Abstract
Endangered forest–grassland mosaics interspersed with expanding agriculture and silviculture occur across many parts of the world, including the southern Brazilian highlands. This natural mosaic ecosystem is thought to reflect alternative stable states driven by threshold responses of recruitment to fire and moisture regimes. The role of adaptive human behavior in such systems remains understudied, despite its pervasiveness and the fact that such ecosystems can exhibit complex dynamics. We develop a nonlinear mathematical model of coupled human–environment dynamics in mosaic systems and social processes regarding conservation and economic land valuation. Our objective is to better understand how the coupled dynamics respond to changes in ecological and social conditions. The model is parameterized with southern Brazilian data on mosaic ecology, land-use profits, and questionnaire results concerning landowner preferences and conservation values. We find that the mosaic presently resides at a crucial juncture where relatively small changes in social conditions can generate a wide variety of possible outcomes, including complete loss of mosaics; large-amplitude, long-term oscillations between land states that preclude ecosystem stability; and conservation of the mosaic even to the exclusion of agriculture/silviculture. In general, increasing the time horizon used for conservation decision making is more likely to maintain mosaic stability. In contrast, increasing the inherent conservation value of either forests or grasslands is more likely to induce large oscillations—especially for forests—due to feedback from rarity-based conservation decisions. Given the potential for complex dynamics, empirically grounded nonlinear dynamical models should play a larger role in policy formulation for human–environment mosaic ecosystems.
Keywords: Human–environment coupling, forest–grassland mosaics, ecosystem services valuation, southern Brazil
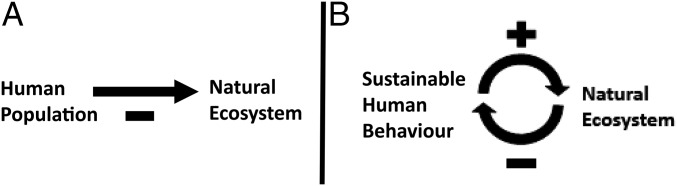
Historically, humans have manipulated their environment beyond sustainable levels, leading to local or regional collapses in resources or even civilizations (1–3). The current paradigm of the human–environment relationship is a dominant one-way deleterious impact of humans on natural ecosystems (Fig. 1A) (4). Human activities, including agriculture, forestry, and livestock management, contribute to widespread conversion of natural ecosystems to cultivated areas at the expense of ecosystem services (5, 6), and as human populations continue to expand the scope and magnitude of human influence on natural ecosystems also grows (7).
Fig. 1.
Human–environment coupling. The negative relationship between humans and natural ecosystems is driven primarily by competition with agricultural land (A). Alternatively, sustainable human behavior (i.e., valuation of natural ecosystem services) leads to positive changes in natural ecosystems when natural ecosystems are rare (B), whereas the perception of abundant natural ecosystems results in a decreased desire to conserve natural ecosystems.
Although it is clear that this negative paradigm needs to be superseded (1, 4), it has been argued that this can only happen given an understanding of the coupled interactions between human behavior and ecosystem dynamics (8–12). As previous research on human–environment systems has found, the importance of human behavior in conservation biology is undeniable, suggesting that an integrated human–environment approach is necessary for successful conservation (13–16). In addition to consumption, human-environment interactions can be motivated by endangerment of species and ecosystems, ecosystem services valuation, and related policies and subsidies (17). This can in turn have a positive impact on natural systems. For example, when natural land becomes rare, individuals, policies, and subsidization support conservation (positive feedback) (Fig. 1B). Once the natural system is restored to a certain level it may no longer remain a conservation priority, allowing for greater resource extraction (negative feedback).
This negative feedback loop is exemplified by the process of sustainable forest management (e.g., protected areas, harvesting limits, reforestation projects, and import regulations) but can also occur in other human–environment systems (e.g., fisheries, hunting quotas, and endangered species recovery) (18). One well-known case is the recovery of the dry tropical forests of Costa Rica. Similar to many tropical countries, Costa Rica experienced rapid deforestation for agricultural and livestock purposes. However, Costa Rica is notable in its vigorous implementation of national conservation policies to restore forests, including payments for environmental services, protected areas, and restrictions on timber extraction (19, 20). Although regional and national successes are evident, many other natural areas of the tropics and subtropics remain a conservation priority due to overwhelming endangerment and a poor understanding of coupled human–environment systems (21, 22).
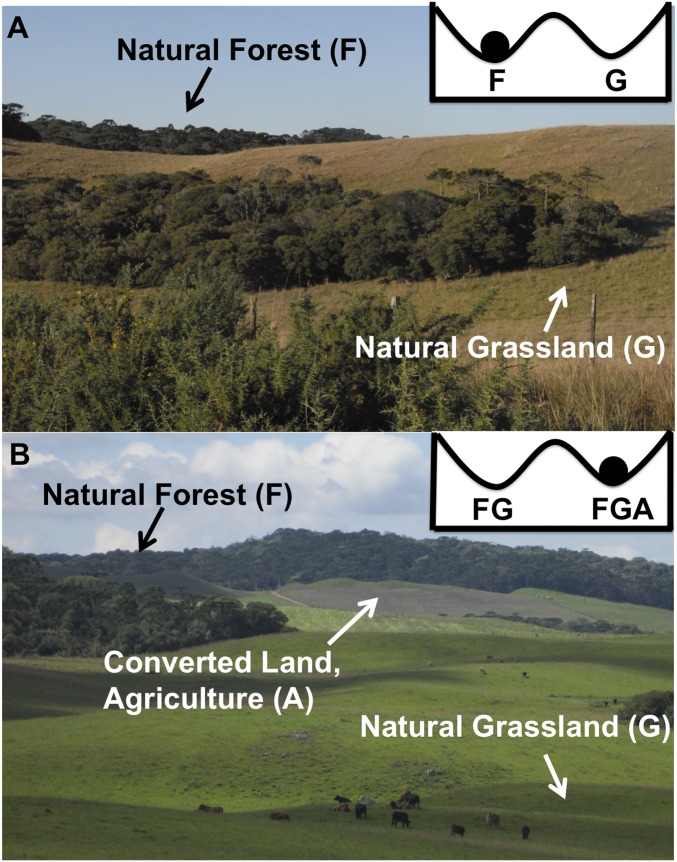
When natural ecosystems occur as alternative stable states, an understanding of human–environment relationships can become even more complicated (23). One such example is forest–grassland mosaics (Fig. 2), where natural grassland and natural forest coexist and can alternate in dominance over time based on positive feedbacks in threshold responses to disturbance regimes (11, 24–27). Pollen and charcoal records reveal vegetation changes following climate shifts and environmental condition shifts on a millennial time scale, whereas human activities can cause dramatically different states within centuries (28–30). The expansion of human influence (both positive and negative) has the potential to catastrophically affect the stability dynamics of mosaic ecosystems (11, 31, 32). In southern Brazil, as in many other parts of the world (e.g., South Western Ghats montane forests in India and the Jos Plateau forest–grassland mosaic in Nigeria), these forest–grassland ecosystems are doubly endangered in the sense that both the natural grasslands and the natural forests are extremely rare (33, 34). Grassland conservation is often overshadowed by forest conservation, because individuals perceive forest as having higher aesthetic value (21, 35). Brazil’s Forest Code (BFC)—the law that protects all natural vegetation in Brazil—reflects this perceived bias in the valuation of forest (35), such that individuals are often unaware or unwilling to protect grassland.
Fig. 2.
Bistability in forest–grassland-agriculture system. (A) The image depicts a natural mosaic in southern Brazil without human influence, where dominant Araucaria forest () or dominant Campos grassland () are alternative stable states. (B) An example of bistability in a mosaic of natural forest () and natural grassland () with converted land (agriculture, ). The alternative stable states are dominant converted land (agriculture and/or silviculture, ) and dominant forest ().
In earlier work using a relatively simple model we suggested that introducing strong human coupling (through harvesting and other human impacts) removes bistability in these mosaics (11). Other researchers have also pointed out that attempting to manage natural ecosystems without appreciating the potentially large role played by alternative stable states may lead to unforeseen collapses in ecosystems due to the presence of tipping points (28). Therefore, in systems where both bistability and strong human influence are present, there is value in developing coupled human–environment system models. With increased awareness of the possible effects of human interventions, we can examine sustainability in the context of both naturally occurring and human-influenced regime shifts.
Here we couple human social dynamics, in terms of imitation, conservation values, and economic gains, with an ecological model of a forest–grassland mosaic. The objective of this work is to understand the dynamics arising from coupling between decision making about land conversion (a complex process that considers both human values and economic gains) and bistable mosaic ecosystems and draw conclusions about potential land-use policy implications. We investigate how this coupling might lead to outcomes that cannot be understood when these systems are considered in isolation. We evaluate the effectiveness of conservation values, discount rates, and discount time horizons at maintaining natural mosaics. We use empirical data and questionnaire results from a human-dominated forest–grassland mosaic system in southern Brazil as a case study to parameterize our model. Modeling approaches for nonlinear dynamical systems vary across a spectrum from simple dynamical models that can be analyzed by pencil and paper (or chalk and chalkboard) to detailed statistical models and spatially explicit, stochastic, agent-based models. Simple dynamical models allow us to explicitly describe underlying mechanisms in complex biological systems, thereby “enabling meaningful comparison between the consequences of basic assumptions and empirical facts” and allowing space for a parsimonious description to emerge, although oversimplification may prevent researchers from answering ecological questions (36). Most coupled human–environment systems models are relatively detailed agent-based models, whereas differential equation models of intermediate complexity are seldom used. Because of this gap in the “ecology” of human–environment system models, and according to the data that were available to us, we opted to develop a differential equation model of intermediate complexity. The model is described in the following section.
Methods Overview
Study System.
The dominant land cover of the southeastern Brazilian highland region has historically alternated between forest and grassland with changes in climate and greater human inhabitance (i.e., fire and grazing) (24, 37). Paleoecological records provide a historical range of vegetation patterns and natural disturbance regimes, which are used to infer potential multiple stable states.
The forest–grassland mosaics of southern Brazil (23° to 30°S and 55° to 48°W) are among the most diverse in the world (24). The Campos grasslands and the Atlantic forest are rich in species diversity (38, 39). In addition, the Atlantic forest is host to many endemic species—including the endangered Araucaria angustifolia tree species. Over the past 30 to 60 y, converted (agriculture and/or silviculture) land use has expanded into these ecological hotspots of southern Brazil (24, 38). This provides an opportunity to study a natural mosaic ecosystem under rapidly evolving (and growing) human influence. Thus, the Campos–Araucaria mosaic constitutes an important case study for anthropogenically disturbed mosaic ecosystems.
Environment (Land-Use and Natural Dynamics) Model.
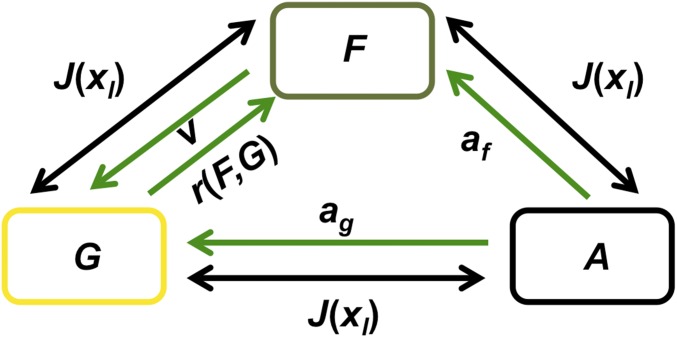
We build on previous models of forest–grassland dynamics (11, 40, 41), where biophysical processes regulate changes between grassland and forest. We model transitions between forest (), grassland (), and converted land (agriculture and/or silviculture, ), based on landowner preference for each state (, , or ). A conceptual diagram of the model is presented in Fig. 3 and the equations for land-cover dynamics are
| [1] |
| [2] |
| [3] |
Parameter definitions and baseline values are provided in Table S1. Landowner preferences are reflected by the quantities , , and , representing the proportion of the landowner population preferring more forest (forest-preferrers), grassland (grass-preferrers), and converted land (convert-preferrers) on their property, respectively, compared with current land composition. In the fully coupled human–environment system, these quantities become model variables (discussed in the next section). Natural processes governing change in natural land cover are the recruitment rate () and natural disturbance rate (). Abandonment and reversion of plantation to forest cover () and crops to grassland () occur at a constant rate. is the land conversion rate as a function of landowner preferences for each land-cover state, . All land cover is assumed to be composed of either forest, grassland, or converted land (agriculture and/or silviculture), such that . Therefore, can be obtained from the relation , and we only need to solve Eqs. 1 and 2.
Fig. 3.
Conceptual diagram. Land states in our study region include forest (), grassland (), or converted land (agriculture and/or silviculture, ). Green arrows represent environmental drivers (natural disturbance (), recruitment () (Eq. 4), and abandonment () in Eqs. 1–3. Black arrows represent human influence, driven by landowner preference for each state [, Eq. 9], based on a valuation of penalties (, Eq. 8), profits, and conservation values in Eqs. 7 and 8.
Table S1.
Model parameters from empirical data and questionnaire results
| Parameter | Description | Range | Value simulated | Sources |
| Natural disturbance rate | 0.02 | 43 | ||
| , | Converted land abandonment to and | , | 41, 47 | |
| Soil moisture content | 0.2 | 45 | ||
| Maximum recruitment rate | 0.2 | 44 | ||
| Recruitment threshold constant | 0.149 | Calibrated | ||
| Recruitment transition constant | 0.0667 | 40 | ||
| Annual profit from natural land and | , | 47, 62, 63 | ||
| Annual profit from agricultural land | 1 | 47, 62 | ||
| Annual profit from plantation on 7-y rotation | , , , | 66 | ||
| Penalty for noncompliance with BFC | 0.01 | 65, 66 | ||
| Social learning constant | 0.42 | 11, 47 | ||
| , | Conservation value of natural land () | , | 11, 47 | |
| Economic discount rate | 0.1 | 49, 50, 69 | ||
| Conservation discount rate | 0.001 | 14, 15, 51 | ||
| Discount time horizon for economic gains | 7 | 53 | ||
| Discount time horizon for conservation values | 28 | 14 | ||
| Diffusion of practices threshold | 0.5 | 54, 67, 68 | ||
| Diffusion of practices rate | 0.075 | 54, 67, 68 | ||
| Maximum potential human influence | 54, 67, 68 |
Parameter selection and calibration is described in SI Materials and Methods
We use a sigmoidal function to represent the recruitment rate, whereby recruitment of forest is high when and low when (11, 42). The function reflects a fire-limited recruitment threshold where soil moisture also helps determine the threshold. The recruitment function is parameterized using data on soil moisture content () and natural land cover ( and ) (see SI Materials and Methods for details):
| [4] |
The maximum recruitment rate, , is limited by environmental conditions and herbivory (43, 44). represents the soil moisture content of the region or land patch and its value is obtained directly from empirical studies (45). controls the location of the threshold in the recruitment function and its value is determined by calibrating the model to published data on forest thresholds, moisture availability and fuel load (40). controls the steepness of the recruitment curve.
Human Behavior Model.
Conversion of rural land is greatly influenced by values associated with the landscape (46). Human influence on land-cover dynamics (see below, Eq. 9) is modeled as a function of landowner preferences for each land-cover state, (), (see the previous subsection for a definition). Landowner preferences and parameterization of the human behavioral model are gleaned from questionnaire responses (47); details are provided in SI Materials and Methods The behavioral model equations are given by
| [5] |
| [6] |
We note that , hence an equation for is not needed. Also, we have used . is the rate at which landowners sample others and adopt their preference, if the utility for changing preferences is higher. The values of and are described as utilities via the term , where and is the land-cover state or . reflects both economic gains () and rarity-based conservation () according to
| [7] |
Human behavior is in part driven by the perceived future gains for their present actions (41, 47, 48), prompting the use of a discount factor. is the economic discount rate (49, 50) and is the conservation discount rate. We assume (see SI Materials and Methods for discussion and justification) (51, 52). The discounting time horizon and are the amount of foresight applied to decisions for economic and conservation utilities, respectively (14, 53).
The decision to convert natural land into agriculture and/or silviculture is determined by economic gains and compliance with minimum natural vegetation requirements. The function was parameterized using data on profits from crops () and plantations () and penalties () for not adhering to BFC. The converted land utility is given by
| [8] |
is a piecewise function, such that when the legal reserve requirements are met by landowners () there is no penalty, ; otherwise , reflects a monetary penalty, (see SI Materials and Methods for the equation). The method for discounting annual plantation profits () is similar to , and , although the profit depends on the stage in the harvest rotation cycle and represents a sum of gains and losses; full details are given in SI Materials and Methods.
The diffusion of practices among individuals follows a sigmoidal curve because the initial proportion of adopters is minimal but gradually gathers momentum as individuals imitate others (54) (SI Materials and Methods). represents land conversion to , , or :
| [9] |
is the maximum potential influence of landowners. is the threshold proportion of landowners preferring land cover , , or for which land conversion, , is , and controls the steepness of the curve.
Analysis.
We construct parameter planes for conservation values , discount rates , and discount time horizons to determine the land-state dynamical regimes for a range of initial conditions after 1,000 y. Each simulation is run under weak, moderate, and strong human influence, to show varying degrees of human–environment interactions. For details on parameter ranges used in our base case (São Francisco de Paula) see SI Materials and Methods. Model simulations use ode45 in MATLAB (ode15s was used to check for consistency) (details in Dataset S1). We use a burn-in time of 5,000 y to allow sufficient time for damped oscillations to settle down to an equilibrium state. After 5,000 y, model simulations indicate either an equilibrium point or stable limit cycles. After burn-in, the time series were used to confirm land-cover dynamics at various points in the parameter planes and at the edges between land states.
SI Materials and Methods
Environment (Land-Use and Natural Dynamics) Model.
In the first set of Eqs. 1–3, the terms and parameters are based on earlier work by Innes et al. (11), although the functional form of recruitment (, explained in next paragraph) and the functional form of human influence (, explained below) are markedly different. We have also included a third land-cover state, converted land (), because in recent years the forest–grassland mosaic landscape, characteristic of southern Brazil, has experienced gains in converted land (i.e., plantations and crops) (47). Shifts in land use between converted land and natural land are often motivated by economic gains and demand for food. Any nonpecuniary transitions from converted land to natural land are defined as abandonment, for reasons such as poor soil fertility, climate shifts, and modernization.
The natural mosaic exists as alternative stable states—dominant grassland and dominant forest—driven by nonlinear recruitment (Fig. 2A). The positive feedback loop between recruitment and the proportion of grass cover/tree cover is governed by a fire threshold response. Once tree cover exceeds , the frequency and severity of fire are diminished, thus allowing seedlings to develop (42). However, the location of the threshold is also thought to be influenced by moisture availability, which alters fire intensity and ultimately probability of topkill (40).
Similar to previous work (40), Eq. 4 of the main text represents recruitment in terms of grass cover, with an implicit fire-driven threshold where the location of the threshold depends on moisture availability. As mentioned in the main text, (together with ) controls the location of the threshold in the recruitment function. is a calibrated parameter that reflects the empirical recruitment threshold in relation to grass biomass. To account for the impact of moisture availability on fire and grass cover, we incorporate a moisture-limited threshold response to forest recruitment through the term . is the soil moisture content, the value of which is obtained from the literature (45) and in our given study region , hence the term (). Soil moisture content here is used as a proxy for rainfall and thereby fuel moisture, which are both shown to alter the transition between grasses and trees (40, 42, 56). In tree–grass systems regulated by fire, there is a general consensus that tree cover less than 40% ( or ) suppresses recruitment (11, 42). Once this threshold is crossed, trees proliferate, resulting in maximal forest recruitment (). Therefore, is calibrated to obtain , for , and , for . Furthermore, the forest recruitment threshold is related to the proportion of grasses in natural ecosystems, hence the term representing implicit fire response. We assume that forest is incapable of natural expansion into converted land, as farming practices suppress forest succession; therefore, when and are small, recruitment is limited.
Human Behavior Model.
The expansion of converted lands introduces an additional alternative stable state (dominant converted land, ) that is driven primarily by profits (Fig. 2B). Converted land is primarily driven by economic gains (47), whereas natural land-cover states appeal to cultural and ecological interests, in addition to monetary gains. In Eqs. 5 and 6, landowners are assumed to share information among each other, as indicated in the questionnaire responses (47). Imitation is a common behavior among individuals, particularly within groups that share interests (13, 57, 58). Finally, landowners consider the value of each state and select the most profitable state or the natural state with the highest likelihood of extinction.
In Eq. 7, the utility functions for natural forest () and natural grassland () capture economic gains (, ) and conservation values (, ). The term , reflects the conservation value of and , which are determined by the relative abundance of either natural state. Human behavior is in part driven by the perceived future gains for their present actions (41, 59–61), which requires the use of a discount factor. A discount factor is applied in economics (including behavioral economics) to align future profits with present values, in an effort to explain preferences in consumption, monetary inflation, and uncertainties in the future.
From Satake and Rudel (41) we set the discount factor at and for monetary gains and conservation values, respectively. A significant body of environmental economic literature prescribes a small discount rate for conservation projects or values (14, 52, 59–61), as a means to minimize land conversion. However, there are also empirical and theoretical reasons to think that individuals use lower discount rates in their decision making for conservation projects than they do for strictly economic activities. For instance, Broome (51) aptly summarizes the Hotelling rule, whereby it is argued that interest rates for scarce, nonreproducible resources (e.g., rare ecosystems) are lower than for conventional commodities produced within an economic system, because nonreproducible resources—unlike many commodities—cannot be converted into a greater quantity of future nonreproducible resources, by definition. Therefore, the discount rate for nonreproducible resources should be lower (or even zero) than for conventional commodities (51). In the context of the southern Brazilian forest–grassland mosaic, it has also been noted that gaucho culture favors grassland (24) and natural ecosystems ( and ) not only because they provide valuable environmental services but also because they are important to gaucho heritage. Therefore, for our study system we expect that individuals will use a lower discounting rate for the value of future grassland–forest mosaics than for economic gains. Nonetheless, individuals are generally shortsighted and impatient, making decisions with respect to financial returns based on harvest rotations and the perceived risk of ecological crises impacting the next generation (59).
In Eq. 8, annual profits from crops () are estimated from the 2006 agricultural census of the Instituto Brasileiro de Geografia e Estatística (62). The profit from crops () and the profit from plantation management () are greater than either natural land state (, ), we estimate (62, 63). An economic discount rate is applied to annual profits from crops analogous to natural forest and grassland utilities (Eq. 7). The penalty for noncompliance with the legal reserve requirements is reflected by :
| [S1] |
where is the monetary penalty for noncompliance with land requirements (64, 65). BFC requires 20% of land in Pampa and Atlantic Forest biomes to be conserved in a natural state (65). When natural land exceeds the legal reserve quotas, landowners are not penalized and is zero.
Plantation forestry requires a greater initial investment and generates delayed returns. The harvest rotation cycle leads to net losses when establishing (, at time ) and maintaining (, at time ) the plantation (62, 66). Trees generate revenue in later years through thinning for charcoal (, at time ), whereas the greatest gains occur at the time of final harvest (, at time ).
| [S2] |
The sigmoidal function used to describe the relationship between landowner decision-making processes and land-use change (Eq. 9) is seen in the adoption practices of a broad range of innovations from the agriculture industry to technology launches (67, 68). The parameter values for and are estimated from Rogers (54). The sharpest increase in land-use change occurs between 16 to 84% of the landowner population preferring land cover (); in such cases changes in land composition increase rapidly as preference for a given land state increases. When preference for a land cover is below <16%, landowners are not motivated to make changes to their land composition. Contrarily, above 84% of the landowner population preferring land cover , most landowners have already altered their land composition.
Results
Base Case.
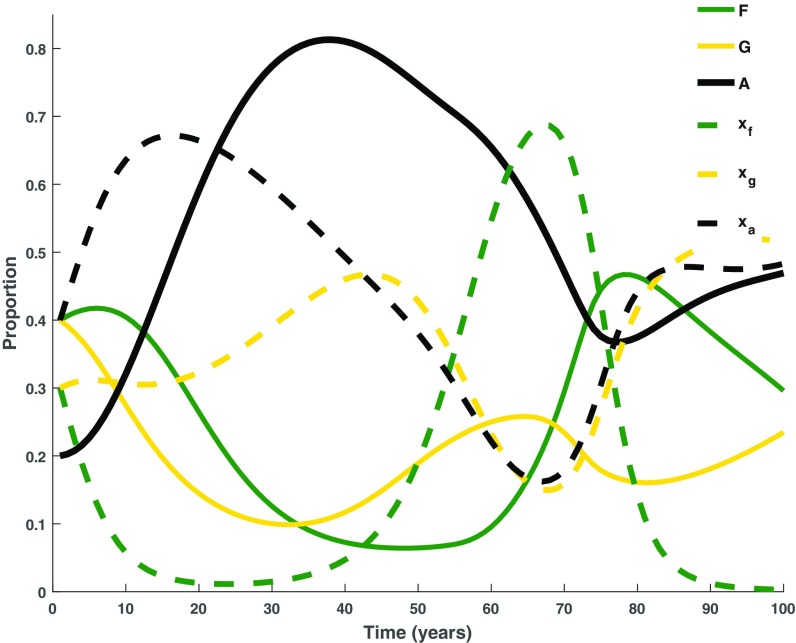
At base-case parameter values, in the short term (100 y) the model predicts that land conversion will continue to grow over the next 40 y at the expense of forest () and grassland () (Fig. 4). Converted land () increases to just above , after which the compliance penalty () and the rarity of natural land motivates an increase in conservationist behavior and therefore a decrease in the value of , below that of and . As a result, and eventually return to ∼20% cover each, at which point they are no longer perceived as rare or endangered and the cycle continues. Interestingly, the increase in forest-preferrers () is greater than the increase in grass-preferrers () over this period, despite being the least profitable land cover and despite having the same initial cover. The large fluctuations in are due to the negative feedback loop, which increases when becomes rare and forest conservation values () are high (Fig. 1B). The proportions of and are moderated by their stable profits, so that their oscillations are less dramatic than those of . Also, the amplitude of oscillations in and exceeds that of land cover (), because social dynamics occur at more rapid timescales than forest dynamics. In our questionnaire responses (47) we observe that landowner preference is not always reflected in actual land use, but there is a correlation between preference and actual land composition. To this effect, , , and shadow oscillations in , , and , following a lag of 15 to 20 y (Fig. 4).
Fig. 4.
Base-case land composition. In the short term, our study region continues to exist in an unstable forest–grassland–converted land () state. The proportion of forest (), grassland (), and converted land (agriculture and/or silviculture, ) are determined by proportions of landowners preferring forest (), grassland (), and converted land (). The preference is driven by utility (i.e., conservation values and profits). Table S1 gives parameter values for the time series simulation.
Changing Conservation Values.
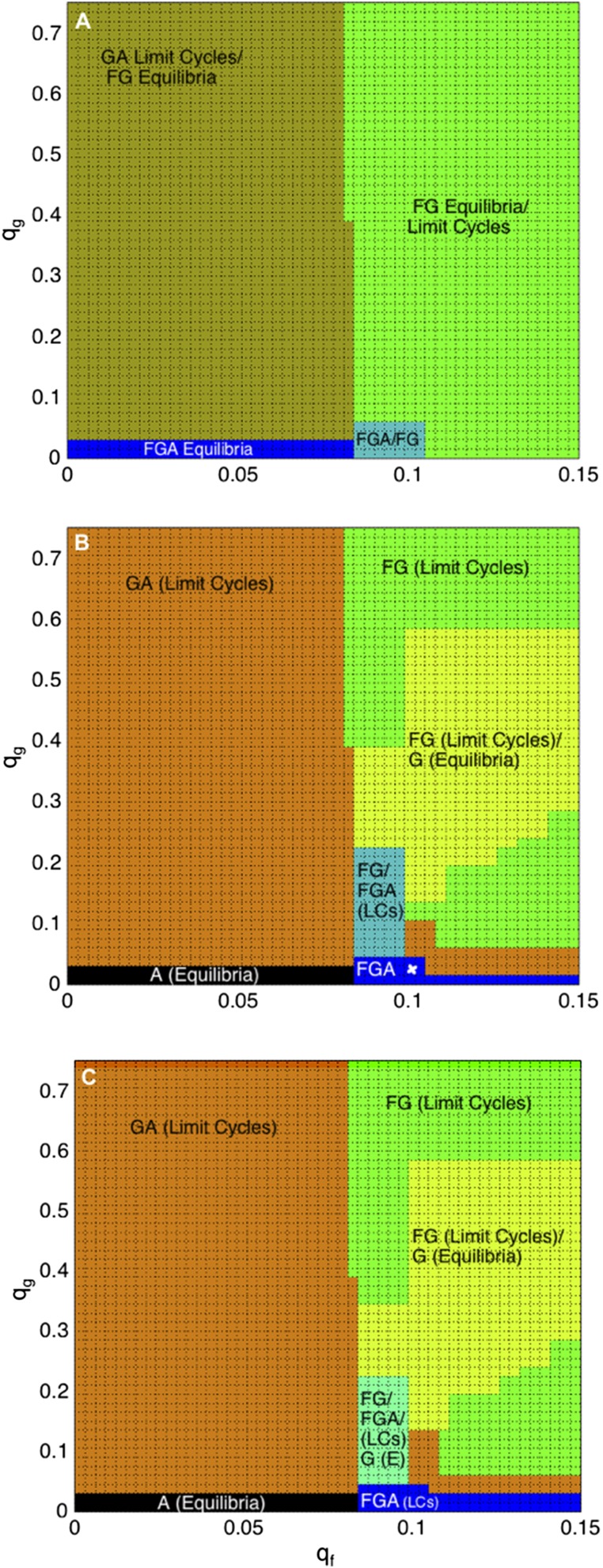
According to our base-case parameter values, the forest–grassland mosaic in southern Brazil currently exists in a region of parameter space where small changes in conservation values and could lead the system into various, dramatically different dynamical regimes (Fig. 5B). Moreover, increasing the conservation value of forest () has less predictable consequences for forest cover () than increasing the conservation value of grassland () has on increasing grassland cover () (Fig. 5). A larger increase in is required to conserve forest than the increase in required to conserve grassland. Under moderate and strong human influence, increasing the conservation value of grassland beyond causes a change from converted land (agriculture and/or silviculture, ) to a state where can coexist with (and, in the case of weak human influence, with as well). In the case of increasing , there exists a critical threshold at , below which is nonexistent (Fig. 5 B and C). Additional increases in can actually result in the exclusion of (for moderate and strong human influence). This occurs because extreme oscillations can put the proportion of forest-preferrers () close to zero, risking the extinction of this subpopulation. These dynamics exemplify the law of unintended consequences.
Fig. 5.
Increasing the grassland conservation value () leads to land compositions with grassland (), for all conservation values, except and , for weak (A), moderate (B), and strong (C) human influence scenarios. Natural forests () rely on forest conservation values () to increase the utility of , and therefore must be large enough to counterbalance the utility of grassland () and converted land (agriculture and/or silviculture, ). The small white x in B marks current conditions in our study region of São Francisco de Paula.
Moreover, increasing can increase both natural states ( and ), because increased utility for natural vegetation outweighs the utility from . is both profitable and culturally significant, which increases the likelihood of the system’s being in the state (Fig. 5). In contrast, relies upon rarity-based conservation feedbacks due to lack of profitability and therefore is more susceptible to temporal variability and the types of dynamics observed in the parameter plane as increases. Conservation values are not the sole factors maintaining natural forest–grassland mosaic systems. Because utility is the dominant force in the system, discount rates and discount time horizons have an important role in determining vegetation cover, which we will see in the next sections.
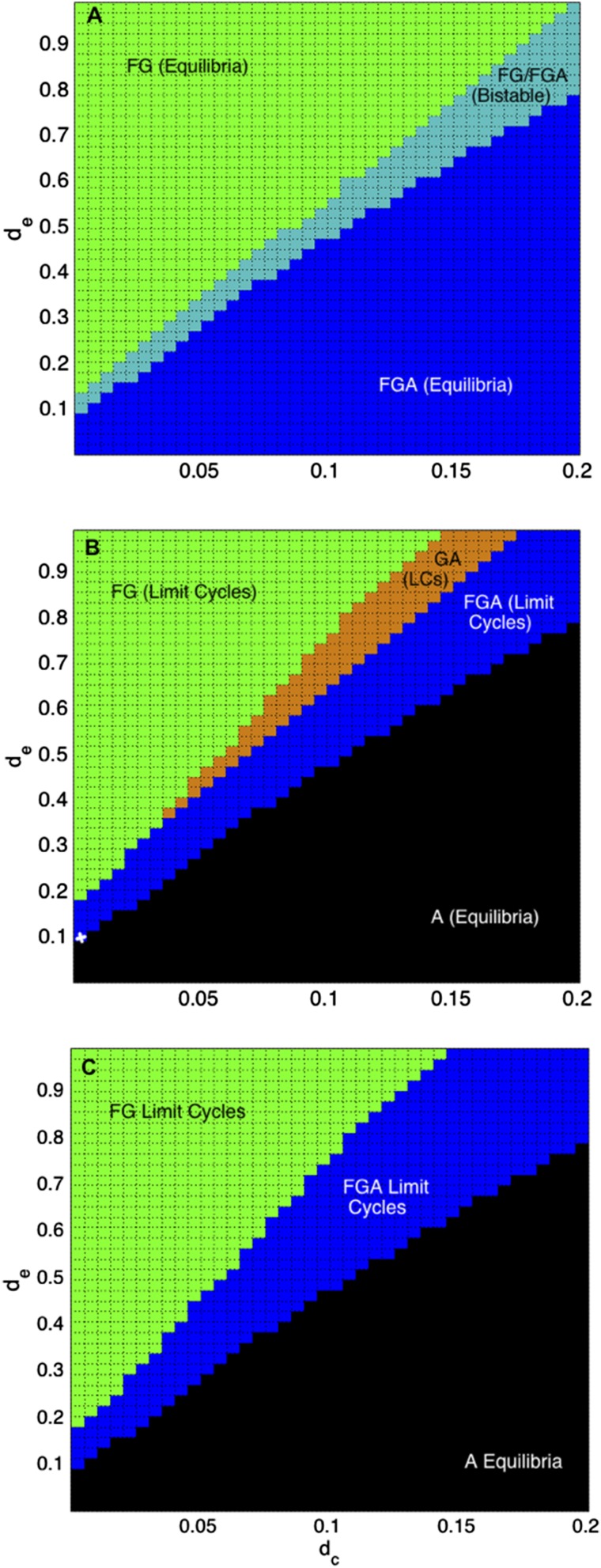
Changing Economic and Conservation Discount Rates.
We find that increasing economic discount rates () can dampen oscillations and regenerate natural land cover, because future profits from land conversion are not strongly influential (Fig. 6). In contrast, low and very high conservation discount rates () increase the tendency of land cover toward converted land (agriculture and/or silviculture, ), because the long-term value from conservation efforts is not strongly influential. Both and are equally important in determining the land-cover dynamics. The land cover depends on the ratio between and . When , dominates, otherwise the natural ecosystem mosaic () dominates. When , the land composition tends to a combination of and , either as bistable states, in the case of weak human influence (Fig. 6A), or limit cycles, for strong and moderate human influence scenarios (Fig. 6 B and C). Although is probably less than , it is not clear on which side of the threshold human populations fall. Moreover, the discount time horizons () alter the threshold ratio for discount rates.
Fig. 6.
Economic discount rates () six times greater than conservation discount rates () promote a natural composition, with forest and grassland (), and results in dominant converted land (agriculture and/or silviculture) cover ( or ), for weak (A), moderate (B), and strong (C) human influence scenarios. Moderate human influence (B) has an additional region of limit cycles when has the highest utility. Weak human influence (A) results in equilibrium states and for all possible discount rates, whereas moderate (B) and strong (C) human influence scenarios result in limit cycles and only stable state. The small white in B marks current conditions in our study region of São Francisco de Paula.
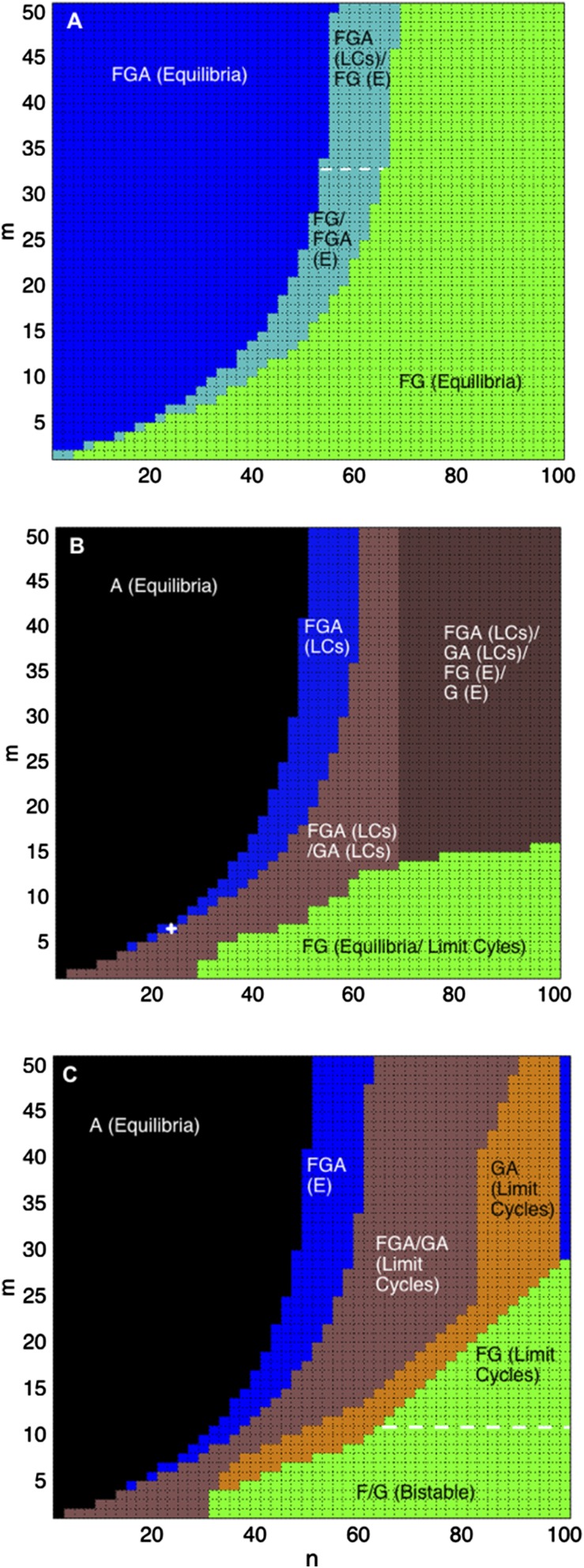
Changing Economic and Conservation Discounting Time Horizons.
Similar to discount rates, the inclusion of a long conservation discount time horizon (large ) can maintain and improve natural land cover (Fig. 7). In addition, large reintroduces bistability for the strong human influence scenario (Fig. 7C). Instead of being driven by recruitment, as in the natural mosaic without converted land (agriculture and/or silviculture, ), drives changes in natural land through rarity-based decision making. When or is rare, increases the utility of and above that of , which in turn increases the proportion of landowners preferring natural land cover (, ). Furthermore, when human influence is strong, landowner preference is reflected in land composition, thereby increasing the proportion of natural land. The discount time horizon for conservation () has a much greater influence on land-cover dynamics than the economic discount time horizon (). More specifically, a conservation discount time horizon of at least 45 y is required to maintain natural states and results in a system dominated by natural mosaics (Fig. 7 A and C). The moderate human influence scenario is an exception, where high and values lead to a proliferation in outcomes in the system (Fig. 7B; see also the next section) whereby the initial conditions strongly influence the land-cover stability dynamics for the moderate scenario. The system can be driven by rarity, such that when initial is rare, is the resulting stable land cover and when is rare, is the resulting stable land cover. Alternatively, when rarity is not a concern for landowners (), the system can be driven by profits, resulting in or limit cycles, or recruitment dynamics, resulting in alternating stable states, and , driven by the fire threshold.
Fig. 7.
A conservation discount time horizon () less than 45 y promotes a composition dominated by converted land ( or ), for weak (A), moderate (B), and strong (C) human influence scenarios. Longer conservation time horizons promote natural land cover. Weak human influence (A) results in equilibrium states and for all possible discount time horizons, whereas moderate (B) and strong (C) human influence scenarios result in limit cycles and a converted land (agriculture and/or silviculture) only stable state. Strong human influence has an additional region of bistability, composed of dominant forest or grassland only (), driven by rarity. The small white in B marks current conditions in our study region of São Francisco de Paula.
Extent of Human Influence.
As mentioned previously, incorporating anthropogenic activity into a natural mosaic ecosystem increases the long-term instability in the system by introducing prolonged oscillatory cycles (Fig. 4). In the weak human influence scenario, natural processes counterbalance anthropogenic activities, resulting in dominant natural land cover and the characteristic bistable dynamics of natural forest–grassland mosaic systems (), with the additional outcome of bistability with converted land (, Figs. 5A, 6A, and 7A).
The most complex interactions occur in the moderate human influence scenario, which allows feedbacks from both natural processes (recruitment) and human values (land rarity, profits, and environmental services) (Figs. 5B, 6B, and 7B), whereas the strong human influence scenario is primarily driven by the costs and benefits of human values, promoting the expansion of converted land (agriculture and/or silviculture, ) and grassland () (Figs. 5C, 6C, and 7C). Near base-case parameter values (for slightly smaller conservation values, lower economic discount rates/higher conservation discount rates, and a longer conservation time horizon than the base case) there exists a stable state with minimal or (Figs. 6 B and C and 7 B and C). In such a state, abandonment ( and ) prevents either natural ecosystem state ( or ) from complete extinction; however, weinterpret this dependence on transient abandonment processes to signify that the natural land cover is relatively degraded.
Under moderate and strong human influence, the stable state is , and occurs in less than 50% of parameter space (Figs. 5 B and C, 6 B and C, and 7 B and C). Moderate and strong human influence removes bistability from the forest–grassland mosaic system (11), except for long conservation discount time horizons () and short economic discount time horizons (). Instead, we find that human influence replaces bistability, the existence of alternative stable states, with a stable state and an alternative limit cycle or multiple alternative limit cycles. In the strong human influence case, we exist near a threshold where a slight change in parameter values can cause a slip into dominance of at the expense of and .
Discussion
Coupling human and environment models allows us to examine feedbacks between the two subsystems, resulting in dynamics that cannot and often should not be studied in isolation, especially for systems under threat by human activities, such as mosaic ecosystems. Our simulations show how tightly these systems are coupled and how important feedbacks can be. As Stern (15) states, “environmentally significant behavior is dauntingly complex, both in its variety and in the causal influences on it.” Dynamical system approaches using relatively simpler mathematical models to complement detailed agent-based models—such as the one we explored in this paper—can provide a level of clarity regarding feedbacks and complex nonlinear processes that is often harder to capture using agent-based models. When data are available to parameterize such models, as in our case study, models can provide insight and potentially lead to policy changes.
Our analysis finds the southern Brazilian forest–grassland to be in a region where many possibilities may unfold in the future. For instance, a relatively small drop in forest conservation values could easily push the system into a region where forest and possibly also grassland are lost.
The model predicts that current trends toward more conversion of endangered land states to agriculture and silviculture can be mitigated through changes in attitude (valuation of ecosystem services) and discounting conservation utilities less than economic gains, but our results indicate that cultivating a conservation mindset in the population requires moderate to strong conservation values and long-term conservation foresight (conservation discount time horizon), looking many decades into the future. Our results also indicate that the effects of increasing grassland conservation values are more straightforward than the effects of increasing forest conservation values, due to the necessity of rarity-based feedbacks in sustaining forests. Increasing forest conservation values can remove converted land (agriculture and/or silviculture) from the forest–grassland mosaic, promoting either forest or grassland states. Unlike grassland conservation, forest conservation maintains alternative natural states (e.g., grassland), by reducing the relative utility of converted land. We can relate this finding back to BFC; the implied bias in BFC toward forest valuation may not be as detrimental to other natural vegetation types (grassland) as first thought, because, as we show, the conservation of forests alone can promote alternative natural land cover (e.g., grassland) in mosaic ecosystems.
A recent theoretical model shows that strong human influence precludes bistability in forest–grassland mosaic systems (11). We expanded on this previous work by including agriculture and silviculture, as well as other realistic characteristics of human decision making, such as discounting. An unexpected result of including discount time horizon (foresight) is the reintroduction of forest–grassland bistability under strong human influence. In our model, bistability is restored when individuals make decisions with long-term conservation goals, but instead of being driven by natural recruitment, the bistability is human-originated, according to rarity of natural land. When conservation foresight is significantly greater than economic foresight, landowner preference for natural systems exceeds the preference for more profitable land compositions. Furthermore, our assumption that landowners use rarity-based conservation creates a threshold response in natural land-cover stability—when initial forest cover is low, forest dominates and when initial grassland cover is low, grassland dominates.
The introduction of utilities and landowner perceptions often results in long-term, damped oscillations, suggesting that studying transient states may be important (55). Moreover, increasing conservation values would have a double benefit; this would not only improve the utilities for future land states and thus increase their average cover, it would also stabilize overall dynamics by increasing the incremental difference between utilities. Improving parameter estimates is therefore a suitable avenue for future work. As with any model, determining whether our predictions are robust to our assumptions would require further empirical validation against other datasets and relaxing simplifying assumptions through developing more complicated models, such as including spatial structure and stochasticity. This is also a suitable avenue for future work.
The dominant paradigm of human–environment interactions has been negative for most of human history, as we have already noted in the Introduction. However, human–environment relationships are not unidirectional and our ability to adapt also applies to our relationship with the environment. Assuming the complete extinction of endangered ecosystems, such as forest–grassland mosaics, based on past trends therefore neglects an important aspect of human–environment interactions and assumes that humans are not able to adapt. Our research demonstrates how nonlinear, coupled human–environment systems can exhibit complex dynamics due to multiple interacting social and natural feedbacks. The endpoints of such systems are not known, but modeling can help us see what the outlines of such endpoints might be. Empirically grounded simulation models such as we have developed here may be useful for guiding future land use and conservation policies.
SI Parameter Selection
Base Case Study.
The São Francisco de Paula region contains natural forest–grassland mosaic interspersed with lands converted to agriculture and silviculture (). We parameterize the model with data (Table S1) on profit and conservation values obtained from the literature and a detailed questionnaire about landowner behavior from the São Francisco de Paula region (47). We set the base-case parameters using landowner-preferred composition for each land-cover type (, , or ) and the current land composition. For each parameter there is a range of empirically plausible values that can be obtained from the literature. The model-simulated composition should reflect what we see in our study region, if our assumptions are correct. Therefore, we calibrated all model parameters except the economic discount rate to fit the regional land composition, , , , while ensuring the calibrated parameter value remained within the empirically plausible values (47). The base-case economic discount rate was set at 10% (50) and the conservation discount rate, referred to as discounting well-being in the literature, is set at 0.1% (52). The majority of the farms (crops or livestock) in southern Brazil, where forest–grassland mosaics occur, are small family farms (<40 ha) and the revenue generated from farm activities accounts for the majority of the landowner’s income (62). There has been significant expansion of converted land in the past few decades (35, 38, 47), suggesting that human influence dominates over ecological processes. Therefore, we choose a value of (i.e., moderate) to represent the base-case assumption for the strength of human influence. At this value for human influence, human behavior and natural forest recruitment both affect the land composition. We explore the effectiveness of variations in conservation values, discount rates, and discounting time horizons at maintaining natural land cover across a spectrum of human influence scenarios to increase the scope of our model. In the following subsections we describe how we choose the parameter ranges for constructing parameter planes that allow us to understand how model dynamics vary as parameters are increased or decreased.
Changing Conservation Values.
We simulate the influence of conservation values (,) as a function of rarity, as a way of evaluating sustainable decision-making practices. The range of values for and are estimates based on questionnaire results describing landowner preferences (47) and data on profits from natural lands (62). In our study region (São Francisco de Paula), the percentage of landowners preferring forest, grassland, and converted land are 30, 28, and 42%, respectively. Furthermore, 79% of landowners would rather restore Campos grassland than Araucaria forest, because produces greater profits. Therefore, we assume . Utilities represent the combined value of each land state from financial gains and conservation values, and therefore we use the landowner preference and profit to estimate conservation ranges for and . Profits generated from natural forest are considerably lower than profits from natural grassland and converted land, yet the proportion of natural forest is the largest in our study area. We observe from questionnaire results that individuals cite conservation as a motivation for preserving forest on their lands and, moreover, legal restrictions on A. angustifolia harvesting (which in turn reflect conservation values of the voting public) limit how many trees landowners can fell. Therefore, we set greater than the profit (), . Grasslands hold conservation value, in addition to being profitable, and therefore we set . The range for exceeds estimates from the questionnaire data; however, the large range for is to explore potential interesting changes in land cover for grassland conservation values that exceed . We expect that increasing conservation values will increase the proportion of natural land cover.
Changing Economic and Conservation Discount Rates.
We simulate land-cover dynamics over a range of economic discount rates () and conservation discount rates () to explore possible mechanisms that drive landowner preference. There is a wealth of literature suggesting that should be as close to zero as possible to accommodate the uncertainty associated with environmental time scales (14, 52, 59, 60), whereas experimental studies suggest that can be as high as 43% (49). Theoretically, scarce commodities, such as endangered species, should not be discounted because future quantities will not exceed those of the present (51). Land-cover dynamics for and are represented in a parameter plane. High discount rates typically lead to land exploitation, because the present holds more value than the future (41, 69). Thus, we expect that increases in and will decrease the proportion natural states.
Discounting Economic and Conservation Time Horizons.
Discount time horizons (i.e., foresight) can contribute to sustainable land management practices (14, 60). We use a parameter plane to explore the effectiveness of discount time horizons at maintaining natural land cover. Natural systems are evaluated over a much greater time scale compared with industrial or anthropogenic activities, and therefore we simulate conservation discount time horizons () from 1 to 100 and economic discount time horizons () from 1 to 50 (53). The impact of increasing discount time horizons on land-cover dynamics is highly dependent on the assumptions made by landowners. Therefore, assuming constant values over the discount time period, increasing discount time horizons () should maintain the current land composition.
Weak, Moderate, and Strong Human Influence.
Aside from the moderate human influence scenario we evaluate two alternative scenarios: weak and strong. Weak human influence is set at , such that maximum potential human influence is less than maximum potential recruitment. Strong human influence () represents intense management system, where human values outweigh the feedbacks from natural processes. Thus, strong human influence should shift the system toward the land composition with the greatest utility and weak human influence should converge to a natural forest–grassland mosaic. From previous work (11), we expect that increasing human influence will preclude bistability. We also run simulations under no human influence () to confirm natural mosaic bistability.
In the absence of human influence, conservation values, discount rates, and discount time horizons have no impact on land-cover changes. Instead, the positive feedback between recruitment and fire is the major determining factor in land-cover changes, and as such the system is bistable for all possible human-based parameters.
Supplementary Material
Acknowledgments
We thank G. Hailu, S. A. Levin, and V. de Patta Pillar for discussions. This work was supported by a James S. McDonnell Foundation Complex Systems Award (to M.A.), Natural Sciences and Engineering Research Council (NSERC) Discovery grants (to M.A. and C.T.B.), and NSERC Collaborative Research and Training Experience (CREATE) scholarships (to K.A.H.).
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Coupled Human and Environmental Systems,” held March 14–15, 2016, at the National Academy of Sciences in Washington, DC. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Coupled_Human_and_Environmental_Systems.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604987113/-/DCSupplemental.
References
- 1.Dearing JA, Battarbee RW, Dikau R, Larocque I, Oldfield F. Human–environment interactions: Learning from the past. Reg Environ Change. 2006;6(1-2):1–16. [Google Scholar]
- 2.Glaser M, Krause G, Ratter BM, Welp M. Human-Nature Interactions in the Anthropocene: Potentials of Social-Ecological Systems Analysis. Routledge; London: 2012. [Google Scholar]
- 3.Motesharrei S, Rivas J, Kalnay E. Human and nature dynamics (HANDY): Modeling inequality and use of resources in the collapse or sustainability of societies. Ecol Econ. 2014;101:90–102. [Google Scholar]
- 4.Steffen W, et al. Planetary boundaries: Guiding human development on a changing planet. Science. 2015;347(6223):1259855. doi: 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- 5.Goudie A. The Human Impact on the Natural Environment. MIT Press; Cambridge, MA: 2000. [Google Scholar]
- 6.Foley JA, et al. Global consequences of land use. Science. 2005;309(5734):570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- 7.Ellis EC, Ramankutty N. Putting people in the map: Anthropogenic biomes of the world. Front Ecol Environ. 2008;6(8):439–447. [Google Scholar]
- 8.Olsson P, Folke C, Berkes F. Adaptive comanagement for building resilience in social–ecological systems. Environ Manag. 2004;34(1):75–90. doi: 10.1007/s00267-003-0101-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, et al. Complexity of coupled human and natural systems. Science. 2007;317(5844):1513–1516. doi: 10.1126/science.1144004. [DOI] [PubMed] [Google Scholar]
- 10.Tavoni A, Schlüter M, Levin S. The survival of the conformist: Social pressure and renewable resource management. J Theor Biol. 2012;299:152–161. doi: 10.1016/j.jtbi.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Innes C, Anand M, Bauch CT. The impact of human-environment interactions on the stability of forest-grassland mosaic ecosystems. Sci Rep. 2013;3:2689. doi: 10.1038/srep02689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson KA, Anand M, Bauch CT. Carrot or stick? Modelling how landowner behavioural responses can cause incentive-based forest governance to backfire. PLoS One. 2013;8(10):e77735. doi: 10.1371/journal.pone.0077735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz PW. Conservation means behavior. Conserv Biol. 2011;25(6):1080–1083. doi: 10.1111/j.1523-1739.2011.01766.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, et al. A topology of time-scale mismatches and behavioral interventions to diagnose and solve conservation problems. Conserv Biol. 2015;30(1):42–49. doi: 10.1111/cobi.12632. [DOI] [PubMed] [Google Scholar]
- 15.Stern PC. Toward a coherent theory of environmentally significant behavior. J Soc Issues. 2000;56(3):407–424. [Google Scholar]
- 16.Balmford A, Cowling RM. Fusion or failure? The future of conservation biology. Conserv Biol. 2006;20(3):692–695. doi: 10.1111/j.1523-1739.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 17.Ostrom E, Burger J, Field CB, Norgaard RB, Policansky D. Revisiting the commons: Local lessons, global challenges. Science. 1999;284(5412):278–282. doi: 10.1126/science.284.5412.278. [DOI] [PubMed] [Google Scholar]
- 18.Sodhi NS, Butler R, Laurance WF, Gibson L. Conservation successes at micro-, meso- and macroscales. Trends Ecol Evol. 2011;26(11):585–594. doi: 10.1016/j.tree.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Calvo-Alvarado J, McLennan B, Sánchez-Azofeifa A, Garvin T. Deforestation and forest restoration in Guanacaste, Costa Rica: Putting conservation policies in context. Forest Ecol Manag. 2009;258:931–940. [Google Scholar]
- 20.Kok K, Farrow A, Veldkamp A, Verburg PH. A method and application of multi-scale validation in spatial land-use models. Agr Ecosyst Environ. 2001;85:223–238. [Google Scholar]
- 21.Bond WJ, Parr CL. Beyond the forest edge: Ecology, diversity and conservation of the grassy biomes. Biol Conservat. 2010;4:2395–2404. [Google Scholar]
- 22.Walter H, Breckle SW. Ecological Systems of the Geobiosphere: 2 Tropical and Subtropical Zonobiomes. Vol 2 Springer; New York: 2013. [Google Scholar]
- 23.Beisner BE, Haydon DT, Cuddington K. Alternative stable states in ecology. Front Ecol Environ. 2003;1(7):376–382. [Google Scholar]
- 24.Behling H, Pillar VD. Late Quaternary vegetation, biodiversity and fire dynamics on the southern Brazilian highland and their implication for conservation and management of modern Araucaria forest and grassland ecosystems. Philos Trans R Soc Lond B Biol Sci. 2007;362:243–251. doi: 10.1098/rstb.2006.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheffer M. Terrestrial Ecosystems. Princeton Univ Press; Princeton: 2009. pp. 216–239. [Google Scholar]
- 26.Blanco CC, et al. Feedbacks between vegetation and disturbance processes promote long-term persistence of forest–grassland mosaics in south Brazil. Ecol Model. 2014;291:224–232. [Google Scholar]
- 27.Silva LC, Anand M. Mechanisms of Araucaria (Atlantic) forest expansion into southern Brazilian grasslands. Ecosystems. 2011;14(8):1354–1371. [Google Scholar]
- 28.Suding KN, Gross KL, Houseman GR. Alternative states and positive feedbacks in restoration ecology. Trends Ecol Evol. 2004;19(1):46–53. doi: 10.1016/j.tree.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Carson JF, et al. Environmental impact of geometric earthwork construction in pre-Columbian Amazonia. Proc Natl Acad Sci USA. 2014;111(29):10497–10502. doi: 10.1073/pnas.1321770111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidwell SM. Biology in the anthropocene: Challenges and insights from young fossil records. Proc Natl Acad Sci USA. 2015;112(16):4922–4929. doi: 10.1073/pnas.1403660112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenton TM, et al. Tipping elements in the Earth’s climate system. Proc Natl Acad Sci USA. 2008;105(6):1786–1793. doi: 10.1073/pnas.0705414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindenmayer DB, Hobbs RJ, Likens GE, Krebs CJ, Banks SC. Newly discovered landscape traps produce regime shifts in wet forests. Proc Natl Acad Sci USA. 2011;108(38):15887–15891. doi: 10.1073/pnas.1110245108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson DM, Dinerstein E. The Global 200: A representation approach to conserving the Earth’s most biologically valuable ecoregions. Conservat Biol. 1998;12(3):502–515. [Google Scholar]
- 34.Das A, Nagendra H, Anand M, Bunyan M. Topographic and bioclimatic determinants of the occurrence of forest and grassland in tropical montane forest-grassland mosaics of the Western Ghats, India. PLoS One. 2015;10(6):e0130566. doi: 10.1371/journal.pone.0130566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overbeck GE, et al. Restoration ecology in Brazil–Time to step out of the forest. Natureza Conservação. 2013;11:92–95. [Google Scholar]
- 36.May RM. Uses and abuses of mathematics in biology. Science. 2004;303(5659):790–793. doi: 10.1126/science.1094442. [DOI] [PubMed] [Google Scholar]
- 37.Silva LC, Anand M, Oliveira JM, Pillar VD. Past century changes in Araucaria angustifolia (Bertol.) Kuntze water use efficiency and growth in forest and grassland ecosystems of southern Brazil: Implications for forest expansion. Glob Change Biol. 2009;15(10):2387–2396. [Google Scholar]
- 38.Overbeck GE, et al. Brazil’s neglected biome: The South Brazilian Campos. Perspect Plant Ecol. 2007;9:101–116. [Google Scholar]
- 39.Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:858–863. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 40.Higgins SI, Bond WJ, Trollope WSW. Fire, resprouting and variability: A recipe for grass-tree coexistence in savanna. J Ecol. 2000;88:213–229. [Google Scholar]
- 41.Satake A, Rudel TK. Modeling the forest transition: Forest scarcity and ecosystem service hypotheses. Ecol Appl. 2007;17(7):2024–2036. doi: 10.1890/07-0283.1. [DOI] [PubMed] [Google Scholar]
- 42.Archibald S, Roy DP, van Wilgen BW, Scholes RJ. What limits fire? An examination of drivers of burnt area in southern Africa. Glob Change Biol. 2009;15:613–630. [Google Scholar]
- 43.Iob G, Vieira EM. Seed predation of Araucaria angustifolia (Araucariaceae) in the Brazilian Araucaria Forest: Influence of deposition site and comparative role of small and ’large’ mammals. Plant Ecol. 2008;198:185–196. [Google Scholar]
- 44.Nazareno AG, dos Reis MS. At risk of population decline? An ecological and genetic approach to the threatened palm species Butia eriospatha (Arecaceae) of southern Brazil. J Hered. 2014;105:120–129. doi: 10.1093/jhered/est065. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira RS, et al. Deep root function in soil water dynamics in cerrado savannas of central Brazil. Funct Ecol. 2005;19(4):574–581. [Google Scholar]
- 46.Anderson NM, Williams KJH, Ford RM. Community perceptions of plantation forestry: The association between place meanings and social representations of a contentious rural land use. J Environ Psychol. 2013;34:121–136. [Google Scholar]
- 47.Henderson KA, et al. Landowner perceptions of the value of natural forest and natural grasslands in a mosaic ecosystem in southern Brazil. Sustain Sci. 2015;11:1–10. doi: 10.1007/s11625-015-0319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levin SA. Public goods in relation to competition, cooperation, and spite. Proc Natl Acad Sci USA. 2014;111(Suppl 3):10838–10845. doi: 10.1073/pnas.1400830111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duquette E, Higgins N, Horowitz J. Farmer discount rates: Experimental evidence. Am J Agr Econ. 2011;94(2):451–456. [Google Scholar]
- 50.Oliveira AD, Leite AP, Botelho SA, Scolforo JRS. Avaliação econômica da vegetação de cerrado submetida a diferentes regimes de manejo e de povoamentos de eucalipto plantado em monocultivo. Cerne. 1998;4(1):24–57. [Google Scholar]
- 51.Broome J. Discounting the future. Philos Publ Aff. 1994;23(2):128–156. [Google Scholar]
- 52.Stern NH. The Economics of Climate Change: The Stern Review. Cambridge Univ Press; Cambridge, UK: 2007. [Google Scholar]
- 53.Rout TM, Walshe T. Accounting for time preference in management decisions: An application to invasive species. J Multi-Criteria Decis Anal. 2013;20(3-4):197–211. [Google Scholar]
- 54.Rogers EM. Elements of Diffusion. 3rd Ed. Free Press; New York: 1983. pp. 1–37. [Google Scholar]
- 55.Hastings A. Transients: The key to long-term ecological understanding? Trends Ecol Evol. 2004;19:39–45. doi: 10.1016/j.tree.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Staver AC, Archibald S, Levin S. Tree cover in sub-Saharan Africa: Rainfall and fire constrain forest and savanna as alternative stable states. Ecology. 2011;92(5):1063–1072. doi: 10.1890/10-1684.1. [DOI] [PubMed] [Google Scholar]
- 57.Cialdini RB, Kallgren CA, Reno RR. A focus theory of normative conduct: A theoretical refinement and reevaluation of the role of norms in human behavior. Adv Exp Soc Psychol. 1991;24:201–293. [Google Scholar]
- 58.Bauch CT, Bhattacharyya S. Evolutionary game theory and social learning can determine how vaccine scares unfold. PLoS Comput Biol. 2012;8:e1002452. doi: 10.1371/journal.pcbi.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Markandya A, Pearce DW. Development, the environment and the social rate of discounting. World Bank Res Obs. 1991;6(2):137–152. [Google Scholar]
- 60.Norgaard RB, Howarth RB. Ecological Economics: The Science and Management of Sustainability. Columbia Univ Press; New York: 1991. Sustainability and discounting the future; pp. 88–101. [Google Scholar]
- 61.Weitzman ML. 2001. Gamma discounting. Am Econ Rev pp 260–271.
- 62.IBGE 2012. Censo agropecuário: 2006: Brasil, grandes regiões e unidades da federção: Segunda apurção (Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro)
- 63.Lampert VD, et al. Development and application of a bioeconomic efficiency index for beef cattle production in Rio Grande do Sul, Brazil. Revista Brasileira de Zootecnia. 2012;41(3):775–782. [Google Scholar]
- 64.Silva JS, Ranieri VEL. The legal reserve areas compensation mechanism and its economic and environmental implications. Ambiente & Sociedade. 2014;17(1):115–132. [Google Scholar]
- 65.Federal Government of Brazil . Lei n° 12.727, de 25 de maio de 2012. União; Brasilia: 2012. [Google Scholar]
- 66.Cubbage F, et al. Timber investment returns for selected plantations and native forests in South America and the Southern United States. New Forests. 2007;33(3):237–255. [Google Scholar]
- 67.Gray V. Innovation in the states: A diffusion study. Am Polit Sci Rev. 1973;67(4):1174–1185. [Google Scholar]
- 68.Armstrong JS. Principles of Forecasting: A Handbook for Researchers and Practitioners. Vol 30 Springer; New York: 2001. [Google Scholar]
- 69.Pearce DW. The economic value of forest ecosystems. Ecosys Health. 2001;7(4):284–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.