed HO, Dennis JH, Ballal SG. The accuracy of self-reported high noise exposure level and hearing loss in a working population in Eastern Saudi Arabia. Int J Hyg Environ Health. 2004;207:227–34. doi: 10.1078/1438-4639-00291. [DOI] [PubMed] [Google Scholar]
- 51.Lipsey MW. Identifying interesting variables and analysis opportunities. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed. New York: Russell Sage Foundation; 2009. p. 149. [Google Scholar]
- 52.Verma R, Balhara YPS, Gupta CS. Gender differences in stress response: Role of developmental and biological determinants. Ind Psychiatry J. 2011;20:4–10. doi: 10.4103/0972-6748.98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martuzzi M, Tickner JA, editors. The precautionary principle: Protecting public health, the environment and the future of our children. Copenhagen: WHO; 2004. [Google Scholar]


