The nitrogen cycle is a key biogeochemical cycle on Earth, as nitrogen is an essential nutrient required for all life-forms. The largest source of bioavailable nitrogen originates from biological and synthetic nitrogen (N2) fixation, whereby N2 is converted to ammonia (NH3), which can then be incorporated into biomass by plants. In the developing world, soils often do not contain enough nitrogen to give large crop yields, so fertilizers, produced by synthetic nitrogen fixation, are critically important to supplement this lack of nitrogen. On the other hand, in the developed world, overfertilization is a common problem (1). In the soil, NH3-oxidizing bacteria (AOB) and archaea compete for the uptake of NH3 with plants, aerobically oxidizing it to nitrite () or nitrate (). The first step of this process is catalyzed by ammonia monooxygenase, producing hydroxylamine (NH2OH). Hydroxylamine oxidoreductase (HAO) then further oxidizes NH2OH to (2). These oxidation reactions provide soil microbes with reducing equivalents for ATP synthesis. Complementary to this process is denitrification, which ultimately reduces back down to N2 during anaerobic respiration, producing nitric oxide (NO) and nitrous oxide (N2O) in intermediate steps (3).
Release of both NO and N2O during these transformations is of global consequence, as NO participates in the depletion of the ozone layer, whereas N2O has become the third most significant greenhouse gas, with a global warming potential that is 300 times that of CO2 (4). With the increase in the use of fertilizer since the preindustrial era, global N2O emissions have increased substantially. Understanding the processes that generate N2O from fertilizer in soil and seawater is therefore essential in devising practical strategies to minimize its production.
AOBs are of particular interest in this regard, as they are capable not only of nitrification but also of denitrification, termed nitrifier denitrification. These AOBs have been implicated as significant contributors in increased N2O emissions (5, 6). Previous work has found that AOBs express nitrite and nitrous oxide reductases under anaerobic conditions, protecting against accumulation, and producing N2O as a direct product (7). Other studies have implicated aerobic NH2OH oxidation as another source of trace amounts of NO and N2O (8). Typically, NH2OH is oxidized to by HAOs, as mentioned above. These enzymes contain a catalytic heme P460 cofactor in the active site. It has been postulated that HAOs could incompletely oxidize NH2OH to HNO, which, after release from the active site, could then dimerize and dehydrate to form N2O and H2O. NO is another potential incomplete oxidation product of NH2OH, which could serve as a substrate for denitrification to produce N2O, or escape into the atmosphere (8). Now, work by Lancaster and coworkers (9) has revealed a potential pathway for the direct production of N2O by HAOs.
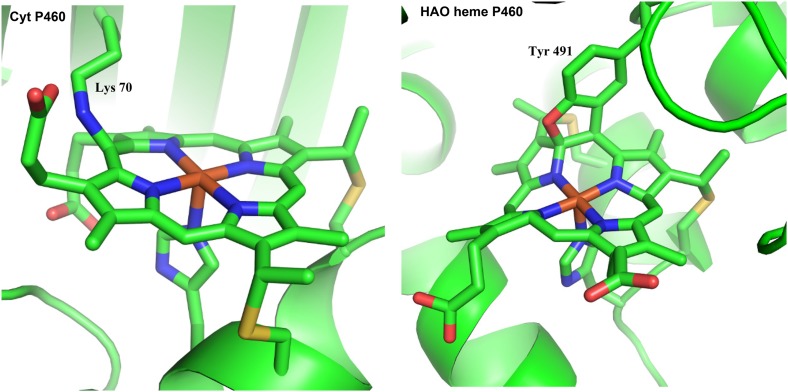
To better understand NH2OH oxidation, the Lancaster group studied the enzyme cyt P460 from the AOB Nitrosomonas europaea (10). N. europaea cyt P460 exists as a 36-kDa homodimer with a c-type heme in the active site that has an unusual N−C cross-link from the heme 13′ mesocarbon to the amine of Lys70 as shown in Fig. 1, Left (11). In contrast, the heme P460 cofactor in the active site of HAOs is doubly cross-linked by Tyr491 at the 5′ mesocarbon and the pyrrole α-carbon positions (Fig. 1, Right) (12). Despite this structural difference, studies on cyt P460s from other AOBs, Methylococcus capsulatus and Kuenenia stuttgartiensis, have broadly implicated cyt P460s in NH2OH oxidation (13, 14). Nevertheless, it still remains an open question whether the results for the N. europaea cyt P460 discussed herein can simply be transferred to HAOs. Further studies on HAOs will be necessary to confirm this.
Fig. 1.
PyMOL depiction of the N. europaea cyt P460 active site [Protein Data Bank (PDB) ID code 2JE2, Left] and of the N. europaea HAO heme P460 active site (PDB ID code 4N4N, Right).
In their PNAS paper, Lancaster and coworkers (9) report that the aerobic oxidation of NH2OH by cyt P460 does not result in stoichiometric conversion to but, instead, leads to the production of 70% and 30% N2O. This finding prompted further studies under anaerobic conditions that point toward a direct enzymatic pathway for stoichiometric N2O production from the cyt P460 catalyzed oxidation of NH2OH. N2O analysis of solutions of the enzyme by GC, with varying concentrations of either NH2OH or the two-electron oxidant phenazine methosulfate (PMS), reveals the stoichiometry for this reaction to be 2:1 of NH2OH and PMS to N2O, respectively, suggesting that the reaction requires two equivalents of NH2OH and four oxidizing equivalents to generate one molecule of N2O,
| [1] |
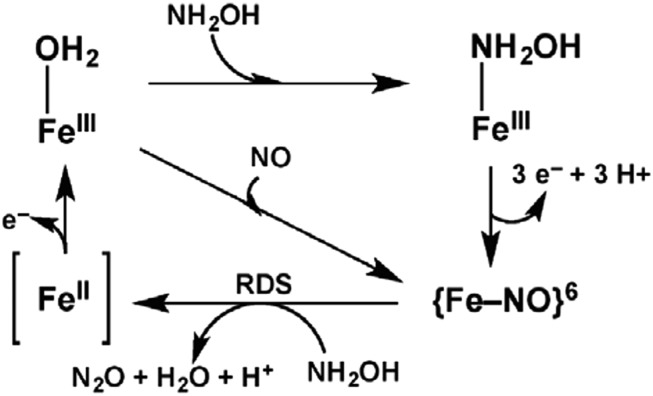
Mechanistic details of this reaction were obtained by UV/visible and EPR studies (see Fig. 2). Spectra obtained for the as-isolated cyt P460 show the heme in the high-spin ferric state with a Soret band at 440 nm and g values of 6.57, 5.09, and 1.97. It is proposed that the active site is six-coordinate, with a likely solvent H2O loosely bound at the active site (FeIII−H2O). Upon addition of NH2OH to cyt P460, the 440-nm Soret band associated with the FeIII−H2O resting state shifts to 445 nm, and Q bands broaden with the formation of an isosbestic point at 438 nm, suggesting a single-step conversion. EPR of the product revealed a 95% conversion to a low-spin species, consistent with the displacement of the bound water molecule with the stronger field NH2OH ligand. The remaining 5% are converted to an off-path and nonproductive species, a ferrous NO complex, or {FeNO}7 in Enemark−Feltham notation (15). Because the electronic structures of transition metal nitrosyls can be ambiguous (3), this notation is useful in counting electrons of transition metal NO complexes by treating the metal−NO unit as a single entity. Here, the superscript “7” corresponds to the sum of iron d- and NO π*-electrons.
Fig. 2.
Proposed mechanism of NH2OH oxidation by N. europaea cyt P460. The square brackets indicate that the ferrous product has not been directly observed so far. Reproduced from ref. 9.
Addition of the oxidant [Ru(NH3)6]Cl3 and excess NH2OH to the FeIII−NH2OH complex results in the single-step formation of an intermediate, which exhibits a Soret band at 455 nm and is EPR-silent. This intermediate accumulates, suggesting that its decay is the rate-limiting step and, thereby, allowing it to be studied in detail. This species reacts with hydroxylamine in the next step of the reaction with a second-order rate constant, kobs(2) = 0.07 mM−1⋅min−1.
UV/visible data indicate that this intermediate is oxidized by 3e− relative to the FeIII−NH2OH adduct. Lancaster and coworkers propose that this species is a diamagnetic ferric NO complex, or {FeNO}6 in the Enemark−Feltham notation (see Fig. 2). This idea was confirmed by independently producing the {FeNO}6 species by reacting the resting FeIII−H2O complex with the NO donor PROLI-NONOate [1-(hydroxy-NNO-azoxy)-L-proline] in an NO-shunted pathway. The resulting complex has an identical UV/visible signature to the observed intermediate and was shown to be stable both in solution and in the presence of excess oxidant. Importantly, addition of varying concentrations of NH2OH to the {FeNO}6 complex, generated by reaction of the enzyme with NO, shows the same second-order decay (0.07 ± 0.01 mM−1⋅min−1), confirming that the intermediate that accumulates in the rate-determining step of cyt P460 catalysis is, in fact, the {FeNO}6 complex.
To confirm that N2O is the product of the reaction between the {FeNO}6 intermediate and NH2OH, the FeIII−H2O complex was reacted with varying concentrations of NO (provided by PROLI-NONOate) and NH2OH, and the N2O yield was determined. A clear 1:1 stoichiometry is observed between the NO added (and, correspondingly, the {FeNO}6 generated) and N2O produced. When using isotopically labeled 15NH2OH, 14/15N2O is produced only in the presence of cyt P460, confirming that the N−N coupling reaction occurs between the {Fe14NO}6 complex and 15NH2OH. The product of this coupling reaction should be a ferrous complex (see Fig. 2); however, this species was never observed directly. Under the reaction conditions used in this study, this species is rapidly oxidized to FeIII due to the presence of excess oxidant (kobs > 1,100 s−1), which prevents its characterization.
Finally, Lancaster and coworkers examined the reversibility of NO binding in the NO shunt by generating the {FeNO}6 complex with one equivalent PROLI-NONOate. Upon exposure to O2, the UV/visible features of the {FeNO}6 complex disappeared while the FeIII−H2O signal appeared. Additionally, is produced as the oxidized product.
In summary, the paper by the Lancaster group (9) describes a new, direct pathway of N2O production from cyt P460s via oxidation of NH2OH that has thus far been overlooked in the field. This result implies that N2O production by AOBs does not require nitrifier denitrification. In fact, these findings provide a rationale for previous reports of increased N2O production under anaerobic conditions and high concentrations of NH3, where nitrifier denitrification is not promoted (16). The second-order decay of the {FeNO}6 intermediate suggests that the N. europaea cyt P460 may be responsible for detoxifying high levels of NH2OH. Taking these ideas a step further, it seems possible that production by HAOs may not actually be the catalytic function of HAOs under high NH2OH concentrations but, instead, that N2O is the main product and that the observed nitrite is a byproduct of NO oxidation to in the presence of O2—a potentially game-changing result. These findings prompt further studies on HAOs to better understand their catalytic function and to determine how these enzymes regulate NO and production. All of these results have dramatic implications for the function of HAOs in nitrification, the potentially underestimated role of nitrification in N2O production, and the significance of NH2OH in the nitrogen cycle. The mechanistic understanding of NH2OH oxidation seems now essential in designing ways to minimize N2O release from agricultural soils and wastewater treatment plants.
Footnotes
The authors declare no conflict of interest.
See companion article on page 14704.
References
- 1.Lehnert N, Coruzzi G, Hegg E, Seefeldt L, Stein L. NSF Workshop Report: Feeding the World in the 21st Century: Grand Challenges in the Nitrogen Cycle. Natl Sci Found; Arlington, VA: 2016. Available at https://www.nsf.gov/mps/che/workshops/nsf_nitrogen_report_int.pdf. Accessed December 7, 2016. [Google Scholar]
- 2.Heil J, Vereecken H, Brüggemann N. A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil. Eur J Soil Sci. 2016;67(1):23–39. [Google Scholar]
- 3.Lehnert N, Berto T, Galinato M, Goodrich L. The role of heme-nitrosyls in the biosynthesis, transport, sensing, and detoxification of nitric oxide (NO) in biological systems: Enzymes and model complexes. In: Kadish K, Smith K, Guilard R, editors. The Handbook of Porphyrin Science. Vol 14. World Sci; Singapore: 2011. pp. 1–247. [Google Scholar]
- 4.Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326(5949):123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- 5.Ni B-J, Peng L, Law Y, Guo J, Yuan Z. Modeling of nitrous oxide production by autotrophic ammonia-oxidizing bacteria with multiple production pathways. Environ Sci Technol. 2014;48(7):3916–3924. doi: 10.1021/es405592h. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Garcia O, Villas-Boas SG, Swift S, Chandran K, Singhal N. Clarifying the regulation of NO/N2O production in Nitrosomonas europaea during anoxic-oxic transition via flux balance analysis of a metabolic network model. Water Res. 2014;60:267–277. doi: 10.1016/j.watres.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 7.Beaumont HJE, van Schooten B, Lens SI, Westerhoff HV, van Spanning RJM. Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J Bacteriol. 2004;186(13):4417–4421. doi: 10.1128/JB.186.13.4417-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper AB, Terry KR. Hydroxylamine oxidoreductase of Nitrosomonas. Production of nitric oxide from hydroxylamine. Biochim Biophys Acta. 1979;571(1):12–20. doi: 10.1016/0005-2744(79)90220-1. [DOI] [PubMed] [Google Scholar]
- 9.Caranto JD, Vilbert AC, Lancaster KM. Nitrosomonas europaea cytochrome P460 is a direct link between nitrification and nitrous oxide emission. Proc Natl Acad Sci USA. 2016;113:14704–14709. doi: 10.1073/pnas.1611051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmore BO, Pearson AR, Wilmot CM, Hooper AB. Expression, purification, crystallization and preliminary X-ray diffraction of a novel Nitrosomonas europaea cytochrome, cytochrome P460. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62(Pt 4):395–398. doi: 10.1107/S1744309106008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson AR, et al. The crystal structure of cytochrome P460 of Nitrosomonas europaea reveals a novel cytochrome fold and heme-protein cross-link. Biochemistry. 2007;46(28):8340–8349. doi: 10.1021/bi700086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi N, Moriyama H, Fujiwara T, Fukumori Y, Tanaka N. The 2.8 Å structure of hydroxylamine oxidoreductase from a nitrifying chemoautotrophic bacterium, Nitrosomonas europaea. Nat Struct Biol. 1997;4(4):276–284. doi: 10.1038/nsb0497-276. [DOI] [PubMed] [Google Scholar]
- 13.Zahn JA, Duncan C, DiSpirito AA. Oxidation of hydroxylamine by cytochrome P-460 of the obligate methylotroph Methylococcus capsulatus Bath. J Bacteriol. 1994;176(19):5879–5887. doi: 10.1128/jb.176.19.5879-5887.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maalcke WJ, et al. Structural basis of biological NO generation by octaheme oxidoreductases. J Biol Chem. 2014;289(3):1228–1242. doi: 10.1074/jbc.M113.525147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enemark JH, Feltham RD. Principles of structure, bonding, and reactivity for metal nitrosyl complexes. Coord Chem Rev. 1974;13(4):339–406. [Google Scholar]
- 16.Law Y, Ni B-J, Lant P, Yuan Z. N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res. 2012;46(10):3409–3419. doi: 10.1016/j.watres.2012.03.043. [DOI] [PubMed] [Google Scholar]