Tooth development in sharks
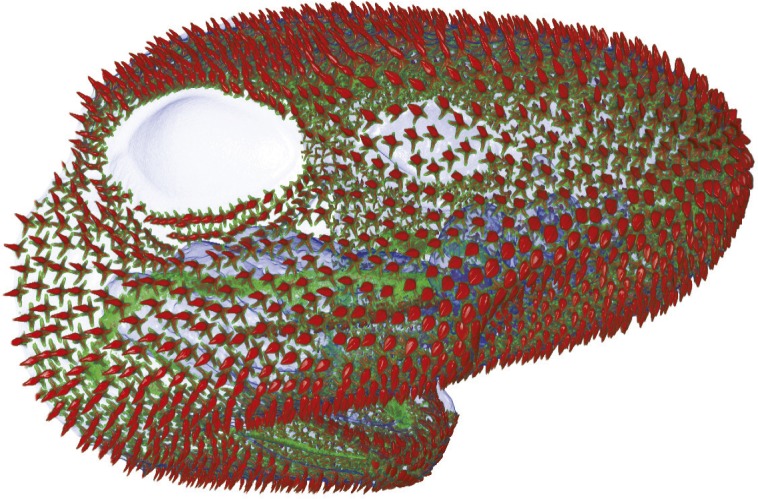
X-ray microscopy scan of hatchling catshark head.
Teeth and skin denticles, which are flat, hard, tooth-shaped structures, are part of a group of organs called odontodes. The evolutionary origin of teeth and their relationship to other odontode structures remains unclear. Kyle Martin et al. (pp. 14769–14774) compared the cellular and molecular mechanisms that shape the development of teeth and denticles in the catshark (Scyliorhinus canicula), an organism that has maintained both forms of odontodes. Gene expression profiles revealed a remarkable conservation of gene expression patterns during the development of teeth and denticles, suggesting that a shared gene regulatory network drives the development of all odontodes. Genetic studies of developing catshark jaws revealed that the epithelial stem cell marker sox2 is expressed during tooth, but not denticle, development and is associated with the rapid and continuous regenerative capacity of shark teeth. Dendrites do not regenerate and develop only after injury or when they are lost. In addition, the authors found that teeth and anteriormost oral taste buds, which constantly regenerate, emerge from the same field of epithelial sox2+ progenitor cells. According to the authors, truly regenerative teeth may have arisen when the gene regulatory network for producing simple denticles combined with the network for producing the highly regenerative taste buds in the mouth. — L.C.
Gut microbiota and chromatin landscape
The human gut harbors a large and complex microbial community. These microorganisms are known to influence human metabolism and immunity, and one theory suggests that they induce epigenetic changes in host cells, modifying the chromatin landscape and altering gene expression. To determine whether the microbiota influences chromatin accessibility in the intestine’s immune cells, Nicholas Semenkovich et al. (pp. 14805–14810) characterized transcriptional regulatory regions known as enhancers in intraepithelial lymphocytes from three groups of mice: conventionally raised mice exposed to microbes in their environment from the time of birth, germ-free mice reared under sterile conditions, and germ-free mice colonized at weaning with gut microbiota harvested from their conventionally raised counterparts. Using high-throughput sequencing that defines the openness of chromatin in enhancer regions, the authors identified signaling networks, metabolic pathways, and enhancer-associated transcription factors that are affected by gut microbiota. Furthermore, the authors report that the vast majority of changes in accessibility occur in preexisting enhancers. These observations add to a growing body of work suggesting that microbial communities effect epigenetic changes that can imprint host cells with a history of microbial exposure, according to the authors. — T.J.
Genetic factors tied to chronic mucocutaneous candidiasis
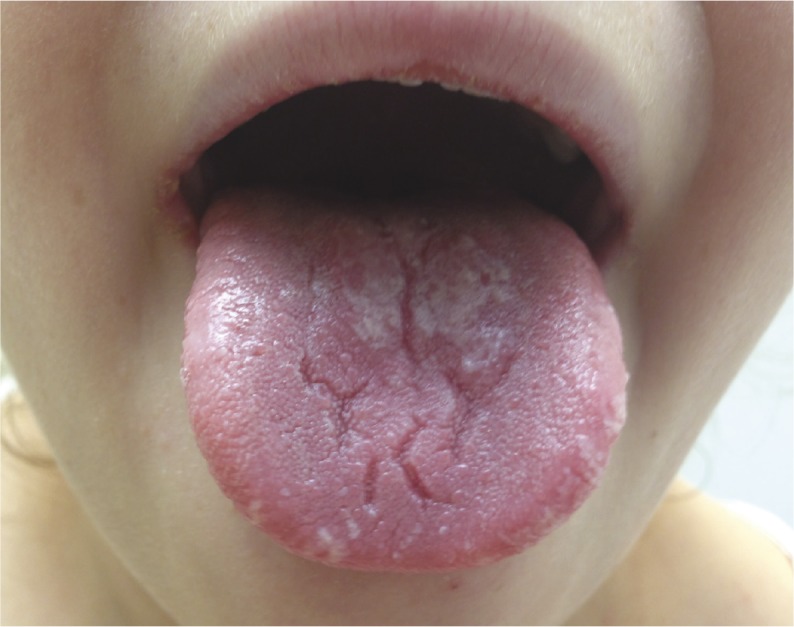
Chronic oral candidiasis in a patient with inherited IL-17RA deficiency.
Chronic mucocutaneous candidiasis (CMC) is a hereditary disorder characterized by chronic infection of the nails, skins, and mucosae by the commensal fungus Candida. Previously, researchers reported a link between autosomal recessive (AR) IL-17 receptor A (IL-17RA) deficiency and CMC infection, but evidence to draw firm conclusions about hereditary factors affecting CMC infection is lacking. To better understand genetic predisposition to CMC, Romain Lévy et al. (pp. E8277–E8285) analyzed genomic DNA extracted from the blood cells of 21 early-onset CMC patients who were 2 to 35 years of age and of various ethnic backgrounds. Additionally, 14 patients had staphylococcal skin disease and 8 patients were prone to bacterial infections of the respiratory tract. The authors report that all 21 patients had AR IL-17RA deficiency and were homozygous for 1 of 12 different IL17RA alleles. A number of the alleles affected the expression of AR IL-17RA on leukocytes and dermal fibroblasts, and prevented cellular responses initiated by IL-17 cytokines. According to the authors, human IL-17RA might be involved in immunity against Candida infection, and might be relevant to the genetic diagnosis of CMC and related fungal or bacterial infections. — C.S.


