Significance
Histone deacetylases (HDACs) belong to a large protein family in plants, and little is known about how target specificity of each HDAC is achieved. We show that a paired SANT (SWI3/DAD2/N-CoR/TFIII-B) domain-containing protein, POWERDRESS, specifically acts with HDA9 to confer the deacetylation of histone H3 lysine residues at a set of genomic targets to regulate various biological processes. Our study elucidates the functional correlation between SANT domain-containing proteins and HDACs in plants.
Keywords: SANT domain, POWERDRESS, HDA9, histone deacetylation, AGL19
Abstract
Histone acetylation is a major epigenetic control mechanism that is tightly linked to the promotion of gene expression. Histone acetylation levels are balanced through the opposing activities of histone acetyltransferases (HATs) and histone deacetylases (HDACs). Arabidopsis HDAC genes (AtHDACs) compose a large gene family, and distinct phenotypes among AtHDAC mutants reflect the functional specificity of individual AtHDACs. However, the mechanisms underlying this functional diversity are largely unknown. Here, we show that POWERDRESS (PWR), a SANT (SWI3/DAD2/N-CoR/TFIII-B) domain protein, interacts with HDA9 and promotes histone H3 deacetylation, possibly by facilitating HDA9 function at target regions. The developmental phenotypes of pwr and hda9 mutants were highly similar. Three lysine residues (K9, K14, and K27) of H3 retained hyperacetylation status in both pwr and hda9 mutants. Genome-wide H3K9 and H3K14 acetylation profiling revealed elevated acetylation at largely overlapping sets of target genes in the two mutants. Highly similar gene-expression profiles in the two mutants correlated with the histone H3 acetylation status in the pwr and hda9 mutants. In addition, PWR and HDA9 modulated flowering time by repressing AGAMOUS-LIKE 19 expression through histone H3 deacetylation in the same genetic pathway. Finally, PWR was shown to physically interact with HDA9, and its SANT2 domain, which is homologous to that of subunits in animal HDAC complexes, showed specific binding affinity to acetylated histone H3. We therefore propose that PWR acts as a subunit in a complex with HDA9 to result in lysine deacetylation of histone H3 at specific genomic targets.
Posttranslational modifications of histones—including acetylation, methylation, phosphorylation, and ubiquitination—play important roles in plant development, genome integrity, and stress responses. Histone acetylation/deacetylation, a reversible process, promotes/represses gene expression (1) and occurs at lysine residues within histone N-terminal tails. The histone acetylation status is regulated by counteracting enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). The 18 HDACs identified in Arabidopsis (2) can be categorized into three groups based on phylogenetic analysis: reduced potassium dependency-3/histone deacetylase-1 (RPD3/HDA1), histone deacetylase-2 (HD2), and silent information regulator-2 (SIR2)-like (3). Twelve HDACs belong to the RPD3/HDA1 group (3) and are involved in various biological processes, such as organ development, reproductive processes, hormone signaling, and DNA methylation (4–9). They can be further classified into three classes based on sequence homology (3). The HD2 group is plant-specific and includes four HDACs that act in plant development and stress responses (10–13). The two HDACs encoded by the SIR2 family genes in Arabidopsis, SRT1 and SRT2, regulate mitochondrial energy metabolism and cellular dedifferentiation, respectively (14, 15).
In general, histone-modifying enzymes are components of multisubunit protein complexes, and interaction partners are thought to modulate enzymatic activity, substrate binding specificity, and cofactor recruitment. HDAC-interacting proteins in Arabidopsis include chromatin-modifying enzymes and transcription factors. The interaction partners responsible for specific biological functions of HDACs are best understood for HDA6 and HDA19 belonging to the RPD3/HDA1-class I genes (HDA6, HDA7, HDA9, and HDA19). HDA6 controls flowering time, stress response, and gene silencing through its interacting partners (13, 16–20). HDA6 associates with histone demethylase and FLOWERING LOCUS D, as well as homologs of the human histone binding proteins RbAp46/48, FVE, and MSI5 to ensure proper flowering time (16, 18, 19, 21). In addition, HDA6 physically interacts with the DNA methyltransferase MET1 and regulates a subset of transposons and repeats (17). HDA6 and HDA19 also form complexes with various transcription factors (22–26). The corepressor TOPLESS complexes with HDA6 and PSEUDO RESPONSE REGULATORs to control circadian clock function (23). HDA19 participates in brassinosteroid signaling and basal defense through its interaction with the transcription factors BRASSINAZOLE RESISTANT1 (BZR1) and WRKY 38/62, respectively (24, 26). The interacting partners of HDA9 have been elusive.
SANT (SWI3/DAD2/N-CoR/TFIII-B) domain-containing proteins exist as subunits of many chromatin remodeling complexes, such as histone acetylases, HDACs, and ATP-dependent chromatin-remodeling enzymes in yeast and animals (27, 28). The SANT domain was first described in nuclear receptor corepressors (N-CoR) and later found in the subunits of other chromatin-modifying complexes and transcription factors, including ADA, SWI-SNF, and TFIII-B (27). SANT domain function is tightly linked to enzymatic activity and substrate affinity. Deletion of the SANT domain in ADA2, a subunit of HATs, results in attenuated HAT activity and binding ability to unacetylated histone H3 tails (29, 30). Paired SANT domains (SANT1 and SANT2) are present in the corepressors SMRT (silencing mediator of retinoid and thyroid receptors), N-CoR (an HDAC3 complex subunit), and CoREST (an HDAC1 complex subunit) (27, 31). The two SANT domains have distinct roles in terms of HDAC function: SANT1 is responsible for HDAC activity and protein interaction, whereas SANT2 is necessary for substrate recognition (31–33). In contrast to the in-depth study of SANT domain-containing proteins in yeast and animals, the functions of SANT domain-containing proteins and their interaction partners in plants remain unclear.
POWERDRESS (PWR) encodes a protein with two SANT domains in Arabidopsis (34). A pwr mutant was isolated as an enhancer of ag-10, a weak allele of AGAMOUS (AG); the pwr ag-10 double-mutant had prolonged floral stem cell activity, suggesting that PWR promotes the termination of floral stem cell fate. The pwr single-mutant exhibited other developmental defects, including bulged silique tips and early flowering. The broad spectrum of developmental defects of the pwr mutant indicates that PWR is involved in diverse biological processes. Although PWR’s role in floral stem cell regulation can be explained by its positive effects on the expression of a subset of MIR172 genes and CRABS CLAW (CRC), how it affects other aspects of plant development is unknown. Furthermore, the molecular function of PWR in gene regulation is unknown.
Here, we show that PWR acts in association with HDA9 to repress gene expression through histone deacetylation. The SANT2 domain of PWR binds acetylated histone H3, including acetylated H3K9 and H3K14. The high degree of similarity between pwr and hda9 mutants in terms of both morphological phenotypes and the hyperacetylation status of histone H3 suggests a functional link between PWR and HDA9. Indeed, PWR and HDA9 participate in histone H3K9 and H3K14 deacetylation and repression of gene expression at highly overlapping sets of genomic targets. One such target is AGL19, the misregulation of which was previously associated with the early-flowering phenotype of hda9 mutants (35). We show that PWR and HDA9 act together on AGL19 in the regulation of flowering time. Moreover, PWR and HDA9 were found to physically interact. Taken together, these findings suggest that a PWR–HDA9 complex regulates histone H3 deacetylation to control various biological processes.
Results
The PWR SANT2 Domain Preferentially Binds Modified Histone H3.
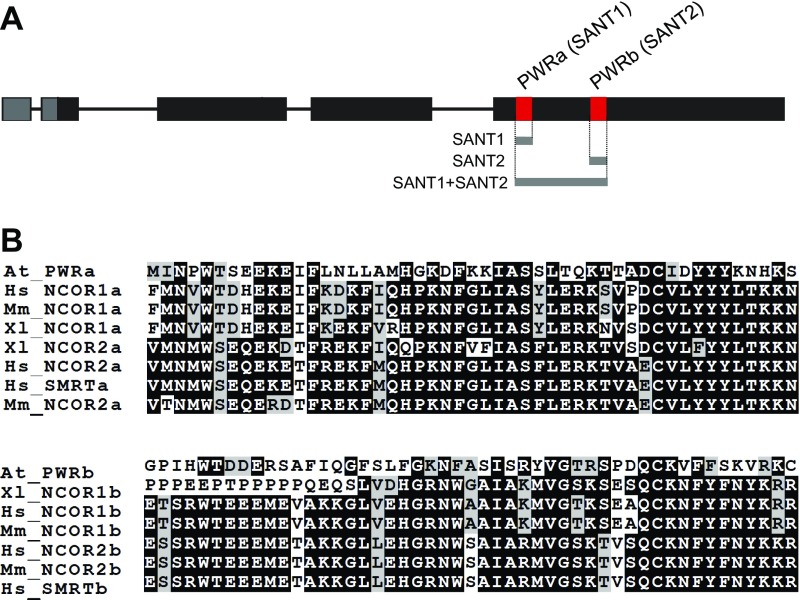
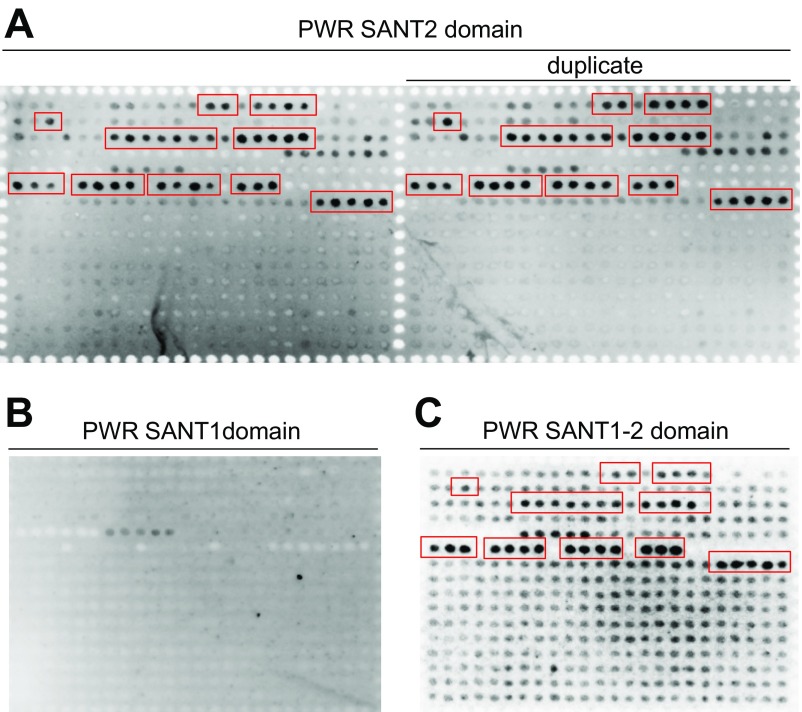
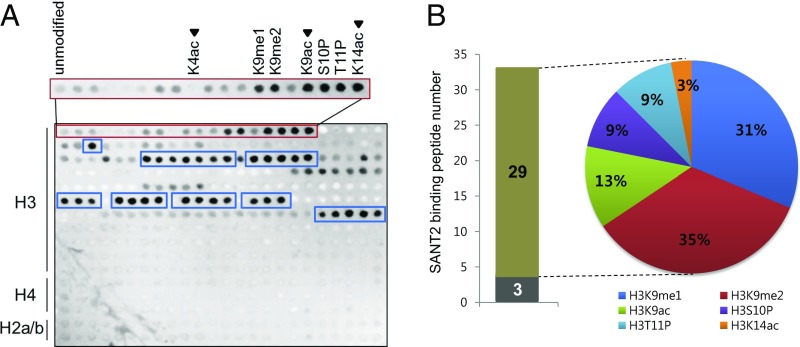
We reported previously that PWR regulates floral stem cell fate through promotion of miR172 and CRC expression (34), but its molecular function was unknown. The paired SANT domains (SANT1 and SANT2), a distinct feature of PWR, were found to have high sequence similarity with the animal HDAC subunits SMRT and N-CoR (52% and 63% similarity, respectively) (Fig. S1). The SANT1 and SANT2 domains of SMRT are necessary for HDAC activity and binding to unmodified histone tails, respectively (32). To test whether the PWR SANT domains have similar functions to those of the SMRT SANT domains, a histone peptide array was probed with purified recombinant His-PWR SANT1, His-PWR SANT2, and His-PWR SANT1+SANT2 (Fig. S2). Although the PWR SANT1 domain did not bind any histone peptides (Fig. S2B), the SANT2 domain strongly bound to a subset of modified histone H3 but did not show any affinity for histone H4 or H2A/B; this binding preference was perfectly repeated in duplicated arrays in the same chip (Fig. 1A and Fig. S2A). The PWR SANT1+SANT2 also bound the same peptides (Fig. S2C). Because SANT1 did not bind histone peptides, the SANT1+SANT2 data independently verified the SANT2 binding specificities. Among monomodified H3, PWR SANT2 selectively bound to K9me1, K9me2, K9ac, S10P, T11P, and K14ac compared with unmodified H3 or H3K4ac. It also interacted with 32 kinds of multimodified histone H3, 29 of which contained at least one of the above modifications bound by His-PWR SANT2 (Fig. 1 and Table S1). Modifications at the H3K9 residue were the most preferred by the PWR SANT2 domain among the histone H3 monomodifications (∼70%) (Fig. 1B). This finding suggests that the PWR SANT2 domain recognizes modified histone H3 and raises the possibility that PWR is functionally relevant in histone modifications.
Fig. S1.
Features of the PWR SANT domains. (A) A schematic gene diagram of PWR showing the regions encoding two SANT domains in close proximity within the last exon. Gray bars indicate PWR SANT domains used to probe histone peptide arrays. (B) Sequence homology analysis of the two SANT domains in PWR and those from animal homologs also containing paired SANT domains. Identical and similar amino acids are highlighted in black and gray, respectively. The analysis was performed using the Clustal W program.
Fig. S2.
Histone peptide array analysis of the binding preference of the PWR SANT domains to histone peptides. Histone peptide array was performed to evaluate the binding affinity of the SANT2 domain in duplicate (A), SANT1 domain (B), and SANT1+2 (C) to various modified histone peptides. Red boxes represent PWR SANT2- or SANT1+SANT2-bound, modified histone H3 peptides.
Fig. 1.
The PWR SANT2 domain interacts with a subset of acetylated lysine residues of histone H3. (A) Histone peptide array analysis of the binding of the PWR SANT2 domain to histone peptides. Darker spots indicate stronger interaction between the PWR SANT2 domain and histone peptides. The red box includes unmodified and monomodified histone H3 peptides. PWR SANT2 binds strongly to six monomodified histone H3 peptides (K9me1, K9me2, K9ac, S10P, T11P, and K14ac). Note that, of the acetylated marks, PWR SANT2 binds K9ac and K14ac, but not K4ac (arrowheads). PWR SANT2-bound, multimodified histone H3 peptides are marked by blue boxes. (B) Graph and pie chart showing the representation of the six PWR SANT2-bound, monomodified histone H3 peptide species among 32 PWR SANT2-bound, multimodified histone H3 peptides. The gray and brown bars show the number of PWR SANT2-bound, multimodified histone H3 peptides that do not contain and contain PWR SANT2-bound monomodified histone H3 peptide species, respectively. The percentages in the pie chart indicate the occurrence rate of PWR SANT2-bound monomodified histone H3 peptide species.
Table S1.
List of PWR SANT2-bound, multimodified histone H3 peptides
| No. | PWR SANT2-bound, multimodified histone H3 peptides |
| 1 | H3-R2me2a K4me2 |
| 2 | H3-R8me2a K9me3 |
| 3 | H3-R8me2a K9ac |
| 4 | H3-R8me2a S10P |
| 5 | H3-R8me2a T11P |
| 6 | H3-R8Citr K9me1 |
| 7 | H3-R8Citr K9me2 |
| 8 | H3-R8Citr K9me3 |
| 9 | H3-R8Citr S10P |
| 10 | H3-R8Citr T11P |
| 11 | H3-K9me1 S10P |
| 12 | H3-K9me1 T11P |
| 13 | H3-K9me1 K14ac |
| 14 | H3-R2me2a K4me2 K9ac |
| 15 | H3-R2me2a K4me3 K9ac |
| 16 | H3-R2me2a K4ac K9ac |
| 17 | H3-K4me2 R8me2s K9me1 |
| 18 | H3-K4me3 R8me2s K9me1 |
| 19 | H3-K4ac R8me2s K9me1 |
| 20 | H3-K4me1 R8me2a K9me1 |
| 21 | H3-K4me3 R8me2a K9me1 |
| 22 | H3-K4ac R8me2a K9me1 |
| 23 | H3-K4me1 R8me2s K9me2 |
| 24 | H3-K4me2 R8me2s K9me2 |
| 25 | H3-K4ac R8me2s K9me2 |
| 26 | H3-K4me1 R8me2a K9me2 |
| 27 | H3-K4me2 R8me2a K9me2 |
| 28 | H3-R2me2s K4me1 R8me2s K9me2 |
| 29 | H3-R2me2s K4me2 R8me2s K9me2 |
| 30 | H3-R2me2s K4me3 R8me2s K9me2 |
| 31 | H3-R2me2s K4ac R8me2s K9me2 |
| 32 | H3-R2me2a K4me1 R8me2s K9me2 |
Bold indicates PWR SANT2-bound, monomodified histone H3 peptide species.
PWR Specifically Promotes Histone H3 Deacetylation.
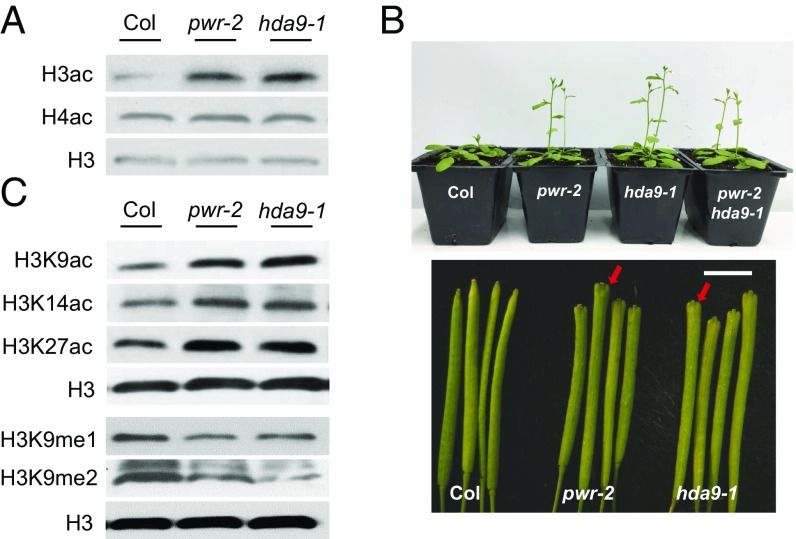
In light of the sequence similarity of the PWR SANT2 domain to that of HDAC subunits and its affinity for H3K9ac and H3K14ac, the effect of PWR on histone H3 acetylation status was analyzed. Histone H3 and H4 acetylation levels were examined by Western blotting using pan-acetylated H3 and H4 antibodies. Consistent with the histone peptide array results, hyperacetylation of histone H3 was observed in pwr-2, whereas no difference was observed for histone H4 acetylation (Fig. 2A). The levels of histone H3 acetylation at K9, K14, and K27 were all increased in pwr-2 (Fig. 2C). These results suggest that PWR may contribute to the regulation of histone H3 acetylation.
Fig. 2.
PWR and HDA9 are specifically required for histone H3 deacetylation. (A and C) Western blot analysis of histone H3 acetylation levels. Total protein extract was resolved on an SDS/PAGE gel and blotted against pan-acetylated H3 (A) and acetylated-H3K9/H3K14/H3K27 and mono/dimethylated-H3K9antibodies (C). H3 was used as an internal control. (B) Phenotypes of pwr-2, hda9-1, and pwr-2 hda9-1. Plants were grown under continuous light. Note the enlarged silique tips in pwr-2 and hda9-1 (arrows). (Scale bar, 4 mm.)
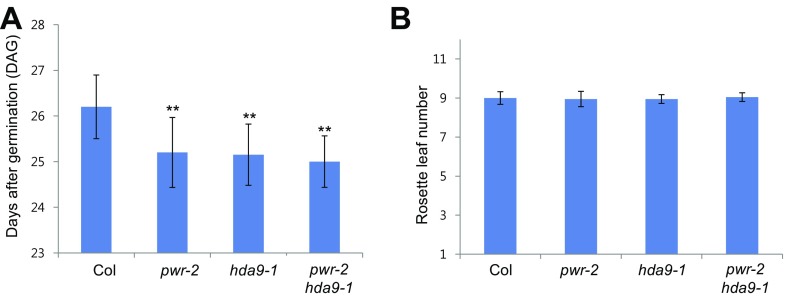
To investigate the functional correlation between PWR and HDACs, we screened several hda mutants for similarities with pwr mutants in morphology. From both the screen and published reports, the phenotype of hda9 mutants was similar to that of pwr mutants, including early flowering and slightly bulged silique tips (Fig. 2B and Fig. S3) (35, 36). The early flowering phenotype was much milder under continuous light compared with short-day conditions (35, 36), such that pwr-2 and hda9-1 mutants bolted about 1.5 d earlier than wild type, with a similar number of rosette leaves to that in wild type at bolting (Fig. S3). Additionally, the acetylation levels of histone pan-H3, but not pan-H4, were increased in the hda9 mutant (Fig. 2A). H3K9 and H3K14 acetylation levels were also increased in hda9 (Fig. 2C). Hyperacetylation of H3K27 in the hda9 mutant was reported in a previous study (35) and was also observed here (Fig. 2C). The similar morphological and histone acetylation phenotypes of the two mutants led us to hypothesize that PWR and HDA9 act closely to deacetylate histone H3 at specific target genes.
Fig. S3.
Flowering time of Col, pwr-2, hda9-1, and pwr-2 hda9-1 under continuous light condition. Flowering time was determined by DAG (A) and rosette leaf number at bolting (B). Twenty plants were surveyed. **P = 0.
PWR and HDA9 Enhance Histone H3 Deacetylation at Largely Overlapping Sets of Targets.
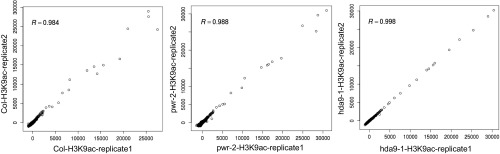
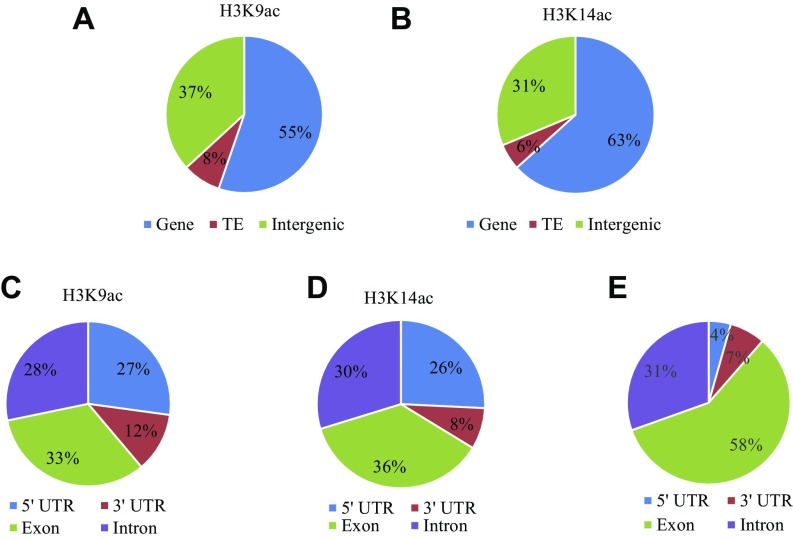
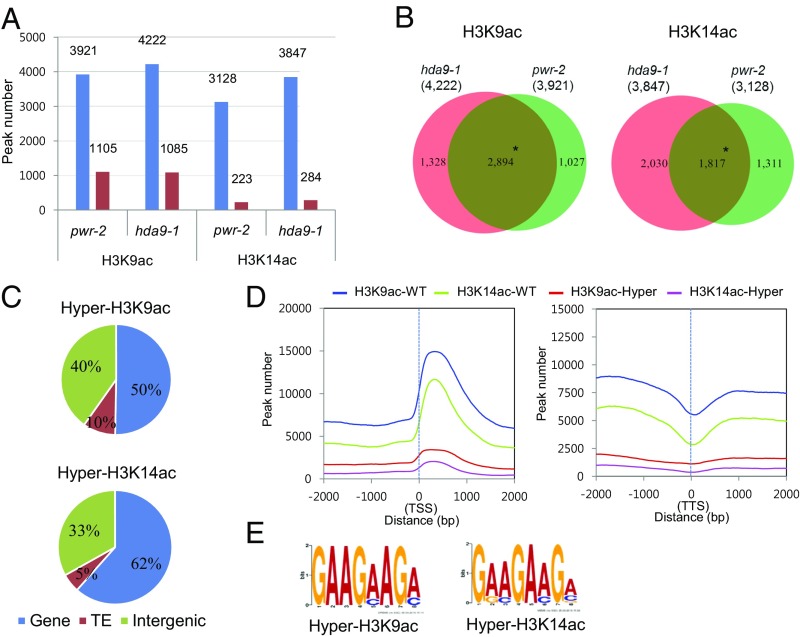
To determine whether PWR promotes deacetylation at specific genomic targets and, if so, whether PWR and HDA9 affect the same targets, a global analysis of the acetylation status of histone H3K9 and H3K14 was conducted. Chromatin immunoprecipitation (ChIP) high-throughput sequencing (ChIP-seq) was performed in wild type, pwr-2, and hda9-1 using antibodies against H3K9ac and H3K14ac. For H3K9ac ChIP-seq, two biological replicates were performed; Pearson’s correlation analysis showed that the replicates were reasonably reproducible (Fig. S4). H3K14ac ChIP-seq was carried out with one replicate, but randomly selected observations were later validated by ChIP-quantitative PCR (ChIP-qPCR) (Fig. S5). In wild type, a total of 12,650 H3K9ac peaks were found. As observed before (37, 38), the peaks were mostly in genes or intergenic regions (Fig. S6A). For the H3K14ac ChIP-seq, a total of 11,141 H3K14ac peaks were found. The genomic distribution of H3K14ac was similar to that of H3K9ac, but a stronger enrichment for genic regions than H3K9ac was found (Fig. S6B). Within genes, H3K9ac and H3K14ac marks were strongly enriched in 5′UTRs, which constitute 4% of all genes (Fig. S6E) but more than 25% of H3K9ac- or H3K14ac-containing genic regions (Fig. S6 C and D). In addition, H3K9 and H3K14 acetylation was centered at ∼300 bp downstream of the transcriptional start site (TSS) and markedly dropped off at the transcription termination site (TTS) (Fig. 3D).
Fig. S4.
Scatterplots showing the correlation between two ChIP-seq replicates. The x and y axes represent normalized signal intensity from replicate 1 and the replicate 2 in log2 scale, respectively.
Fig. S5.
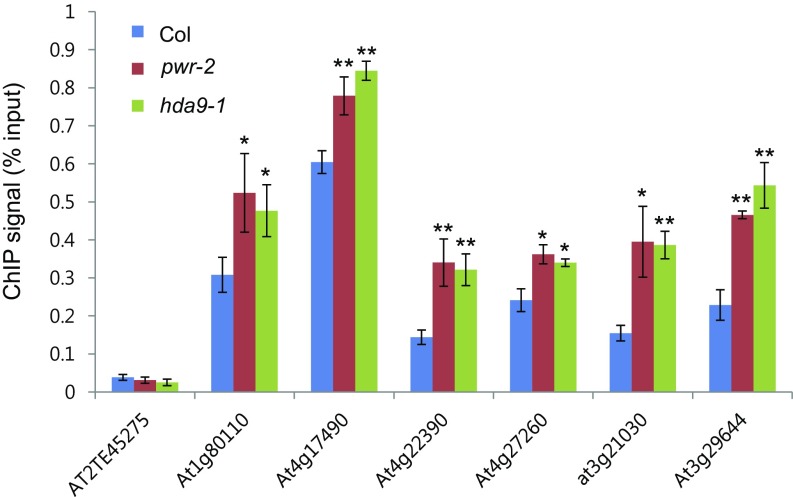
Validation of H3K14ac ChIP-seq data. H3K14ac levels of randomly selected hyperacetylation peaks were determined by ChIP-qPCR in pwr-2 and hda9-1. AT2TE45275 was used as a negative control. Error bars indicate SD from three biological replicates. *P < 0.05 and **P < 0.01.
Fig. S6.
Pie charts showing the genomic features at the H3K9ac and H3K14ac peaks from wild type. (A and B) Distribution of H3K9ac (A) and H3K14ac (B) peaks among genes, TEs, and intergenic regions in the genome. (C–E) Distribution of H3K9ac (C) and H3K14ac (D) peaks across different features within genes. The proportions of various features (5′UTR, 3′UTR, exon, and intron) in all genes are shown in E. The H3K9ac and H3L14ac marks are highly enriched in the 5′UTR, as reflected by the higher proportions of 5′UTRs in H3K9ac and H3K14ac regions compared with the genome average (E).
Fig. 3.
Hyperacetylated H3K9 and H3K14 regions in pwr and hda9 significantly overlap. (A) Histogram showing ChIP-seq–derived differentially acetylated regions for H3K9 and H3K14 in pwr-2 and hda9-1. Blue and red bars represent hyper- and hypoacetylation, respectively. (B) Venn diagram showing the overlap of hyperacetylation ChIP peaks between pwr-2 and hda9-1. *P = 0 (hypergeometric test). (C) Pie charts showing the genomic distribution of overlapping hyperacetylation ChIP peaks in pwr-2 and hda9-1 from B. (D) The distribution of all ChIP peaks in wild type (blue and green curves) and overlapping hyperacetylation ChIP peaks in pwr-2 and hda9-1 (red and magenta curves) around the TSS (Left) and TTS (Right) of protein-coding genes. (E) The motifs significantly enriched in hyperacetylation ChIP peaks in pwr-2 and hda9-1 through MEME-ChIP analysis.
Next, we identified differentially acetylated regions between each mutant and wild type. Among the differentially acetylated regions identified, the majority were hyperacetylated in pwr-2 (H3K9ac: 3,921 of 5,026; H3K14ac: 3,128 of 3,351) and hda9-1 (H3K9ac: 4,222 of 5,307; H3K14ac: 3,847 of 4,131) compared with wild type (Col) (Fig. 3A), which is consistent with the observed increase in H3K9ac and H3K14ac levels in the two mutants (Fig. 2C). The hyperacetylated regions accounted for 30–40% of all ChIP-derived peaks for H3K9 (pwr-2: 3,921 of 12,890; hda9-1: 4,222 of 12,745) and H3K14 (pwr-2: 3,128 of 12,791; hda9-1: 3,847 of 13,122) in the pwr and hda9 mutants, which reflects that PWR and HDA9 contribute to more than one-third of H3K9 and H3K14 deacetylation at a genome-wide level. Comparative analysis of the hyperacetylated regions in pwr-2 and hda9-1 revealed significant overlap: about 70% overlap for H3K9ac (P = 0; hypergeometric test) and 50–60% overlap for H3K14ac (P = 0; hypergeometric test) (Fig. 3B). Further analysis of the overlapping regions of hyperacetylated H3K9 and H3K14 between pwr-2 and hda9-1 revealed that more than 50% corresponded to genes and a minor fraction corresponded to transposable elements (TEs) (Fig. 3C), which is consistent with the nature of H3K9ac and H3K14ac as euchromatic marks. Furthermore, in the overlapping, hyperacetylated regions, H3K9 and H3K14 acetylation was peaking at ∼300 bp downstream of the TSS, as observed in wild type (Fig. 3D). MEME-ChIP analysis (39) was performed to identify overrepresented motifs among the genomic targets of PWR and HDA9 in terms of histone H3K9 and H3K14 deacetylation. Similarly, motifs GAAG(A/C)AG(A/C) and G(A/G)(A/C)GA(A/C)G(A/C/G) were identified as the most significant motifs for H3K9ac and H3K14ac marks, respectively, from overlapping hyperacetylation regions between pwr-2 and hda9-1 (Fig. 3E). These findings indicate that PWR and HDA9 regulate histone H3 deacetylation mainly at actively transcribed regions and that the functions of these two genes are tightly correlated.
PWR and HDA9 Share a Significant Number of Target Genes.
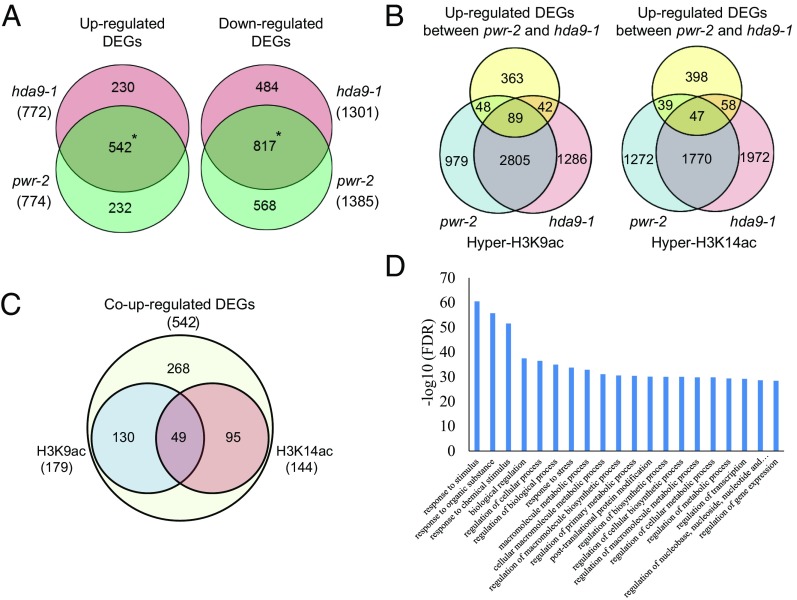
The studies above revealed hyperacetylation at common genomic sites in the pwr and hda9 mutants, raising the possibility that the two genes regulate similar sets of target genes. Transcriptome analysis was performed to examine the correlation between the histone acetylation status and gene-expression levels of the targets of PWR and HDA9. In pwr-2 and hda9-1, 2,159 and 2,073 differentially expressed genes (DEGs) were identified, respectively (Fig. 4A). Among the DEGs, 774 and 1,385 genes were increased and reduced in pwr-2, respectively, and 772 and 1,301 genes were increased and reduced in hda9-1, respectively (Fig. 4A). There was a significant overlap between the DEGs identified in pwr-2 and hda9-1 [542 increased DEGs and 817 reduced DEGs, P = 0 (hypergeometric test)] (Fig. 4A), indicating that PWR and HDA9 act in the same regulatory pathway. Gene ontology (GO) analysis revealed that the up-regulated genes in pwr-2 and hda9-1 were mainly involved in stress responses (Fig. 4D). Further analysis was performed to assess the correlation between overlapping up-regulated DEGs and ChIP-derived hyperacetylated regions in the corresponding gene body and promoter regions (up to 2-kb upstream of the TSSs). Of the 542 genes identified as up-regulated DEGs in both mutants (i.e., co–up-regulated DEGs), 179 and 144 genes were hyperacetylated at H3K9 and H3K14, respectively, in the gene body or promoter regions in pwr-2 or hda9-1 (Fig. 4B). About 50% of the hyperacetylated DEGs for H3K9 and H3K14 overlapped between pwr-2 and hda9-1 (H3K9: 89 of 137 in pwr-2 and 89 of 131 in hda9-1; H3K14: 47 of 86 in pwr-2 and 47 of 105 in hda9-1) (Fig. 4B). Furthermore, 274 of the 542 co–up-regulated DEGs retained hyperacetylation status for either H3K9 or H3K14 (Fig. 4C). About 30% (49 of 179 for H3K9 and 49 of 144 for H3K14) had both H3K9 and H3K14 hyperacetylation (Fig. 4C), suggesting some degree of functional specificity between H3K9 and H3K14 acetylation of target genes. Taken together, these findings indicate that histone deacetylation at either H3K9 or H3K14 through PWR and HDA9 is significantly correlated with gene expression at the target loci.
Fig. 4.
Histone deacetylation through PWR and HDA9 correlates with gene expression. (A) Venn diagrams showing the overlapping up-regulated (Left) and down-regulated (Right) DEGs identified in pwr-2 and hda9-1 (i.e., coregulated DEGs). *P = 0 (hypergeometric test). (B) Venn diagrams showing the overlap of co–up-regulated DEGs and hyperacetylated H3K9 (Left) or hyperacetylated H3K14 (Right) in pwr-2 and hda9-1. (C) Venn diagram showing the status of H3K9 or H3K14 hyperacetylation in either pwr-2 or hda9-1 among the 542 co–up-regulated DEGs. (D) GO term analysis of co–up-regulated DEGs in pwr-2 and hda9-1. FDR, false-discovery rate.
PWR and HDA9 Act in the Same Genetic Pathway Through AGL19 to Ensure Proper Flowering Time.
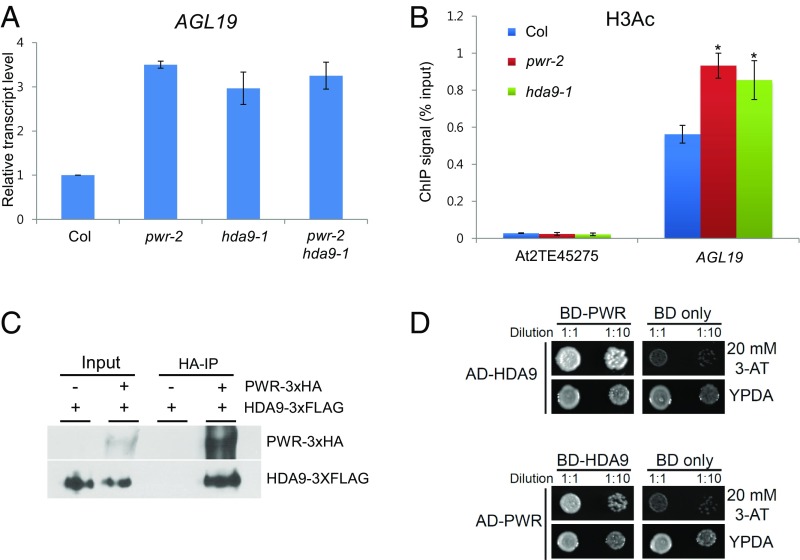
One of the obvious phenotypes of pwr and hda9 mutants is early flowering (Fig. 2B). Several studies have shown that early flowering in hda9 is because of increased AGL19 levels, which results from increased levels of histone acetylation and Pol II occupancy at the locus (35, 36). To investigate whether PWR acts in the AGL19-mediated flowering-time pathway through HDA9 function, we first examined AGL19 transcript levels in pwr-2. AGL19 expression was higher in pwr-2 as in hda9-1 (Fig. 5A). Next, the acetylation level of histone H3 was tested in pwr-2 and hda9-1 by ChIP-qPCR, and the analysis revealed increased histone H3 acetylation in pwr-2 and hda9-1 (Fig. 5B). Finally, no synergetic effect was observed in the pwr-2 hda9-1 double-mutant compared with either single-mutant in terms of AGL19 transcript levels and flowering time (Figs. 2B and 5A and Fig. S3). Taken together, these findings indicate that PWR and HDA9 act in the same genetic pathway in the regulation of flowering time.
Fig. 5.
The PWR–HDA9 complex regulates flowering time through AGL19. (A) Quantitative RT-PCR analysis of AGL19 expression in pwr-2, hda9-1, and pwr-2 hda9-1. AGL19 transcript levels were normalized to UBQ5 and compared with Col (wild type). (B) The acetylation status of histone H3 at the AGL19 locus in pwr-2 and hda9-1 determined by ChIP. AT2TE45275 was used as a negative control. Error bars indicate SD derived from two biological replicates. *P < 0.05. (C) Coimmunoprecipitation (co-IP) experiment showing the interaction between PWR-3xHA and HDA9-3XFLAG in a tobacco transient-expression assay. (D) Yeast two-hybrid confirmation of PWR–HDA9 interaction. The interaction was tested with SD-Leu/-Trp/-His media with 20 mM 3-amino-1,2,4-triazole (3-AT). Yeast grown on normal media (YPDA) served as a control. AD, activation domain; BD, binding domain.
PWR Physically Interacts with HDA9.
Our results indicate that the functions of PWR and HDA9 are tightly correlated: they share histone deacetylation targets and regulate some of the same biological processes. Because N-CoR/SMRT acts together with HDAC3 as a subunit of the complex (32), we investigated whether PWR is associated with HDA9. HA epitope-fused PWR (PWR-3xHA) and FLAG epitope-tagged HDA9 (HDA9-3xFLAG) were transiently expressed in Nicotiana benthamiana. Immunoprecipitation was performed with anti-HA antibodies to pull down PWR-3xHA followed by Western blotting to detect HDA9-3xFLAG. Indeed, HDA9-FLAG was enriched in the PWR-3xHA immunoprecipitate (Fig. 5C). To confirm the interaction between PWR and HDA9, we performed a yeast two-hybrid assay and PWR was found to interact with HDA9 (Fig. 5D). These results suggest that PWR interacts with HDA9 and may function in the same complex.
Discussion
The presence of a pair of SANT domains in PWR and their high sequence similarity to those of animal HDAC subunits were a major clue in the investigation of the molecular function of PWR. We found that the PWR SANT2 domain recognizes histones, as does the SANT2 motif of the HDAC-associated subunit SMRT/N-CoR. This finding prompted further studies on PWR and HDACs and led to the conclusion that PWR acts together with HDA9 to deacetylate H3K9 and H3K14 at numerous genes. Unlike the specific binding of the SMRT SANT2 domain to an unacetylated histone H4 tail (32), the PWR SANT2 domain preferentially binds to acetylated histone H3. Yu et al. (32) proposed that the SMRT SANT2 domain binds to unacetylated histone H4, possibly to recruit other cofactors or to prohibit further modification. In contrast, the binding of the PWR SANT2 domain to targets probably promotes histone H3 deacetylation in plants. The PWR SANT2 domain exhibited specificity for modified histone H3 but not H4 or H2A/B (Fig. 1A), which is consistent with the hyperacetylation status in pwr-2 and hda9-1 being specific for histone H3 and not H4 (35 and present study). Given the interaction between PWR and HDA9 and the significant overlap in their deacetylation targets in the genome, we propose that the PWR SANT2 domain confers substrate binding preferences to HDA9.
HDA9 regulates several biological processes, such as flowering time, seed dormancy, and stress responses (14, 35, 36, 40, 41). Although previous studies have shown that development and stress-response genes are up-regulated and this is accompanied by hyperacetylation in the hda9 mutant, there have been no reports about HDA9-interacting proteins required for its histone deacetylation function. The physical interaction between HDA9 and PWR and the similar morphological and molecular defects in the pwr and hda9 mutants support the hypothesis that PWR and HDA9 act in the same complex. However, it is not clear whether HDA9 is the only interacting partner of PWR. The histone peptide array showed that in addition to acetylated histone H3, the PWR SANT2 domain also bound to other modified histone H3, such as H3K9me1 and H3K9me2 (Fig. 1A), and the levels of H3K9me1 and H3K9me2 were reduced in pwr-2 and hda9-1 (Fig. 2C). This finding raises two possibilities. First, the PWR–HDA9 complex coordinates histone acetylation and methylation for transcriptional regulation. For example, PWR may act as a histone code reader of which the SANT2 domain recognizes H3K9me1/me2 and interprets its repressive mode through histone deacetylation by recruiting HDA9. Because H3K9me1/me2 is mainly found at heterochromatin, this may be a mechanism to prevent histone acetylation at such genomic regions. Second, PWR acts in a distinct complex from the one containing HDA9 to regulate other histone modifications. Several histone demethylases are known to interact with N-CoR. For example, JMJD2A, which demethylases di- or trimethylated histone H3K9, was isolated from affinity purification of the N-CoR complex (42).
Our previous study revealed that PWR regulates floral stem cells as a positive regulator of a subset of MIR172 genes and CRC (34). Given our finding that PWR acts in histone H3 deacetylation, the reduced transcript levels of MIR172 and CRC in pwr may be an indirect effect. In fact, there were a large number of down-regulated DEGs observed in pwr-2 and hda9-1 along with significant overlap between them (Fig. 4A). As an example, the level of miR164 was increased in pwr-2 (34) and, consistently, ChIP-seq revealed H3K9 hyperacetylation at MIR164b in both pwr-2 and hda9-1. Therefore, MIR164b could be a direct target of the PWR–HDA9 complex and increased miR164 levels are expected to result in reduced expression of miR164 target genes in pwr and hda9 mutants. It is not yet clear how the PWR–HDA9 complex specifies its targets. Motif analysis uncovered significant enrichment of sequences from the H3K9ac and H3K14ac ChIP-seq peaks (Fig. 3E), which are very similar to the GAAGAA motif. No transcription factors targeting this motif are known, but several studies have proposed that this motif is significantly enriched in splicing junction proximal exons, possibly as an exonic splicing enhancer (43–45). Therefore, the GAAGAA motif present in some genes may be recognized by PWR–HDA9 to regulate their transcription. Alternatively, the GAAGAA motif may simply be associated with histone acetylation in spliced genes, leading to an overrepresentation of this motif in the dataset rather than being directly targeted by the PWR–HDA9 complex.
Materials and Methods
For plant materials, histone peptide array, Western blot analysis, mRNA-seq, ChIP-seq, and other methods, please see SI Materials and Methods. See Table S2 for a list of the primers used. A summary of the ChIP-seq reads is provided in Table S3.
Table S2.
Primers used in this study
| Primer | Sequence | Usage |
| SANT2-F | AAAGGATCCGGTCCTATTCACTGGACAGATGATG | Cloning |
| SANT2-R | AAACTCGAGCTACCCAAGACATTTCCGAACTTTGCTG | Cloning |
| PWR-CDS-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTTATGCCGCAGGATCACGCT | Cloning |
| PWR-CDS-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACGTGGCTGCCTCTGCTACAC | Cloning |
| HDA9-F | CTGCAGATGCGTTCCAAGGACAAAATCT | Cloning |
| HDA9-R | GGATCCGGTGACGCATCGTTATCGTTGTC | Cloning |
| AGL19-F | TCAGCAAGCGAGAGACGAAACATC | qRT-PCR |
| AGL19-R | TGCATCAATGCCTTCTCCAAGCAA | qRT-PCR |
| AGL19-ChIP-F | TTTCTTTCTTTCTCTCCCCTCCTTCAT | ChIP-qPCR |
| AGL19-ChIP-R | ATCTATCTTCTATAAGTGAGTGGAGAGT | ChIP-qPCR |
| N-UBQ5 | GGTGCTAAGAAGAGGAAGAAT | qRT-PCR |
| C-UBQ5 | CTCCTTCTTTCTGGTAAACGT | qRT-PCR |
| At2TE45275-F | ACGTTGACATAATTCCCAAA | ChIP-qPCR |
| At2TE45275-R | TGAGATCAAATCAAAATAAGAGG | ChIP-qPCR |
| At1g80110-F | AGCAAATAAGCAATCAATCG | ChIP-qPCR |
| At1g80110-R | ACGAGGGAGAAGAAGAGTTG | ChIP-qPCR |
| At4g17490-F | ACGAGTTCCACGACGAGT | ChIP-qPCR |
| At4g17490-R | TTGTAGCAGCAGAGGAGAAG | ChIP-qPCR |
| At4g22390-F | CCAAAAACCTCTGTAAAACCT | ChIP-qPCR |
| At4g22390-R | AGTTCGTCTCATCTCATCTCC | ChIP-qPCR |
| At4g27260-F | TCCTTTGCTCTGACTCTTACC | ChIP-qPCR |
| At4g27260-R | AACTTGATGGCTCTGATGAA | ChIP-qPCR |
| At3g21030-F | CAACGACGAATCCAGAGTTA | ChIP-qPCR |
| At3g21030-R | TGAGCAAATCCCAAAGTTC | ChIP-qPCR |
| At3g29644-F | CAGTGGCTGATGTTCTCTGT | ChIP-qPCR |
| At3g29644-R | GCTTTAGTTGATTTGAAGGTTT | ChIP-qPCR |
Table S3.
Summary of ChIP-seq reads
| Sample | Total reads | Genome-aligned reads (% of total) |
| H3K14ac-Col-Input | 15,518,166 | 10,615,589 (68.4) |
| H3K14ac-pwr-2-Input | 15,227,332 | 10,408,650 (68.4) |
| H3K14ac-hda9-1-Input | 12,285,281 | 8,546,933 (69.6) |
| H3K14ac-Col-ChIP | 11,469,414 | 7,346,168 (64.1) |
| H3K14ac-pwr-2-ChIP | 16,311,771 | 10,410,312 (63.8) |
| H3K14ac-hda9-1-ChIP | 11,667,955 | 7,668,366 (65.7) |
| H3K9ac-Col-Input-rep1 | 76,095,338 | 48,967,220 (64.3) |
| H3K9ac-Col-Input-rep2 | 73,988,124 | 47,381,829 (64) |
| H3K9ac-pwr-2-Input-rep1 | 32,612,457 | 20,156,770 (61.8) |
| H3K9ac-pwr-2-Input-rep2 | 28,907,658 | 17,970,232 (62.2) |
| H3K9ac-hda9-1-Input-rep1 | 33,149,537 | 21,211,954 (64) |
| H3K9ac-hda9-1-Input-rep2 | 43,118,979 | 27,694,878 (64.2) |
| H3K9ac-Col-ChIP-rep1 | 67,975,951 | 67,346,244 (99.1) |
| H3K9ac-Col-ChIP-rep2 | 69,372,047 | 68,710,409 (99) |
| H3K9ac-pwr-2-ChIP-rep1 | 32,976,355 | 32,654,209 (99) |
| H3K9ac-pwr-2-ChIP-rep2 | 31,221,673 | 30,927,791 (99.1) |
| H3K9ac-hda9-1-ChIP-rep1 | 39,477,741 | 39,066,347 (99) |
| H3K9ac-hda9-1-ChIP-rep2 | 56,646,443 | 56,063,321 (99) |
SI Materials and Methods
Plant Materials and Growth Conditions.
Arabidopsis plants were grown at 23 °C under continuous light. pwr-2 was described in ref. 34, and hda9-1 was described in ref. 41. All mutants were in the Columbia background. Seeds were surface-sterilized and sown on MS-agar plates. After 2 d of cold treatment, the plated seeds were transferred to 23 °C under long-day (16-h light, 8-h dark) or short-day (8-h light, 16-h dark) conditions.
Plasmid Construction.
For expression of the PWR SANT2 domain in Escherichia coli, the PWR SANT2 domain was amplified from cDNA using the SANT2-F and SANT2-R primers. The PCR fragment was cloned into pET32a at the BamHI and XhoI sites (pET32a-SANT2). To generate the PWR entry clone for in planta PWR expression, the PWR coding region was amplified using PWR-CDS-F and PWR-CDS-R and cloned into pDONR221 by the standard Gateway cloning system (Invitrogen). The PWR coding region was subsequently introduced into the gCsVMV-3xHA-N-1300 destination vector through Gateway cloning to generate gCsVMV-PWR-3xHA. The HDA9 coding region was amplified using HDA9-F and HDA9-R primers and cloned into pCAMBIA1390, which also contained 3xFLAG, using the PstI and BamHI sites to generate pCAMBIA-HDA9-3xFLAG. gCsVMV-PWR-3xHA and pCAMBIA-HDA9-3xFLAG were used for the tobacco transient assay. The primers used for plasmid construction are listed in Table S2.
Recombinant Protein Purification.
pET32a-SANT2 was transformed into the E. coli strain BL21 Star(DE3) for protein expression. E. coli was cultured at 30 °C until the OD reached 0.5. Isfopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM, and the culture was incubated at 16 °C overnight. The recombinant protein was purified under native conditions by affinity chromatography using AKTA explorer (GE Healthcare) and Chelating Sepharose Fast Flow (GE Healthcare) medium. After the extract containing recombinant protein was loaded onto the column, the column was washed four times with the following wash buffers: wash buffer 1 (200 mM NaCl, 50 mM Tris⋅HCl pH 8.0, 40 mM imidazole), wash buffer 2 (200 mM NaCl, 50 mM Tris⋅HCl pH 8.0, 60 mM imidazole), wash buffer 3 (200 mM NaCl, 50 mM Tris⋅HCl pH 8.0, 100 mM imidazole), and wash buffer 4 (200 mM NaCl, 50 mM Tris⋅HCl pH 8.0, 120 mM imidazole). The recombinant protein was eluted with elution buffer (200 mM NaCl, 50 mM Tris⋅HCl pH 8.0, 500 mM imidazole).
Histone Peptide Array Binding Assay.
Two peptide arrays were incubated in blocking solution [TTBS buffer (10 mM Tri-HCl pH 7.5, 0.05% Tween-20, 150 mM NaCl pH 7.5) with 5% (wt/vol) nonfat milk] at 4 °C overnight then washed twice with TTBS buffer and once with interaction buffer [100 mM KCl, 20 mM Hepes pH 7.5, 1 mM EDTA, 0.1 mM DTT, 10% (vol/vol) glycerol]. The arrays were incubated with purified His-PWR-SANT2 protein for 2 h at room temperature followed by three washes with TTBS buffer. Anti-His antibody (1:5,000 dilutions; Abcam, ab184607) was added to the arrays in blocking buffer. After 1 h of incubation at room temperature, the arrays were washed three times with TTBS, and signals were detected using the ECL kit (GE Health, RPN3244) and X-ray film exposure.
Tobacco Transient Expression and Co-IP.
Cultured Agrobacteria harboring gCsVMV-PWR-3xHA, pCAMBIA-HDA9-3xFLAG and P19 were resuspended in infiltration buffer (500 mM Mes pH 5.6, 500 mM MgSO4, 100 mM acetosyringone) in a 1:1:2 ratio. After 2-h incubation, the Agrobacteria mix was infiltrated into tobacco leaf (Nicotiana benthamiana). The leaves were harvested after 3 d, and total protein was extracted in IP buffer (150 mM NaCl, 50 mM Tris⋅HCl pH 7.5, 5 mM EDTA, 1.0% Triton X-100). The protein extract was incubated with anti-HA agarose conjugates (Sigma, A2095) for 2 h at 4 °C, and the beads were washed three times with washing buffer (150 mM NaCl, 50 mM Tris⋅HCl pH 7.5, 5 mM EDTA). HA-HRP antibody (Roche, 12013819001) and FLAG-HRP antibody (Sigma, A8592) were used for Western blots.
Yeast Two-Hybrid Assay.
Yeast two-hybrid assay was performed with the ProQuest Two-Hybrid System (ThermoFisher, PQ10001-01 and PQ10002-01). The AD and BD plasmids were cotransformed into the yeast strain MaV203 and interactions were tested following the manufacturer’s instructions.
Western Blot Assay.
Ten-day-old seedlings were ground in liquid nitrogen and the powder was boiled for 5 min in 1× protein sample buffer. After centrifugation for 1 min at 16,000 × g, the proteins were resolved on a 15% (wt/vol) SDS/PAGE gel and transferred onto nitrocellulose membranes (Bio-Rad). The membranes were blocked in TBST (137 mM NaCl, 20 mM Tris⋅HCl pH 7.6, 0.1% Tween-20) with 5% (wt/vol) nonfat milk for 1 h and probed with antipan-acetylated H3 (Abcam, ab47915), anti-H3K9ac (Abcam, ab10812), H3K14ac (Abcam, ab46984), H3K27ac (Abcam, ab4729), H3K9me1 (Abcam, ab9045), and H3K9me2 (Abcam, ab1220) in TBST. After three washes with TBST, the signals were detected with Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
Real-Time RT-PCR.
Total RNA was extracted from 10- to 14-d-old seedlings using the TRIzol reagent (Invitrogen), and cDNA was synthesized using the SuperScript III kit (Invitrogen) according to the manufacturer’s instructions. qPCR was performed in triplicate on a Bio-Rad CFX 384 Real-Time System using iQ SYBR Green Supermix (Bio-Rad, 170–88802AP). The primers used for real-time RT-PCR are listed in Table S2.
ChIP.
ChIP was performed as previously described (34). Seedlings were ground in liquid nitrogen and cross-linked with 1% formaldehyde in M1 buffer [10 mM phosphate buffer pH 7.0, 0.1 M NaCl, 10 mM mercaptoethanol, 1 M hexylene glycol, protease inhibitor mixture (Roche)] for 10 min. The cross-linking reaction was quenched by adding glycine. The suspension was filtered through four layers of Miracloth, and the filtrate was centrifuged at 13,000 × g for 10 min. The pelleted chromatin was washed three times with M2 buffer (M1 buffer plus 10 mM MgCl2 and 0.5% Triton X-100) and one time with M3 buffer [10 mM phosphate buffer pH 7.0, 0.1 M NaCl, 10 mM mercaptoethanol, protease inhibitor mixture (Roche)]. The chromatin was resuspended in nuclei lysis buffer and sonicated to generate DNA fragments ∼500 bp in length. The lysate was precleared by incubation with 50-µL protein-A agarose beads/salmon sperm DNA (Millipore) for 1 h then incubated with anti-H3K9ac (Abcam, ab10812) and anti-H3K14ac antibodies (Abcam, ab52946) overnight. The beads were washed two times with each of the following solutions: low salt, high salt, LiCl, and TE (34). The bound DNA fragments were recovered and purified using columns from the Qiagen Plasmid Extraction Kit according to the manufacturer’s instructions. Quantitative real-time PCR was performed on bound and input DNAs. The primers used are listed in Table S2.
mRNA-Seq Library Construction and Data Analysis.
RNA purity was determined by assaying 1 µL of total RNA extract on a NanoDrop8000 spectrophotometer. Total RNA integrity was determined using an Agilent Technologies 2100 Bioanalyzer with an RNA integrity number value. mRNA sequencing libraries were prepared according to the manufacturer’s instructions (Illumina Truseq stranded mRNA library prep kit). mRNA was purified and fragmented from total RNA (1 µg) using poly-T oligo-attached magnetic beads and two rounds of purification. Cleaved RNA fragments were reverse transcribed into first strand cDNA using reverse transcriptase, random primers, and dUTP in place of dTTP. (The incorporation of dUTP quenches the second strand during amplification because the polymerase does not incorporate past this nucleotide.) A single adenine base was added to these cDNA fragments, followed by adapter ligation. The products were purified and amplified with PCR to create the final strand-specific cDNA library. The quality of the amplified libraries was verified by capillary electrophoresis (Bioanalyzer, Agilent). After qPCR using SYBR Green PCR Master Mix (Applied Biosystems), the index-tagged libraries were combined in equimolar amounts within a single pool. RNA sequencing was performed using an Illumina NextSEq. 500 system following the provided protocols for 1 × 75 sequencing. Reads were mapped to the TAIR10 Arabidopsis genome using HISAT2 (46) with default settings. Reads that mapped to multiple regions were discarded. Gene-expression levels were determined using a Perl script, and the calculations for differential gene expression were performed using edgeR (47). Genes with counts per million <1 were discarded. Genes with at least a 1.5 fold-change in expression (FDR ≤ 0.05) were considered to be differentially expressed.
ChIP-Seq Library Construction and Data Analysis.
ChIP DNA was quantified using a fluorescence-based quantification method (Qubit, Invitrogen). ChIP-seq libraries were prepared according to the manufacturer’s instructions (Illumina TruSeq ChIP sample prep kit). Input ChIP DNA was blunt-ended and phosphorylated, and a single adenine base was added to the 3′ ends of the fragments in preparation for ligation to an adapter with a single thymine base overhang. The products of this ligation reaction were purified and size-selected (300- to 400-bp fragments) by 2% agarose gel electrophoresis. The size-selected DNA was purified and PCR-amplified to enrich for fragments with adapters on both ends. The quality of the amplified libraries was verified by capillary electrophoresis (Bioanalyzer, Agilent). After qPCR using SYBR Green PCR Master Mix (Applied Biosystems), the index-tagged libraries were combined in equimolar amounts within a single pool. Sequencing was performed using an Illumina NextSEq. 500 system following the provided protocols for 1 × 75 sequencing. The ChIP-seq analysis was performed as previously described (48). Briefly, GAPipeline, the Illumina sequence data analysis pipeline, was used to analyze the raw sequence data. The reads were mapped to the TAIR10 Arabidopsis genome using Bowtie (49). Perfectly and uniquely mapped reads were processed for further analysis. Wiggle (WIG) files of the alignments were generated using bam2wig (https://github.com/MikeAxtell/bam2wig) and visualized using IGV (50). Using the SICER program (51), ChIP-enriched peaks were identified, and qualitative comparisons of binding levels in wild-type and mutant plants were determined. Each peak was positioned to genomic location (TE, genes, or intergenic region), and peaks within genes were further classified as exon, intron, 5′UTR or 3′UTR. The promoter-representing peaks were defined by their position within 2-kb upstream of a TSS. A summary of the reads number is provided in Table S3. Pearson correlation was determined by R statistical software on normalized signal intensity for ChIP peaks in the two biological replicates. Correlation (R) was 0.984, 0.988, and 0.998 for H3K9ac ChIP of Col, pwr-2 and hda9-1, respectively (Fig. S4).
GO and Gene List Overlap Analyses.
GO analysis was performed using agriGO (bioinfo.cau.edu.cn/agriGO/index.php) (52).
Acknowledgments
We thank Dr. Jeongsik Kim for the plasmid gCsVMV-3xHA-N-1300; Dr. Hui Kyung Cho for technical support; and Rae Eden Yumul for editing the manuscript. This work was supported by NIH Grant GM061146 (to Xuemei Chen); Gordon and Betty Moore Foundation Grant GBMF3046 (to Xuemei Chen); Guangdong Innovation Research Team Fund 2014ZT05S078 (to Xuemei Chen); IBS-R013-G2 (to J.M.K. and Y.-J.K.); and National Natural Science Foundation of China Grants 31570372 and 31671777 (to R.W. and L.X.). Work in X.Z.’s laboratory was supported by National Science Foundation CAREER Award MCB-1552455, the Alexander von Humboldt Foundation (Alfred Toepfer Faculty Fellow Award), and US Department of Agriculture and National Institute of Food and Agriculture Grant Hatch 1002874.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE89770).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618618114/-/DCSupplemental.
References
- 1.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12(5):599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 2.Pandey R, et al. Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res. 2002;30(23):5036–5055. doi: 10.1093/nar/gkf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollender C, Liu Z. Histone deacetylase genes in Arabidopsis development. J Integr Plant Biol. 2008;50(7):875–885. doi: 10.1111/j.1744-7909.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, et al. HDA18 affects cell fate in Arabidopsis root epidermis via histone acetylation at four kinase genes. Plant Cell. 2013;25(1):257–269. doi: 10.1105/tpc.112.107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cigliano RA, et al. Histone deacetylase AtHDA7 is required for female gametophyte and embryo development in Arabidopsis. Plant Physiol. 2013;163(1):431–440. doi: 10.1104/pp.113.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou C, Zhang L, Duan J, Miki B, Wu K. HISTONE DEACETYLASE19 is involved in jasmonic acid and ethylene signaling of pathogen response in Arabidopsis. Plant Cell. 2005;17(4):1196–1204. doi: 10.1105/tpc.104.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long JA, Ohno C, Smith ZR, Meyerowitz EM. TOPLESS regulates apical embryonic fate in Arabidopsis. Science. 2006;312(5779):1520–1523. doi: 10.1126/science.1123841. [DOI] [PubMed] [Google Scholar]
- 8.Aufsatz W, Mette MF, van der Winden J, Matzke M, Matzke AJM. HDA6, a putative histone deacetylase needed to enhance DNA methylation induced by double-stranded RNA. EMBO J. 2002;21(24):6832–6841. doi: 10.1093/emboj/cdf663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu H, Cho H, Bae W, Hwang I. Control of early seedling development by BES1/TPL/HDA19-mediated epigenetic regulation of ABI3. Nat Commun. 2014;5:4138. doi: 10.1038/ncomms5138. [DOI] [PubMed] [Google Scholar]
- 10.Wu K, Tian L, Malik K, Brown D, Miki B. Functional analysis of HD2 histone deacetylase homologues in Arabidopsis thaliana. Plant J. 2000;22(1):19–27. doi: 10.1046/j.1365-313x.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhou C, et al. Expression and function of HD2-type histone deacetylases in Arabidopsis development. Plant J. 2004;38(5):715–724. doi: 10.1111/j.1365-313X.2004.02083.x. [DOI] [PubMed] [Google Scholar]
- 12.Sridha S, Wu K. Identification of AtHD2C as a novel regulator of abscisic acid responses in Arabidopsis. Plant J. 2006;46(1):124–133. doi: 10.1111/j.1365-313X.2006.02678.x. [DOI] [PubMed] [Google Scholar]
- 13.Luo M, et al. HD2C interacts with HDA6 and is involved in ABA and salt stress response in Arabidopsis. J Exp Bot. 2012;63(8):3297–3306. doi: 10.1093/jxb/ers059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K, Park OS, Jung SJ, Seo PJ. Histone deacetylation-mediated cellular dedifferentiation in Arabidopsis. J Plant Physiol. 2016;191:95–100. doi: 10.1016/j.jplph.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 15.König AC, et al. The Arabidopsis class II sirtuin is a lysine deacetylase and interacts with mitochondrial energy metabolism. Plant Physiol. 2014;164(3):1401–1414. doi: 10.1104/pp.113.232496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu CW, et al. HISTONE DEACETYLASE6 interacts with FLOWERING LOCUS D and regulates flowering in Arabidopsis. Plant Physiol. 2011;156(1):173–184. doi: 10.1104/pp.111.174417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, et al. HDA6 directly interacts with DNA methyltransferase MET1 and maintains transposable element silencing in Arabidopsis. Plant Physiol. 2012;158(1):119–129. doi: 10.1104/pp.111.184275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu X, et al. Arabidopsis homologs of retinoblastoma-associated protein 46/48 associate with a histone deacetylase to act redundantly in chromatin silencing. PLoS Genet. 2011;7(11):e1002366. doi: 10.1371/journal.pgen.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Michaels SD, Amasino RM. Regulation of flowering time by histone acetylation in Arabidopsis. Science. 2003;302(5651):1751–1754. doi: 10.1126/science.1091109. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, et al. PHYTOCHROME INTERACTING FACTOR3 associates with the histone deacetylase HDA15 in repression of chlorophyll biosynthesis and photosynthesis in etiolated Arabidopsis seedlings. Plant Cell. 2013;25(4):1258–1273. doi: 10.1105/tpc.113.109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu CW, Chang KY, Wu K. Genome-wide analysis of gene regulatory networks of the FVE-HDA6-FLD complex in Arabidopsis. Front Plant Sci. 2016;7:555. doi: 10.3389/fpls.2016.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, et al. HISTONE DEACETYLASE19 interacts with HSL1 and participates in the repression of seed maturation genes in Arabidopsis seedlings. Plant Cell. 2013;25(1):134–148. doi: 10.1105/tpc.112.096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Kim J, Somers DE. Transcriptional corepressor TOPLESS complexes with pseudoresponse regulator proteins and histone deacetylases to regulate circadian transcription. Proc Natl Acad Sci USA. 2013;110(2):761–766. doi: 10.1073/pnas.1215010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, et al. Identification of BZR1-interacting proteins as potential components of the brassinosteroid signaling pathway in Arabidopsis through tandem affinity purification. Mol Cell Proteomics. 2013;12(12):3653–3665. doi: 10.1074/mcp.M113.029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krogan NT, Hogan K, Long JA. APETALA2 negatively regulates multiple floral organ identity genes in Arabidopsis by recruiting the co-repressor TOPLESS and the histone deacetylase HDA19. Development. 2012;139(22):4180–4190. doi: 10.1242/dev.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KC, Lai Z, Fan B, Chen Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell. 2008;20(9):2357–2371. doi: 10.1105/tpc.107.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aasland R, Stewart AF, Gibson T. The SANT domain: A putative DNA-binding domain in the SWI-SNF and ADA complexes, the transcriptional co-repressor N-CoR and TFIIIB. Trends Biochem Sci. 1996;21(3):87–88. [PubMed] [Google Scholar]
- 28.Boyer LA, Latek RR, Peterson CL. The SANT domain: A unique histone-tail-binding module? Nat Rev Mol Cell Biol. 2004;5(2):158–163. doi: 10.1038/nrm1314. [DOI] [PubMed] [Google Scholar]
- 29.Boyer LA, et al. Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol Cell. 2002;10(4):935–942. doi: 10.1016/s1097-2765(02)00634-2. [DOI] [PubMed] [Google Scholar]
- 30.Sterner DE, Wang X, Bloom MH, Simon GM, Berger SL. The SANT domain of Ada2 is required for normal acetylation of histones by the yeast SAGA complex. J Biol Chem. 2002;277(10):8178–8186. doi: 10.1074/jbc.M108601200. [DOI] [PubMed] [Google Scholar]
- 31.You A, Tong JK, Grozinger CM, Schreiber SL. CoREST is an integral component of the CoREST-human histone deacetylase complex. Proc Natl Acad Sci USA. 2001;98(4):1454–1458. doi: 10.1073/pnas.98.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Li Y, Ishizuka T, Guenther MG, Lazar MA. A SANT motif in the SMRT corepressor interprets the histone code and promotes histone deacetylation. EMBO J. 2003;22(13):3403–3410. doi: 10.1093/emboj/cdg326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guenther MG, Barak O, Lazar MA. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol. 2001;21(18):6091–6101. doi: 10.1128/MCB.21.18.6091-6101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yumul RE, et al. POWERDRESS and diversified expression of the MIR172 gene family bolster the floral stem cell network. PLoS Genet. 2013;9(1):e1003218. doi: 10.1371/journal.pgen.1003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim W, Latrasse D, Servet C, Zhou DX. Arabidopsis histone deacetylase HDA9 regulates flowering time through repression of AGL19. Biochem Biophys Res Commun. 2013;432(2):394–398. doi: 10.1016/j.bbrc.2012.11.102. [DOI] [PubMed] [Google Scholar]
- 36.Kang MJ, Jin HS, Noh YS, Noh B. Repression of flowering under a noninductive photoperiod by the HDA9-AGL19-FT module in Arabidopsis. New Phytol. 2015;206(1):281–294. doi: 10.1111/nph.13161. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, et al. Genome-wide profiling of histone H3 lysine 9 acetylation and dimethylation in Arabidopsis reveals correlation between multiple histone marks and gene expression. Plant Mol Biol. 2010;72(6):585–595. doi: 10.1007/s11103-009-9594-7. [DOI] [PubMed] [Google Scholar]
- 38.Sequeira-Mendes J, et al. The functional topography of the Arabidopsis genome is organized in a reduced number of linear motifs of chromatin states. Plant Cell. 2014;26(6):2351–2366. doi: 10.1105/tpc.114.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machanick P, Bailey TL. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics. 2011;27(12):1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, et al. Histone deacetylase HDA9 negatively regulates salt and drought stress responsiveness in Arabidopsis. J Exp Bot. 2016;67(6):1703–1713. doi: 10.1093/jxb/erv562. [DOI] [PubMed] [Google Scholar]
- 41.van Zanten M, et al. HISTONE DEACETYLASE 9 represses seedling traits in Arabidopsis thaliana dry seeds. Plant J. 2014;80(3):475–488. doi: 10.1111/tpj.12646. [DOI] [PubMed] [Google Scholar]
- 42.Zhang D, Yoon HG, Wong J. JMJD2A is a novel N-CoR-interacting protein and is involved in repression of the human transcription factor achaete scute-like homologue 2 (ASCL2/Hash2) Mol Cell Biol. 2005;25(15):6404–6414. doi: 10.1128/MCB.25.15.6404-6414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fairbrother WG, Yeh RF, Sharp PA, Burge CB. Predictive identification of exonic splicing enhancers in human genes. Science. 2002;297(5583):1007–1013. doi: 10.1126/science.1073774. [DOI] [PubMed] [Google Scholar]
- 44.Ramchatesingh J, Zahler AM, Neugebauer KM, Roth MB, Cooper TA. A subset of SR proteins activates splicing of the cardiac troponin T alternative exon by direct interactions with an exonic enhancer. Mol Cell Biol. 1995;15(9):4898–4907. doi: 10.1128/mcb.15.9.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanford JR, et al. Splicing factor SFRS1 recognizes a functionally diverse landscape of RNA transcripts. Genome Res. 2009;19(3):381–394. doi: 10.1101/gr.082503.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D, Langmead B, Salzberg SL. HISAT: A fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C, et al. Concerted genomic targeting of H3K27 demethylase REF6 and chromatin-remodeling ATPase BRM in Arabidopsis. Nat Genet. 2016;48(6):687–693. doi: 10.1038/ng.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson JT, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zang C, et al. A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics. 2009;25(15):1952–1958. doi: 10.1093/bioinformatics/btp340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38(Web Server issue):W64–W70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]