Significance
Myelin allows the rapid conduction of electrical signals and provides axons with metabolic support. It appeared relatively late in evolution in hinge-jawed vertebrates and concomitantly with the emergence of the androgen receptor (AR), hinting at a specific role for androgens in myelination. Here, we show that after demyelination of the central nervous system (CNS), the male gonad, testosterone, and AR favor astrocyte recruitment and myelin regeneration by oligodendrocytes. In their absence, astrocytes remain sparse and peripheral-type remyelination, classically associated with Schwann cells, is predominantly detected. These findings reveal a novel role of androgen signaling in CNS myelin formation and glial cell communication, with possible implications for demyelinating, psychiatric, and age-related disorders.
Keywords: myelin, oligodendrocytes, Schwann cells, testosterone, androgen receptor
Abstract
Lost myelin can be replaced after injury or during demyelinating diseases in a regenerative process called remyelination. In the central nervous system (CNS), the myelin sheaths, which protect axons and allow the fast propagation of electrical impulses, are produced by oligodendrocytes. The abundance and widespread distribution of oligodendrocyte progenitors (OPs) within the adult CNS account for this remarkable regenerative potential. Here, we report a key role for the male gonad, testosterone, and androgen receptor (AR) in CNS remyelination. After lysolecithin-induced demyelination of the male mouse ventral spinal cord white matter, the recruitment of glial fibrillary acidic protein-expressing astrocytes was compromised in the absence of testes and testosterone signaling via AR. Concomitantly, the differentiation of OPs into oligodendrocytes forming myelin basic protein (MBP)+ and proteolipid protein-positive myelin was impaired. Instead, in the absence of astrocytes, axons were remyelinated by protein zero (P0)+ and peripheral myelin protein 22-kDa (PMP22)+ myelin, normally only produced by Schwann cells in the peripheral nervous system. Thus, testosterone favors astrocyte recruitment and spontaneous oligodendrocyte-mediated remyelination. This finding may have important implications for demyelinating diseases, psychiatric disorders, and cognitive aging. The testosterone dependency of CNS oligodendrocyte remyelination may have roots in the evolutionary history of the AR, because the receptor has evolved from an ancestral 3-ketosteroid receptor through gene duplication at the time when myelin appeared in jawed vertebrates.
The remyelination of axons is a complex process, involving interactions between different types of neural cells. It shows a peculiarity that has been observed in both experimental models and demyelinating diseases such as multiple sclerosis (MS) (1). Although most of the remyelination is normally accomplished by oligodendrocytes derived from oligodendrocyte progenitors (OPs), a variable proportion of central nervous system (CNS) axons can be remyelinated by cells with the immunophenotypic and ultrastructural characteristics of Schwann cells, the presence of which is normally limited to the peripheral nervous system (PNS). These cells indeed express protein zero (P0), a sensitive and specific marker of Schwann cells (2). Moreover, analysis by electron microscopy demonstrated that they have the typical morphology of myelinating Schwann cells (1–4). These ultrastructural findings indicate that the expression of P0 by part of the remyelinating cells is not merely ectopic, but that these cells may correspond to authentic Schwann cells. This observation was further supported by their expression of Schwann cell-specific transcription factors (5).
Importantly, there is strong evidence that large numbers of the Schwann cells present in demyelinating CNS lesions are derived from OPs. The first evidence came from the transplantation of purified OPs into demyelinating CNS lesions, in which endogenous remyelination was prevented by X-irradiation (4, 6, 7). Unequivocal confirmation that most Schwann cells contributing to CNS remyelination are indeed derived from OPs has been provided by genetic fate-mapping in transgenic mice (5). This plasticity of OPs with a neuroepithelial origin may come as a surprise, because Schwann cells normally arise from the neural crest, and this problem still presents a matter of controversy. However, under the influence of specific morphogenetic factors, neuroepithelial cells can differentiate into neural crest stem cells and give rise to neural cell types of both CNS and PNS (4, 8).
Astrocytes play an important role in determining the balance between oligodendrocyte and Schwann cell remyelination in the CNS. Thus, within a demyelinated lesion, oligodendrocyte remyelination occurs in regions where astrocytes are present, whereas Schwann cell remyelination preferentially occurs in the absence of astrocytes (7, 9, 10). Moreover, reducing astrocyte activation by a conditional genetic deletion promoted Schwann cell remyelination (1).
Like other members of the steroid receptor family, the androgen receptor (AR) functions as a ligand-activated transcription factor and also directly interacts with components of extranuclear signaling pathways (11, 12). Here, by investigating the role of AR signaling in the spontaneous regeneration of myelin within the adult CNS, we show that the recruitment of glial fibrillary acidic protein (GFAP)+ astrocytes into a demyelinated area of the ventral funiculus of male mice is compromised in the absence of testes, testosterone, or AR. Consistent with a key role of astrocytes in CNS remyelination, in their absence, the newly formed myelin was P0+ and peripheral myelin protein 22-kDa (PMP22)+, most likely reflecting Schwann cell remyelination.
Results
The Role of Testes and Testosterone in the Recruitment of Astrocytes and Spontaneous Remyelination.
We used an experimental model in which acute demyelination is followed by spontaneous remyelination. Axons of the right ventrolateral white matter tract of the adult male mouse spinal cord (ventral funiculus) were locally demyelinated by stereotaxic microinjection of the demyelinating toxin lysolecithin. Within 3 d, the injected area was depleted of astrocytes, oligodendrocytes, and myelin basic protein (MBP), a major component and established marker of CNS myelin (13).
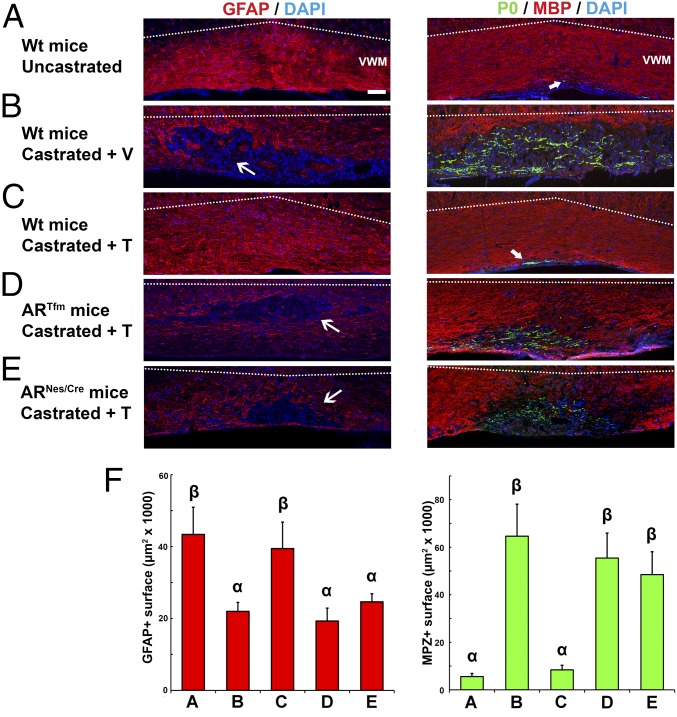
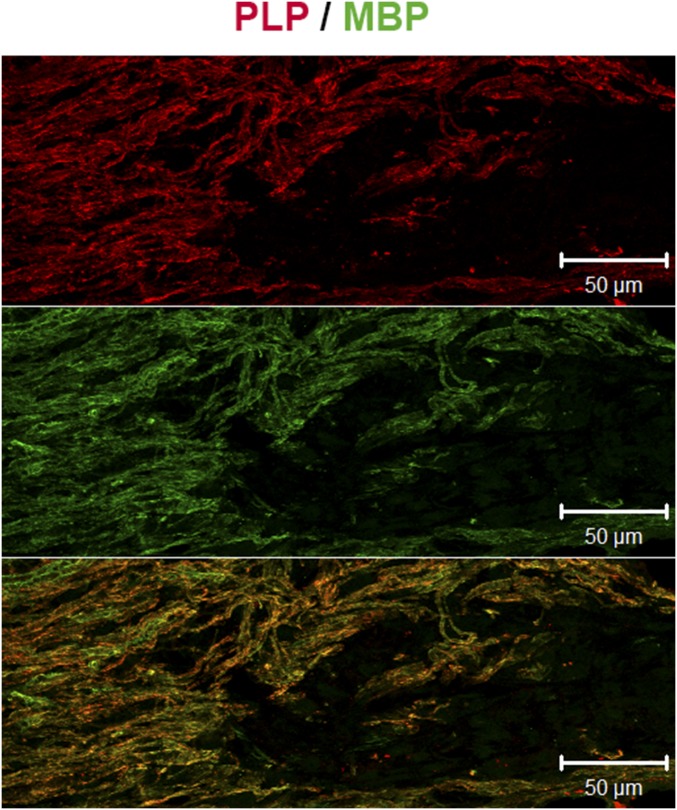
In gonadally intact males, the area of demyelination was rapidly replenished by GFAP+ astrocytes (Fig. 1A, Left). Concomitantly, axons were remyelinated by MBP+ CNS myelin (Fig. 1A, Right). However, after surgical castration, the recruitment of astrocytes was severely impaired (Fig. 1B, Left) and axons were preferentially remyelinated by P0+ PNS-like myelin (Fig. 1B, Right). P0 immunostaining appeared sparse, consistent with previous results showing that Schwann cell-remyelinated areas contain more demyelinated axons than oligodendrocyte-remyelinated ones (1). Similar results were obtained when tissue sections were analyzed with PMP22 antibody, another specific marker of peripheral myelin (14). Immunostaining of MBP remained very sparse within the lesion and perfectly matched immunostaining of proteolipid protein (PLP), another major CNS myelin protein (Fig. S1). Despite the presence of low levels of MBP isoforms in peripheral myelin (14), these isoforms are likely not detected because the monoclonal anti-MBP antibodies used in the present study only weakly stain PNS myelin.
Fig. 1.
Testes, testosterone, and AR are required for the recruitment of astrocytes into a demyelinating lesion, thus determining the balance of central versus peripheral types of remyelination. (A–E, Left) Visualization of astrocytes stained with an antibody to GFAP (red). (A–E, Right) Immunohistochemical staining of MBP+ CNS myelin (red) and P0+ PNS myelin (green) 4 wk after lysolecithin-induced demyelination. Cell nuclei were blue-counterstained with DAPI. Dotted lines delineate the boundary between the ventral white matter (VWM) and top gray matter. (A) Recruitment of astrocytes and recovery of MBP+ CNS myelin in the lesion area of gonadally intact males. Wt, wild type. (Scale bars: 100 μm.) (B) After testes removal and treatment with an empty implant (+ V), astrocytes remained sparse within the remyelinated area and remyelination was mediated by Schwann cells (P0+ myelin). (C) Recruitment of GFAP+ astrocytes and regeneration of MBP+ myelin in castrated males treated with testosterone (+ T). (D and E) Despite testosterone treatment, astrocytes were almost absent within the remyelinated area in ARTfm or ARNesCre mice, and axons were remyelinated by Schwann cells. The thin arrows show the areas depleted in GFAP+ labeling and the thick arrows point out the areas displaying a limited expression of P0. (F, Left) Mean area of GFAP immunolabeling (±SEM) (F5,28 = 5.67, P ≤ 0.001). (F, Right) Mean area of P0 immunolabeling (±SEM) (F5,46 = 3.78, P ≤ 0.01). Letters on top of columns indicate least significant differences (LSDs) [P ≤ 0.001 (Left), P ≤ 0.01 (Right), post hoc LSD tests; n = 5–6].
Fig. S1.
Perfect matching of the immunostaining of CNS myelin with an antibody against PLP (red) or MBP (green). The bottom panel indicates the colocalization of PLP and MBP immunoreactivity.
We then showed that testosterone was the testicular factor necessary for the recruitment of astrocytes and the remyelination of axons by oligodendrocytes. When castrated males received an s.c. Silastic implant filled with testosterone for 4 wk, GFAP+ astrocytes and MBP immunostaining were fully restored within the lesion area (Fig. 1C), as in uncastrated males (Fig. 1A). The implant produced physiological plasma and brain levels of testosterone (10–15 nM) as determined by gas chromatography/mass spectrometry (15).
The role of testosterone treatment in the concomitant replenishment of astrocytes and CNS remyelination is also illustrated in Fig. S2. Although axons were not counted, the staining of large-caliber axons on the sagittal spinal cord sections with an antibody against neurofilament 200 kDa revealed neither a substantial loss of axons after lysolecithin-induced demyelination nor a marked increase in axonal density by testosterone treatment (Fig. S3). These observations point to a crucial role of the male gonads and testosterone in the recruitment of astrocytes and in the balance between CNS- and PNS-like remyelination.
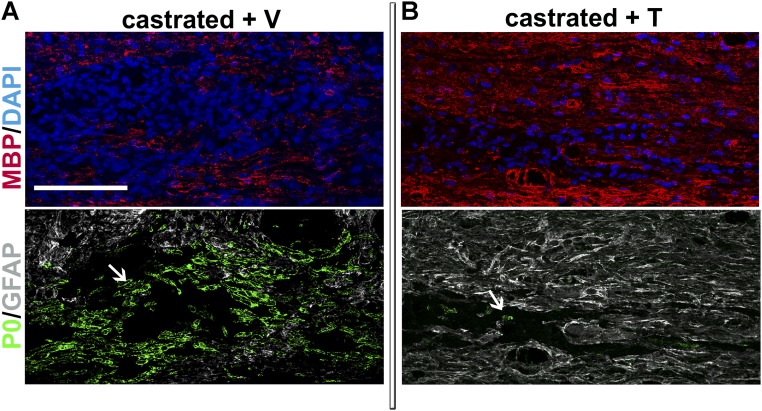
Fig. S2.
In the absence of testes and testosterone, GFAP+ astrocytes are sparse and axons are remyelinated by Schwann cells within the demyelinated lesion. (Top) Myelin formed by oligodendrocytes within the area demyelinated by LPC was stained on sagittal sections with an antibody to MBP (red). Cell nuclei were stained with DAPI in blue. (Bottom) Myelin formed by Schwann cells was stained with an antibody to P0 (green), and astrocytes were stained with an antibody to GFAP (white). (A) When castrated male mice were treated during 4 wk after LPC-induced demyelination with an empty s.c. Silastic implant (+ V), few GFAP+ astrocytes were observed within the lesion area. Axons were preferentially remyelinated by Schwann cells, as indicated by the high expression of P0+ PNS myelin. (B) When castrated males received a testosterone-filled implant (+ T), GFAP+ astrocytes were widely detected within the area of remyelination and axons were preferentially remyelinated by oligodendrocytes, as shown by the high expression of MBP+ CNS myelin. The arrows point out the wide or limited areas expressing the P0 marker in each condition. Representative images from five to six animals are shown. (Scale bar: 100 μm.)
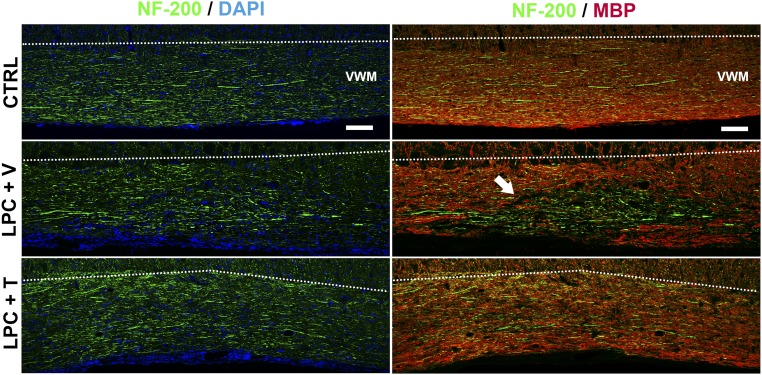
Fig. S3.
Large-caliber axons are largely preserved after lysolecithin-induced demyelination. Immunohistochemical staining of neurofilament 200 kDa (NF-200; green) and MBP (red) in sagittal spinal cord sections. (Left) Cell nuclei were blue-counterstained with DAPI. All male mice were castrated. (Top) Control (CTRL) mice remained unlesioned. Dotted lines delineate the boundary between the ventral white matter (VWM, Bottom) and gray matter (Top). (Scale bars: 100 μm.) (Middle and Bottom) In the other mice, a demyelinating lesion was induced by LPC, and they were treated during 4 wk with an empty (+ V) or testosterone-filled (+ T) s.c. implant. Results confirm the failure of MBP-immunoreactive myelin regeneration in the absence of testosterone and show that NF-200+ axons are largely preserved (as indicated by the arrow). With testosterone treatment, NF-200+ axons are again ensheathed by MBP-immunoreactive myelin. Representative images from five to six animals per group are shown.
The AR Is Required for the Recruitment of Astrocytes and the Spontaneous Regeneration of CNS Myelin.
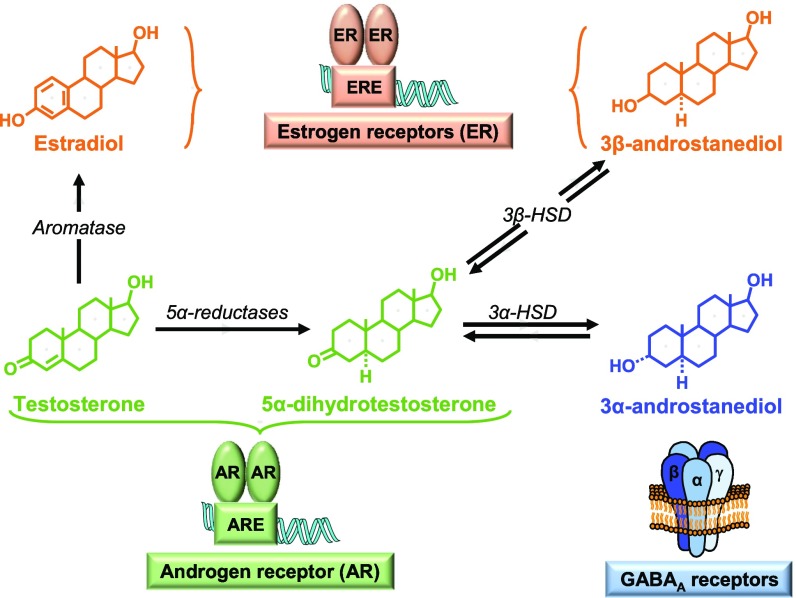
In the CNS, testosterone exerts its effects either after binding to intracellular AR or via its metabolites: estradiol, 3α-androstanediol, and 3β-androstanediol (Fig. S4). Testosterone signaling via its conversion to estradiol may correspond to a phylogenetically ancient mechanism, because the ancestral intracellular steroid receptor was a receptor for estrogens (16). In addition, signaling of steroid metabolites via membrane proteins may have been an early evolutionary feature (17). This possibility led us to investigate whether the testosterone-dependent recruitment of astrocytes and spontaneous regeneration of MBP+ myelin involved AR. We first used ARTfm mice, which display a frameshift mutation in exon 1 of the AR gene, producing a nonfunctional receptor (18). Despite testosterone supplementation during 4 wk, the area of demyelination remained largely depleted of astrocytes (Fig. 1D, Left) in ARTfm mice, and axons were mainly remyelinated by P0+ (Fig. 1D, Right) and PMP22+ myelin.
Fig. S4.
Metabolism and signaling mechanisms of testosterone in the nervous system. In the CNS, testosterone is converted by the aromatase enzyme to estradiol, which activates gene transcription via the binding of estrogen receptors (ERs; comprising ERα and ERβ) to estrogen response element (ERE). The conversion of testosterone to estradiol has a key role in the viability and plasticity of neurons and in cognitive functions (54, 55). In addition, testosterone is converted by the 5α-reductases to its potent metabolite 5α-dihydrotestosterone, which also acts via AR binding to androgen response element (ARE) (56). The 5α-dihydrotestosterone can be further metabolized by hydroxysteroid dehydrogenase (HSD) to 3β-androstanediol or 3α-androstanediol. Whereas 3β-androstanediol is a ligand of ERs, 3α-androstanediol is a positive modulator of γ-aminobutyric acid type A (GABAA) receptors, the main receptors mediating neuronal inhibition in the brain (57–59).
We then tested whether AR expression within the CNS is necessary for the stimulation of remyelination by testosterone. Transgenic ARNesCre mice, displaying selective ablation of the AR in neurons, astrocytes, and oligodendrocytes, but with microglial cells being spared (19), were castrated and received a testosterone-filled s.c. Silastic implant during 4 wk following lysolecithin-induced demyelination. As in ARTfm mice and in contrast to uncastrated wild-type animals, testosterone therapy failed to restore astrocytes and MBP-immunoreactive myelin within the lesion. Instead, PNS-type P0+ remyelination prevailed (Fig. 1E). Thus, testosterone signaling via AR stimulates the recovery of astrocytes and shifts the balance toward the regeneration of CNS myelin.
Testosterone Increased the Number of GFAP+ Astrocytes at the Border of the Demyelinated Area and in Glial Cell Culture.
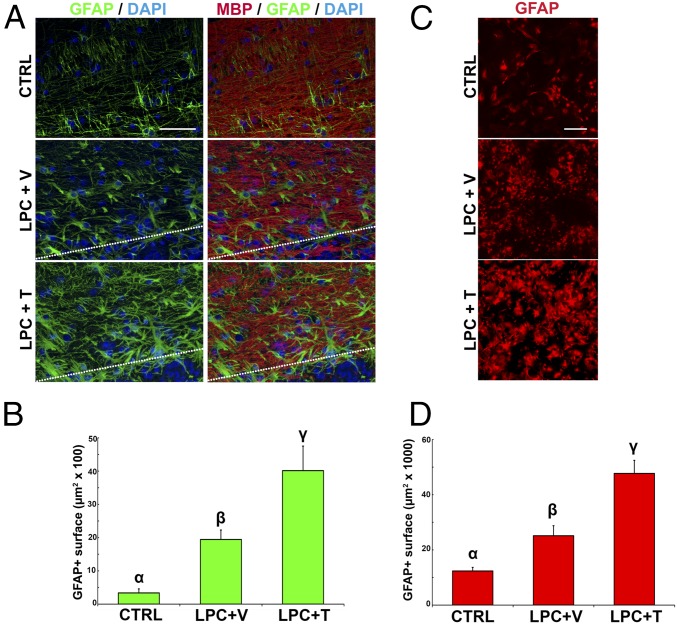
In the vicinity of a demyelinating lesion, new astrocytes are mainly generated from preexisting ones expressing the fibroblast growth factor receptor 3 (5). Therefore, we investigated the effects of testosterone on the rapid astrocytic response after lysolecithin administration. At the top border of the lysolecithin-demyelinated area, the GFAP-immunoreactive surface was slightly increased in castrated males as early as 4 d after lysolecithin injection. However, the extent of GFAP labeling was markedly enhanced following testosterone treatment (Fig. 2 A and B). At 14 d after lysolecithin, the demyelinated lesion was nearly entirely occupied by GFAP+ astrocytes, whereas the remyelination by oligodendrocytes was still incomplete (1), indicating that astrocyte proliferation and recruitment precede the formation of new MBP+ myelin.
Fig. 2.
Testosterone increases the reactivity and number of astrocytes in vivo and in mixed cultures of glial cells. (A, Top) Immunohistochemical staining of reactive GFAP+ astrocytes (green) and MBP+ myelin (red) in the ventrolateral part of the unlesioned male mouse spinal cord [control (CTRL)]. Cell nuclei were blue-counterstained with DAPI. (Scale bar: 50 μm.) (A, Middle and Bottom) GFAP+ astrocytes and MBP+ myelin at the top border of the demyelinated area (dotted line) in castrated male mice that received lysolecithin injection and were treated for 4 d with an empty (+ V) or testosterone filled (+ T) s.c. Silastic implant. (B) Mean area covered by GFAP+ astrocytes (±SEM) (F2,21 = 14.19, P ≤ 0.001). Letters on top of columns indicate LSDs (P ≤ 0.05, post hoc LSD tests; n = 5–6). (C, Top) Immunohistochemical staining of GFAP+ astrocytes (red) in CTRL mixed cultures of glial cells. (C, Middle) Exposing the cultures for 12 h to lysolecithin and treating them for 3 d with vehicle (+ V) increased the density of astrocytes (details are provided in legend of Fig. S5). (C, Bottom) Further increase in the density of astrocytes was observed when cultures were treated for 3 d with testosterone (+ T; 1 μM). (D) Mean area covered by GFAP+ astrocytes (±SEM) (F2,43 = 21.3, P < 0.001). Letters on top of columns indicate LSDs [P ≤ 0.05 (Left), post hoc LSD tests; n = 3–4]. LPC, lysolecithin.
In mixed cultures of glial cells prepared from postnatal day 1–3 male mice, the number of reactive GFAP+ astrocytes was significantly increased 72 h after a 12-h exposure to lysolecithin. When the culture medium was supplemented with testosterone (1 μM) for 72 h after lysolecithin removal, a further increase in GFAP immunostaining was observed (Fig. 2 C and D). Thus, testosterone increased the astrocytic response both in vivo at the border of the lesion and under in vitro conditions in mixed cultures of glial cells.
The Regeneration of MBP-Immunoreactive Myelin Is Accompanied by the Recovery of Mature Oligodendrocytes.
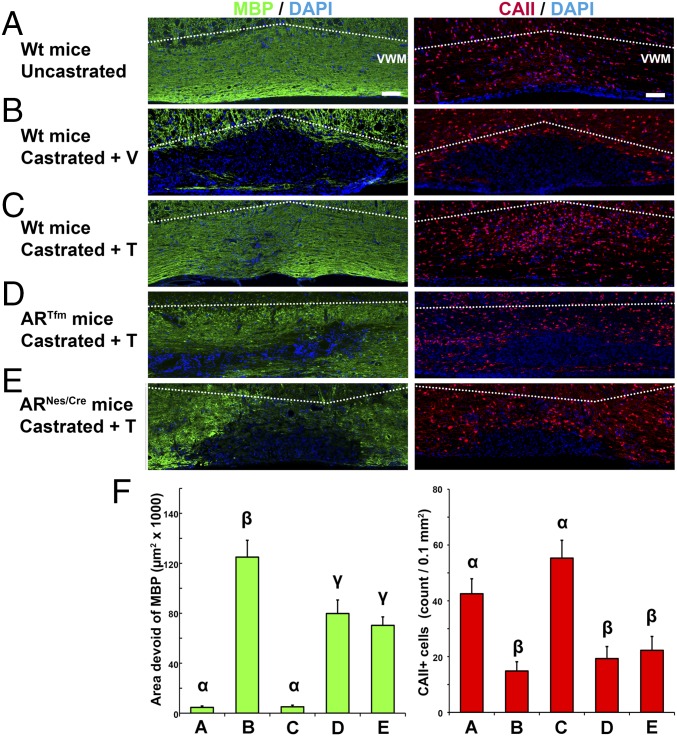
We confirmed that the spontaneous regeneration of MBP-immunoreactive CNS myelin 4 wk after lysolecithin-induced demyelination was also dependent on the presence of testes, testosterone, and the AR. Indeed, in castrated males and in the absence of a functional AR (ARTfm and ARNesCre mice), the recovery of MBP+ myelin was markedly impaired (Fig. 3). Importantly, the regeneration of MBP+ myelin was always accompanied by full replenishment of the demyelinated lesion by oligodendrocytes expressing carbonic anhydrase II (CA II). In contrast, few CA II+ oligodendrocytes were observed after castration or in the absence of AR (Fig. 3). Thus, testosterone-dependent CNS remyelination goes together with the parallel recruitment of both oligodendrocytes and astrocytes.
Fig. 3.
Testes, testosterone, and AR are required for the spontaneous regeneration of CNS myelin by oligodendrocytes. (A–F, Left) MBP-immunoreactive CNS myelin (green) in sagittal sections at 4 wk after lysolecithin microinjection into the right ventrolateral white matter tract of the spinal cord. Cell nuclei were counterstained with DAPI. (A–F, Right) Immunohistochemical staining of CA II+ oligodendrocytes (red). (A) Recovery of MBP and mature oligodendrocytes in gonadally intact Wt mice. Dotted lines delineate the boundary between the ventral white matter (VWM) and the top gray matter. (Scale bars: 100 μm.) (B) Absence of recovery of MBP and oligodendrocytes after the removal of testes and treatment with an empty s.c. implant (+ V). Castration was performed at the age of 4–6 wk, 4 wk before lysolecithin-induced demyelination. (C) Regeneration of MBP-immunoreactive myelin and replenishment of oligodendrocytes in castrated males treated with a testosterone-filled implant (+ T). In ARTfm mice with a nonfunctional AR (D) or in ARNesCre mice with CNS-selective ablation of AR (E), testosterone failed to stimulate CNS remyelination and the replenishment of oligodendrocytes. (F, Left) Mean area devoid of MBP immunostaining (F5,30 = 44.8, P ≤ 0.001). Letters on top of columns indicate LSDs [P ≤ 0.01 (Left), P ≤ 0.05 (Right), post hoc LSD tests; n = 5–6]. (F, Right) Mean number of CA II+ oligodendrocytes (±SEM) within the area of demyelination (F5,34 = 9.2, P ≤ 0.001).
In the Presence of Astrocytes, Testosterone Increases the Proliferation and Differentiation of OPs.
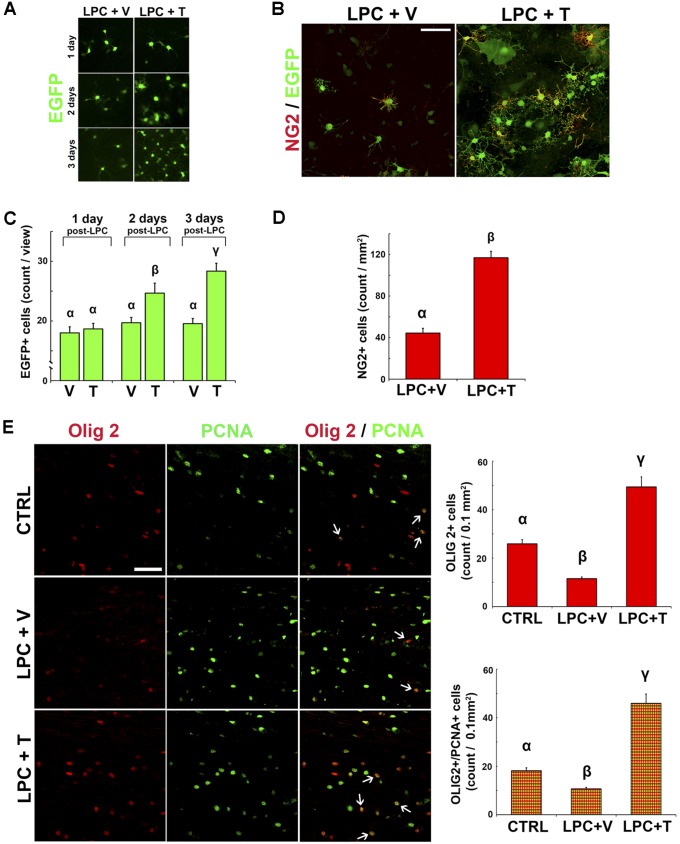
Following oligodendrocyte loss, CNS remyelination requires the recruitment of OPs and their differentiation into mature oligodendrocytes (20). To provide further insight into the mechanisms by which testosterone promotes CNS myelin regeneration in the presence of astrocytes, we first investigated its effect on neonatal OPs in mixed primary glial cell cultures prepared from the brains of postnatal day 1–3 transgenic male mice selectively expressing the enhanced green fluorescent protein (EGFP) in the oligodendrocyte lineage under the control of the mouse Plp gene promoter (Plp-EGFP mice) (21). Confluent cultures, composed of OPs and oligodendrocytes (green fluorescent) and astrocytes, were exposed for 12 h to lysolecithin damage and were then treated with vehicle or testosterone. Three days later, cells were fixed and immunostained with an antibody against the chondroitin sulfate proteoglycan [neural/glial antigen 2 (NG2)], a proven marker of OPs (Fig. S5 A–D).
Fig. S5.
Testosterone increases the density of neonatal OPs in cell culture and of adult OPs in vivo. (A and B) Visualization of a confluent mixed glial cell culture prepared from the postnatal (postnatal days 0–3) brain of Plp-EGFP mice, exposed for 12 h to LPC (0.1 mg/mL culture medium) and then treated during 3 d with vehicle (0.1% ethanol, + V) or testosterone (1 μM, + T). (A) Oligodendroglial cells expressing EGFP were first daily observed. (B) Then, the culture was fixed and NG2+ cells were immunostained. (C) Density of EGFP+ cells in cultures treated for 1, 2, or 3 d with V or T after LPC (F2,28 = 5.53, P ≤ 0.01). (D) Density of NG2+ OPs in cultures treated for 3 d with V or T (F2,15 = 60.2, P ≤ 0.001). (E, Left) Olig2 and proliferating cell nuclear antigen (PCNA) immunostaining performed on sagittal sections of the ventrolateral spinal cord derived from an unlesioned CTRL male (Top) and from animals that received a stereotaxic injection of LPC before being treated for 4 wk with either an empty (+ V) or testosterone-filled (+ T) implant (Middle and Bottom). (E, Right) Histograms indicate the density of Olig2+ cells (Top; F2,12 = 53.5, P ≤ 0.001) and of proliferating Olig2+ cells double-labeled with PCNA (Bottom; F2,12 = 63.7, P ≤ 0.001). Letters on top of columns indicate least significant differences [LSDs; P ≤ 0.01 (Top), P ≤ 0.05 (Bottom), post hoc LSD tests; n = 5–6]. (Scale bars: 50 μm.)
The number of EGFP+ cells was reduced by half after exposure to lysolecithin and remained low in the absence of testosterone. Culture medium supplementation with testosterone (1 μM) significantly increased the number of both EGFP+ and NG2+ cells, which exhibited a differentiated branched morphology (Fig. S5 A–D). Thus, adding testosterone to mixed glial cell cultures increases proliferation and differentiation of neonatal OPs.
Compared with their neonatal counterparts, adult OPs are more quiescent and show other distinct features (22, 23). We thus also examined the impact of testosterone on the oligodendrocyte lineage transcription factor 2 (Olig2)-expressing cells in adult castrated male mice. This transcription factor is confined to the CNS and is critically involved in OP differentiation (24). Four weeks after injection of lysolecithin into the myelinated ventrolateral white matter of the castrated male spinal cord, Olig2+ cells were significantly more abundant in males receiving a testosterone-filled Silastic implant compared with males receiving an empty implant (Fig. S5E). Testosterone replacement indeed significantly increased the number of Olig2+ cells, which even reached a higher density compared with unlesioned control animals. Moreover, double immunolabeling with proliferating cell nuclear antigen showed that testosterone stimulated OP proliferation.
Discussion
Our data establish the importance of the male gonad, testosterone, and AR in the efficient recruitment of astrocytes into a demyelinated lesion, with implications for the balance of oligodendrocyte versus peripheral-type remyelination. In gonadally intact male mice or in castrated animals treated with testosterone, the area of lysolecithin-induced demyelination was replenished with astrocytes after 4 wk. Consequently, axons were mainly remyelinated by oligodendrocytes. On the contrary, in the absence of testosterone or a functional AR, astrocytes remained sparse and P0+ myelin was preferentially detected within the lesion. This observation is consistent with previous studies showing that Schwann cell remyelination occurs in areas of the CNS where astrocytes are absent (9, 10).
Testosterone treatment has recently been shown to stimulate the regeneration of myelin in a mouse model of severe chronic demyelination, where no spontaneous remyelination was observed. Moreover, the strong remyelinating effect of testosterone was AR-dependent (15). These findings already qualified the brain AR as a promising drug target for remyelination therapy. Nevertheless, it came as a surprise in the present study that spontaneous remyelination by oligodendrocytes was markedly inhibited in the absence of testes, testosterone, or AR.
Encoded by a single gene, AR only arose lately in jawed vertebrates, after the cyclostome-gnathosmome divergence (25, 26). This occurrence has allowed androgens to acquire new signaling functions related to reproduction and sexual differentiation (27, 28). In parallel, testosterone and its metabolite 5α-dihydrotestosterone, both ligands of the AR, may also have acquired new functions in the CNS, as suggested by the widespread distribution of AR within the brain and spinal cord (29, 30).
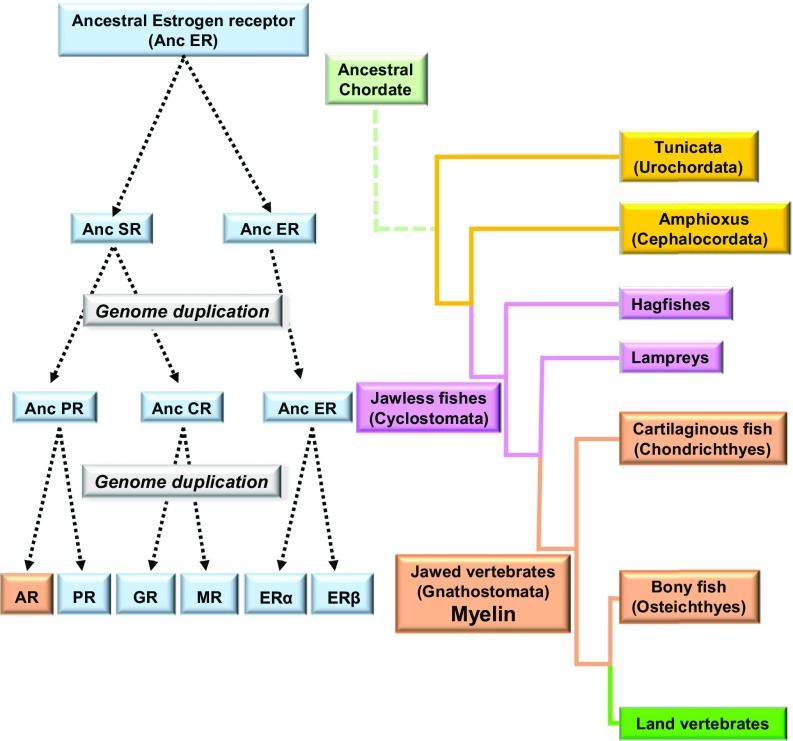
Intriguingly, the evolutionary acquisition of AR was concomitant with the advent of the myelin sheath, constituting one of the most recent structural innovations of the vertebrate nervous system (Fig. 4). Like the AR, myelin first appeared in vertebrates with a hinged jaw, most likely in placoderms, facilitating predatory and escape behaviors and improving information processing by increasing the conduction speed of axons (31). Both myelin and AR are present in extant cartilaginous fishes (sharks, rays), but absent in jawless fishes (hagfishes and lampreys) (28, 32). The parallel evolution of AR and myelin, together with the widespread distribution of AR in the CNS, suggests that androgen signaling may have become involved in the regulation of myelin formation and may play a role in the regenerative capacity of myelin, which is preserved in the adult CNS.
Fig. 4.
Coordinated acquisition of a hinged jaw and myelin, and parallel diversification of steroid receptors during vertebrate evolution. The surrounding of axons with myelin was a prerequisite for the emergence of predation and escape behavior in vertebrates. The evolutionary appearance of myelin indeed parallels the acquisition of a hinged jaw (gnathostomata) (31). The oldest contemporary vertebrates with myelin are cartilaginous fishes (rays and sharks), whereas myelin is absent in jawless fishes (cyclostomata: hagfishes and lampreys) (32). Notably, at the time when a hinged jaw and myelin appeared during vertebrate evolution, the six nuclear steroid hormone receptors diverged from three ancestral receptors during a second round of whole-genome duplication, namely, the AR, progesterone (PR), glucocorticosteroid (GR), mineralocorticosteroid (MR), and the two estrogen (ER) receptors (25, 26). In extant cyclostomata, only ancestral progesterone (Anc PR), corticoid (Anc CR), and estrogen (Anc ER) receptors are present, themselves derived from two ancestral cephalochordata 3-ketosteroid (AncSR) and estrogen (Anc ER) receptors (16). Cartilaginous fishes, the earliest group of living jawed vertebrates with myelinated axons, contain the most ancient type of AR activated by testosterone (27).
The recruitment of astrocytes is likely to play a key role in the remyelinating effect of testosterone because their presence was associated with oligodendrocyte remyelination, whereas P0+/PMP22+ peripheral-type remyelination was observed in their absence. How astrocytes participate in the remyelinating actions of testosterone remains to be clarified. Unfortunately, the quality of presently available AR antibodies did not allow us to study the cellular distribution of the receptor by dual labeling. In previous studies, AR immunoreactivity has been mainly localized to neurons and reactive microglia and to subpopulations of astrocytes and oligodendrocytes (30, 33). In our conditional ARNesCre mice, the AR was selectively inactivated in neurons, astrocytes, and oligodendrocytes, whereas microglial cells were spared (19). Thus, the effect of testosterone observed here does not involve microglial AR. We used the Nestin-Cre mouse strain for two major reasons. First, the high efficiency of the Cre-mediated recombination, which is well characterized for this strain, results in almost complete ablation of the floxed genes in neurons and macroglial cells (15, 19, 34). Then, this choice allowed us to analyze the role of testosterone without any preconceived idea about its neural targets. Nevertheless, cell-specific conditional AR knockout will be needed to identify the direct target cells of androgens. It is conceivable that an androgen-dependent neuronal signal may attract astrocytes to the demyelinating lesion or, alternatively, testosterone may directly act on astrocytes or oligodendrocytes.
Under the present experimental conditions, the magnitude of the effects of castration, testosterone therapy, and AR inactivation on the replenishment of astrocytes and on the balance of oligodendrocyte- versus peripheral-type remyelination was noticeable. However, after a demyelinating lesion induced by the injection of ethidium bromide into the caudal cerebellar peduncle of male or female rats, remyelination by oligodendrocytes or Schwann cells was previously reported not to be affected by gonadectomy (35). The use of different species and different experimental conditions may explain this apparent discrepancy. Alternatively, the effect of castration may also have been masked by the local synthesis of androgens in the brain. Thus, an exercise-dependent increase in androgen biosynthesis within the hippocampus has been shown to stimulate neurogenesis (36). Other “neurosteroids” synthesized within the CNS, such as progesterone, may also positively influence the regeneration of CNS myelin (37, 38). However, in our system, the effect of AR signaling was very robust and AR inactivation was not compensated for by other promyelinating signaling mechanisms.
The multipotency of OPs and their capacity to differentiate into Schwann cells remain amazing (5). How the male gonad-dependent recruitment of astrocytes and its influence on the balance of central- versus peripheral-type remyelination affect functional recovery after a demyelinating event remains to be explored. Moreover, the presence of testes has also been shown to influence the thickness of the myelin sheaths, as well as the number and turnover of oligodendrocytes, which therefore differs between sexes (39).
Neuroprotective and immunosuppressive actions of testosterone have previously been documented in experimental autoimmune encephalomyelitis, a widely used animal model of MS (40). These studies have provided the support for a small clinical pilot study, suggesting that prolonged testosterone therapy of men with MS may have neuroprotective and antiinflammatory effects and also improve cognitive performance (41). The present study may also gain significance from clinical observations showing that (i) the incidence of MS and the relapse rate throughout the disease are lower in men (42); (ii) low testosterone levels are associated with an increased risk of developing MS (43); (iii) levels of testosterone are decreased in men with MS, and reduced levels of testosterone have been associated with disability (44, 45); and (iv) men reach disability milestones more rapidly than women (46).
Testosterone levels progressively decrease with age, but the hormonal decline occurs more rapidly in as many as 20% of aging men (47). Whether age-dependent changes in testosterone are a contributing factor to age-related white matter abnormalities (48) or the reduced regenerative capacity of myelin (49) remains to be explored. Importantly, the influence of androgens on the plasticity and regeneration of myelin may not only have consequences for demyelinating diseases, because recent studies have linked myelin abnormalities to a wide range of psychiatric disorders and cognitive aging (50, 51).
Materials and Methods
Mice.
Wild-type gonadally intact or castrated C57/Bl6 male mice were purchased at the age of 8–12 wk from Janvier Breeding Center. ARNesCre male mice were obtained by crossing floxed AR mice (ARfl/Y) (52) with transgenic mice expressing the Cre recombinase driven by the promoter and the CNS-specific enhancer of rat nestin (Nes), which is selectively active in neuronal and glial precursor cells (34). Both strains were on a C57/BL6 background. The ARNesCre mice have been extensively characterized (19) and were castrated at 2–3 mo of age under ketamine/xylazine anesthesia. ARTfm mice were obtained from René Habert (French Atomic Energy Commission, Fontenay-aux-Roses, France) (18). For in vitro experiments, Plp-EGFP mice were obtained from Wendy Macklin (University of Colorado, Aurora, CO) (21). All animals were housed in standard conditions: a 12-h light/dark cycle with food and water ad libitum. All procedures were performed according to the European Communities Council Directive (86/806/EEC) for the care and use of laboratory animals and were approved by the Regional Ethics Committee CEEA26, Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche. .
Surgical Procedures.
Lysolecithin (Sigma–Aldrich) was microinjected into the right ventral funiculus of the male mouse spinal cord under ketamine/xylazine anesthesia. After shaving the skin at thoracic level T5, a midline incision was made caudally to show thoracolumbar fascia and musculature. Two incisions were performed on the fascia and external edges of dorsal muscles on both sides of the vertebral column that allowed immobilization of the vertebral column on the stereotaxic device (Kopf Instrument). The space between T12 and T13 was identified and carefully dissected until the spinal cord was exposed. The central vein was localized, and the dura mater was carefully pierced using a 32-gauge dental needle. The injection was performed with a fine-tipped glass capillary, elongated until reaching a diameter not exceeding 50 μm (Harvard Apparatus), and mounted on a stereotaxic device after connection to a Hamilton syringe with a plastic tube. By using an infusion pump, 1 μL of 1% lysolecithin solution was unilaterally injected. After suturing the muscles, a Silastic tube, either empty (control) or filled with testosterone (Fluka), was s.c. implanted.
Mixed Glial Cell Cultures.
Male Plp-EGFP mouse pups were used between postnatal days 0 and 3 (53). Briefly, immediately after decapitation, the brain was dissected and placed into Dulbecco’s Modified Eagle Medium (DMEM) supplemented with antibiotics. After the removal of meninges, the brain, including the hemispheres, cerebellum, and cerebral trunk, was placed in fresh DMEM supplemented with antibiotics and 10% (vol/vol) FBS and then passed through a sterile 100-μm nylon mesh. A total of 8 mL of cell suspension was transferred to a poly-l-lysine–coated 100-mm Petri dish. The cells were allowed to grow in DMEM supplemented with 10% (vol/vol) FBS and antibiotics at 37 °C in humidified 5% (vol/vol) CO2. The medium was changed after 4 d, and twice a week later. Cells were used for experiments after 14 d in culture. Before the experimental procedure, cells were transferred to a 24-well plate containing sterilized and poly-l-lysine–coated glass coverslips.
In Vitro Induction of Oligodendrocyte Damage and Hormonal Treatment.
Images of the mixed glial cell cultures were taken under a fluorescent microscope before any treatment and then daily to check viability and morphology of the cells. Lysolecithin (0.1 mg/mL for 12 h) was used for inducing cell damage. After lysolecithin removal, testosterone (1 μM) or vehicle alone (0.1% ethanol) was added to the culture medium. Immunohistochemical analysis was carried out after 4% (wt/vol) paraformaldehyde fixation of the cells for 10 min, PBS washing, incubation for 1 h in 10% (vol/vol) normal goat serum (Vector), and overnight incubation with the primary antibodies at 4 °C.
Histological procedures, antibodies used, image acquisition, and analysis are specified in SI Materials and Methods.
Statistical Analysis.
Statistical analyses were performed using Statistica 12 software (Statsoft, Inc.). All data were given as arithmetic means ± SEM. For the in vivo experiments, each animal group comprised at least five mice. For the in vitro experiments, the groups consisted of at least three wells (average values obtained from three fields of vision per well). Comparisons of means were done by using one-way ANOVA, followed by a least significant difference post hoc test.
SI Materials and Methods
Histological Procedures.
The spinal cord was dissected in one piece along the vertebral column of mice deeply anesthetized and transcardially perfused with 4% (vol/vol) formalin. The tissue was then postfixed for at least 24 h before removal of the vertebral column. After processing in an ethanol/xylene bath and embedding in paraffin blocks, 7-μm tissue sections were cut using a microtome (Leica) and were allowed to dry on glass slides overnight at 37 °C. Immunohistological analysis was performed after deparaffinization and rehydration of the slices. They were then subjected to antigen retrieval by microwaving in citrate buffer (pH 6.0) for 5 min, followed by cooling down at room temperature (RT) for 3 min and reheating for 5 min. Following PBS washing, 10% (vol/vol) normal goat serum solution was applied for 1 h at RT before overnight incubation with the primary antibodies. After incubation with appropriate secondary antibody for 1 h and counterstaining with DAPI (Sigma–Aldrich) for 30 min at RT, the sections were embedded in mounting medium.
Antibodies Used in Histological Procedures.
The following primary antibodies were used: anti-P0 mouse monoclonal (mouse IgG2b hybridoma; kindly provided by Violetta Zujovic, Brain and Spinal Cord Institute, Paris), anti-CA II rabbit polyclonal (provided by M.S.G.), anti-GFAP [one mouse monoclonal (G-3893 clone GA5; Sigma) and two rabbit polyclonal (Z0334; DAKO)], anti-Olig2 rabbit polyclonal (AB9610; Chemicon), anti-NG2 rabbit polyclonal (MAB 5320; Millipore), anti-proliferating cell nuclear antigen mouse monoclonal (sc56; Santa Cruz Biotechnology), anti-MBP rat monoclonal (MAB386; Millipore), and anti-MBP mouse monoclonal (381; Millipore). The following secondary antibodies were used: anti-rabbit Cy3 conjugated, anti-mouse Alexa 488 conjugated, anti-mouse Alexa 633 conjugated, anti-rabbit Alexa 633 conjugated, and anti-rat Cy3 conjugated (Jackson Immunoresearch).
Image Acquisition and Analysis.
Microscopes from Zeiss, Inc. were used: for epifluorescence analysis, an AxioVision A5 microscope with a digital camera with AxioCam MRc5 and Axio-Vision Rel. 4.2 as the operating software for the camera, and for confocal analysis, an LSM 510-Meta Confocor 2 comprising an Axiovert 200M inverted microscope with an LSM Image Browser and Leica TCS SP8 microscope with Leica LAS AF software. Analysis was performed with ImageJ (NIH) software.
Acknowledgments
We thank Wendy B. Macklin (University of Colorado) for sharing Plp-EGFP mice and René Habert for providing Tfm mice (University Paris-Diderot and French Atomic Energy Commission). B.B. was successively supported by the European Leukodystrophy Association (ELA) Foundation (France) and by the Mattern Foundation. The fellowship of S.J. was funded by the Higher Education Commission of Pakistan and the French Embassy in Pakistan. This work was supported by grants from the ELA, French Multiple Sclerosis Foundation (ARSEP), and UK Multiple Sclerosis Society.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1614826113/-/DCSupplemental.
References
- 1.Monteiro de Castro G, Deja NA, Ma D, Zhao C, Franklin RJ. Astrocyte activation via Stat3 signaling determines the balance of oligodendrocyte versus Schwann cell remyelination. Am J Pathol. 2015;185(9):2431–2440. doi: 10.1016/j.ajpath.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keirstead HS, et al. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci. 1999;19(17):7529–7536. doi: 10.1523/JNEUROSCI.19-17-07529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crang AJ, Gilson JM, Li WW, Blakemore WF. The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS. Eur J Neurosci. 2004;20(6):1445–1460. doi: 10.1111/j.1460-9568.2004.03606.x. [DOI] [PubMed] [Google Scholar]
- 4.Talbott JF, et al. Schwann cell-like differentiation by adult oligodendrocyte precursor cells following engraftment into the demyelinated spinal cord is BMP-dependent. Glia. 2006;54(3):147–159. doi: 10.1002/glia.20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zawadzka M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6(6):578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keirstead HS, Morgan SV, Wilby MJ, Fawcett JW. Enhanced axonal regeneration following combined demyelination plus schwann cell transplantation therapy in the injured adult spinal cord. Exp Neurol. 1999;159(1):225–236. doi: 10.1006/exnr.1999.7100. [DOI] [PubMed] [Google Scholar]
- 7.Blakemore WF, Gilson JM, Crang AJ. The presence of astrocytes in areas of demyelination influences remyelination following transplantation of oligodendrocyte progenitors. Exp Neurol. 2003;184(2):955–963. doi: 10.1016/S0014-4886(03)00347-9. [DOI] [PubMed] [Google Scholar]
- 8.Mujtaba T, Mayer-Proschel M, Rao MS. A common neural progenitor for the CNS and PNS. Dev Biol. 1998;200(1):1–15. doi: 10.1006/dbio.1998.8913. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff RH, Franklin RJ. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: A comparative study. Glia. 1999;25(3):216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Talbott JF, et al. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192(1):11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto T, et al. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201–224. doi: 10.1146/annurev-physiol-030212-183656. [DOI] [PubMed] [Google Scholar]
- 12.Levin ER. Extranuclear steroid receptors are essential for steroid hormone actions. Annu Rev Med. 2015;66:271–280. doi: 10.1146/annurev-med-050913-021703. [DOI] [PubMed] [Google Scholar]
- 13.Arnett HA, et al. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306(5704):2111–2115. doi: 10.1126/science.1103709. [DOI] [PubMed] [Google Scholar]
- 14.Hagedorn L, Suter U, Sommer L. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-beta family factors. Development. 1999;126(17):3781–3794. doi: 10.1242/dev.126.17.3781. [DOI] [PubMed] [Google Scholar]
- 15.Hussain R, et al. The neural androgen receptor: A therapeutic target for myelin repair in chronic demyelination. Brain. 2013;136(Pt 1):132–146. doi: 10.1093/brain/aws284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA. 2001;98(10):5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pediaditakis I, et al. Dehydroepiandrosterone: An ancestral ligand of neurotrophin receptors. Endocrinology. 2015;156(1):16–23. doi: 10.1210/en.2014-1596. [DOI] [PubMed] [Google Scholar]
- 18.Merlet J, Racine C, Moreau E, Moreno SG, Habert R. Male fetal germ cells are targets for androgens that physiologically inhibit their proliferation. Proc Natl Acad Sci USA. 2007;104(9):3615–3620. doi: 10.1073/pnas.0611421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raskin K, et al. Conditional inactivation of androgen receptor gene in the nervous system: Effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29(14):4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford AH, Stockley JH, Tripathi RB, Richardson WD, Franklin RJ. Oligodendrocyte progenitors: Adult stem cells of the central nervous system? Exp Neurol. 2014;260:50–55. doi: 10.1016/j.expneurol.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22(3):876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci. 1998;18(12):4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5(3-4):57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fancy SP, Chan JR, Baranzini SE, Franklin RJ, Rowitch DH. Myelin regeneration: A recapitulation of development? Annu Rev Neurosci. 2011;34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- 25.Eick GN, Thornton JW. Evolution of steroid receptors from an estrogen-sensitive ancestral receptor. Mol Cell Endocrinol. 2011;334(1-2):31–38. doi: 10.1016/j.mce.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Baker ME, Nelson DR, Studer RA. Origin of the response to adrenal and sex steroids: Roles of promiscuity and co-evolution of enzymes and steroid receptors. J Steroid Biochem Mol Biol. 2015;151:12–24. doi: 10.1016/j.jsbmb.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Ogino Y, Katoh H, Kuraku S, Yamada G. Evolutionary history and functional characterization of androgen receptor genes in jawed vertebrates. Endocrinology. 2009;150(12):5415–5427. doi: 10.1210/en.2009-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogino Y, et al. Neofunctionalization of androgen receptor by gain-of-function mutations in teleost fish lineage. Mol Biol Evol. 2016;33(1):228–244. doi: 10.1093/molbev/msv218. [DOI] [PubMed] [Google Scholar]
- 29.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 30.DonCarlos LL, et al. Novel cellular phenotypes and subcellular sites for androgen action in the forebrain. Neuroscience. 2006;138(3):801–807. doi: 10.1016/j.neuroscience.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Zalc B, Goujet D, Colman D. The origin of the myelination program in vertebrates. Curr Biol. 2008;18(12):R511–R512. doi: 10.1016/j.cub.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Bullock TH, Moore JK, Fields RD. Evolution of myelin sheaths: Both lamprey and hagfish lack myelin. Neurosci Lett. 1984;48(2):145–148. doi: 10.1016/0304-3940(84)90010-7. [DOI] [PubMed] [Google Scholar]
- 33.Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40(4):446–457. [PubMed] [Google Scholar]
- 34.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23(1):99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 35.Li WW, Penderis J, Zhao C, Schumacher M, Franklin RJM. Females remyelinate more efficiently than males following demyelination in the aged but not young adult CNS. Exp Neurol. 2006;202(1):250–254. doi: 10.1016/j.expneurol.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto M, et al. Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci USA. 2012;109(32):13100–13105. doi: 10.1073/pnas.1210023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain R, et al. Progesterone and Nestorone facilitate axon remyelination: A role for progesterone receptors. Endocrinology. 2011;152(10):3820–3831. doi: 10.1210/en.2011-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Etr M, et al. Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia. 2015;63(1):104–117. doi: 10.1002/glia.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerghet M, et al. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci. 2006;26(5):1439–1447. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: Implications for multiple sclerosis. J Neuroimmunol. 2004;146(1-2):144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Sicotte NL, et al. Testosterone treatment in multiple sclerosis: A pilot study. Arch Neurol. 2007;64(5):683–688. doi: 10.1001/archneur.64.5.683. [DOI] [PubMed] [Google Scholar]
- 42.Kalincik T, et al. MSBase Study Group Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(Pt 12):3609–3617. doi: 10.1093/brain/awt281. [DOI] [PubMed] [Google Scholar]
- 43.Pakpoor J, Goldacre R, Schmierer K, Giovannoni G, Goldacre MJ. Testicular hypofunction and multiple sclerosis risk: A record-linkage study. Ann Neurol. 2014;76(4):625–628. doi: 10.1002/ana.24250. [DOI] [PubMed] [Google Scholar]
- 44.Bove R, et al. Low testosterone is associated with disability in men with multiple sclerosis. Mult Scler. 2014;20(12):1584–1592. doi: 10.1177/1352458514527864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safarinejad MR. Evaluation of endocrine profile, hypothalamic-pituitary-testis axis and semen quality in multiple sclerosis. J Neuroendocrinol. 2008;20(12):1368–1375. doi: 10.1111/j.1365-2826.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 46.Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain. 2006;129(Pt 3):595–605. doi: 10.1093/brain/awh714. [DOI] [PubMed] [Google Scholar]
- 47.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 48.Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442(3):277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- 49.Ruckh JM, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10(1):96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31(7):361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haroutunian V, et al. Myelination, oligodendrocytes, and serious mental illness. Glia. 2014;62(11):1856–1877. doi: 10.1002/glia.22716. [DOI] [PubMed] [Google Scholar]
- 52.De Gendt K, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA. 2004;101(5):1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feutz AC, Pham-Dinh D, Allinquant B, Miehe M, Ghandour MS. An immortalized jimpy oligodendrocyte cell line: Defects in cell cycle and cAMP pathway. Glia. 2001;34(4):241–252. doi: 10.1002/glia.1058. [DOI] [PubMed] [Google Scholar]
- 54.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16(1):17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 55.Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181:209–232. doi: 10.1016/S0079-6123(08)81012-6. [DOI] [PubMed] [Google Scholar]
- 56.van de Wijngaart DJ, Dubbink HJ, van Royen ME, Trapman J, Jenster G. Androgen receptor coregulators: Recruitment via the coactivator binding groove. Mol Cell Endocrinol. 2012;352(1-2):57–69. doi: 10.1016/j.mce.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 58.Reddy DS. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]