Significance
Nitrous oxide (N2O) is a potent ozone-depleting greenhouse gas. This work identifies a means by which N2O is generated during nitrification, or biological ammonia oxidation. Fertilizer use in agriculture stimulates nitrification, thus increasing the volume of N2O emissions worldwide. The results presented herein will inform models and strategies toward optimized, sustainable agriculture. Moreover, these results highlight a rare example of biological N–N bond formation.
Keywords: nitric oxide, nitrification, nitrous oxide, enzymology, bioinorganic chemistry
Abstract
Ammonia oxidizing bacteria (AOB) are major contributors to the emission of nitrous oxide (N2O). It has been proposed that N2O is produced by reduction of NO. Here, we report that the enzyme cytochrome (cyt) P460 from the AOB Nitrosomonas europaea converts hydroxylamine (NH2OH) quantitatively to N2O under anaerobic conditions. Previous literature reported that this enzyme oxidizes NH2OH to nitrite () under aerobic conditions. Although we observe formation under aerobic conditions, its concentration is not stoichiometric with the NH2OH concentration. By contrast, under anaerobic conditions, the enzyme uses 4 oxidizing equivalents (eq) to convert 2 eq of NH2OH to N2O. Enzyme kinetics coupled to UV/visible absorption and electron paramagnetic resonance (EPR) spectroscopies support a mechanism in which an FeIII–NH2OH adduct of cyt P460 is oxidized to an {FeNO}6 unit. This species subsequently undergoes nucleophilic attack by a second equivalent of NH2OH, forming the N–N bond of N2O during a bimolecular, rate-determining step. We propose that results when nitric oxide (NO) dissociates from the {FeNO}6 intermediate and reacts with dioxygen. Thus, is not a direct product of cyt P460 activity. We hypothesize that the cyt P460 oxidation of NH2OH contributes to NO and N2O emissions from nitrifying microorganisms.
Nitrous oxide (N2O) participates in ozone-layer depletion and possesses a global warming potential nearly 300-fold greater than carbon dioxide (1). Atmospheric N2O concentrations have increased ∼120% since the preindustrial era, largely due to the widespread use of fertilizers required to produce sustenance for humans and livestock. N2O is a byproduct of the microbial metabolism of fertilizer components, including ammonia (NH3) and nitrate (); consequently, agricultural soils account for an estimated 60–75% of global N2O emissions. The metabolic pathway by which microorganisms oxidize NH3, nitrification, occurs in two phases, both of which are mediated by autotrophic microorganisms. In the first, NH3-oxidizing bacteria (AOB) or archaea (AOA) oxidize NH3 to nitrite (). In the second, is subsequently oxidized to by -oxidizing bacteria. NH3-oxidizing microbes contribute substantially to global N2O emissions, whereas -oxidizing bacteria produce negligible N2O (2, 3). AOB are proposed to emit N2O either as a byproduct of the nitrification pathway or as a product of the nitrifier denitrification pathway (i.e., the reduction of ) (4–6).
Nitrification of NH3 to occurs in two steps (7, 8). The first step is catalyzed by NH3 monooxygenase, which uses copper (Cu) and dioxygen (O2) to hydroxylate NH3 to hydroxylamine (NH2OH) (9). In AOB, the second step is thought to be the four-electron oxidation of NH2OH to by NH2OH oxidoreductase (HAO). HAO is a multiheme enzyme with eight c-type hemes per subunit: seven are electron transfer cofactors, and the eighth is the so-called P460 active site that contains a unique tyrosine cross-link to the heme ring. The enzyme (or enzymes) that AOA uses to oxidize NH2OH is currently unknown (10).
AOB possess machinery for nitrifier denitrification that reduces to N2O via a nitric oxide (NO) intermediate (11, 12). The archetypal AOB Nitrosomonas europaea possesses genes for a Cu-containing nitrite reductase (NirK) and a membrane-bound, heme-containing NO reductase (NorB). NirK reduces by one electron to NO, whereas NorB catalyzes the two-electron reduction of 2 eq of NO to N2O. Nitrifier denitrification is thought to lead to the increased production of N2O and NO by AOB in microaerophilic or anaerobic conditions (13). However, this pathway does not account for the total AOB N2O emission under aerobic conditions.
Under aerobic conditions, N2O emission from AOB is proposed to result from the incomplete oxidation of NH2OH to either nitroxyl (HNO) or NO. Two eq of HNO rapidly react to form N2O (14), whereas NO is reduced by an NO reductase (12). In support of these hypotheses, both NO and N2O have been observed to form during steady-state turnover of purified HAO under aerobic conditions (15). Although NorB could facilitate this N2O production, a NorB knockout strain also produces N2O at atmospheric O2 concentration, consistent with the presence of an alternate N2O-producing pathway (16).
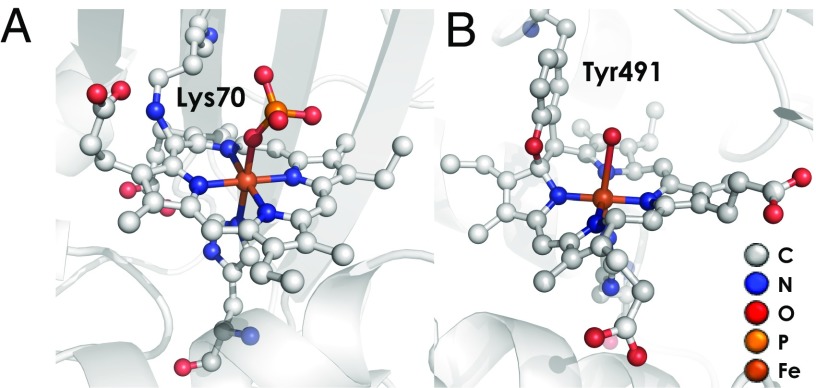
In this study, we demonstrate that there indeed exists a direct enzymatic pathway from NH2OH to N2O, and that this pathway is mediated by cytochrome (cyt) P460, a constitutively expressed (17), soluble, periplasmic metalloenzyme originally isolated from N. europaea (18). N. europaea cyt P460 is a 36-kDa homodimeric protein in which each subunit bears a mono-His c-type heme with an N–C cross-link from the 13′ mesocarbon to the amine of Lys70 (19) (Fig. 1A). Such cross-links alter porphyrin π-conjugation. Moreover, the heme P460 macrocycle exhibits significant ruffling, a common distortion mode for c-type hemes (20, 21). P460 hemes are named for the 460-nm Soret band observed in their ferrous state. The P460 center found in N. europaea HAO differs from cyt P460: it is doubly cross-linked by Tyr491 at the 5′ mesocarbon and an adjacent pyrrole α-carbon (Fig. 1B). Despite lacking homology to HAO (19, 22), the presence of a P460 cofactor in cyt P460 has implicated this enzyme in NH2OH oxidation (23–25).
Fig. 1.
Ferric P460 cofactors in cyt P460 [A, Protein Data Bank (PDB) ID code 2JE2] and HAO (B, PDB ID code 4N4N).
Cyt P460 was previously reported to oxidize NH2OH to (26), implicating all P460 centers in mediating four-electron oxidation of NH2OH. However, an octaheme HAO-like protein from the anaerobic NH3-oxidizing microorganism Kuenenia stuttgartiensis was shown to oxidize NH2OH to NO instead of (27). An X-ray crystal structure of the K. stuttgartiensis HAO-like enzyme features a tyrosine cross-linked active site and heme distribution that was identical to N. europaea HAO. The K. stuttgartiensis enzyme was proposed to yield NO due to the absence of a nearby tyrosine residue that is present in N. europaea HAO. The N. europaea cyt P460 active site also lacks the nearby tyrosine residue and is far more solvent-exposed (19). Herein, we show that N2O is the enzymatic product of anaerobic NH2OH oxidation by N. europaea cyt P460. We demonstrate that this pathway proceeds through an NH2OH-bound form that is oxidized by three electrons to an Fe–NO species that then reacts with NH2OH to form N2O in the rate-determining step.
Results and Discussion
Exclusive Conversion of NH2OH to N2O by Cyt P460 Under Anaerobic Conditions.
Consistent with previous work on the Methylococcus capsulatus cyt P460 (26), under aerobic conditions, cyt P460 reacts with NH2OH in the presence of the oxidant phenazine methosulfate (PMS) to form , as detected by Griess diazotization assays (SI Appendix, Fig. S1A). However, the maximum stoichiometry achieved was 0.7 mol of per mole of NH2OH (SI Appendix, Table ST1). Gas chromatography (GC) analysis reveals that the remainder of the NH2OH is converted to N2O (SI Appendix, Fig. S1A).
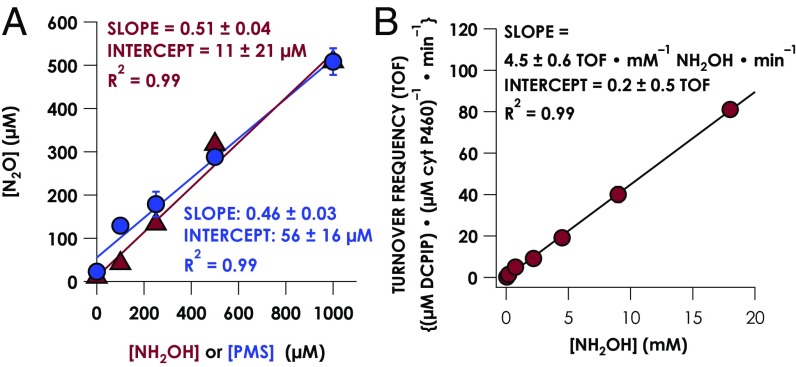
Under anaerobic conditions, no is formed when cyt P460 is treated with NH2OH and PMS. GC analysis (SI Appendix, Fig. S1B) reveals that in the presence of an oxidant ([Ru(NH3)6]Cl3, horse heart cyt c, or PMS), cyt P460 catalyzes the exclusive formation of N2O from NH2OH. In the absence of either enzyme or oxidant, only trace N2O is formed (SI Appendix, Fig. S1). By monitoring the amount of N2O produced under various NH2OH or oxidant concentrations (Fig. 2A), a stoichiometry of 2 NH2OH eq and 4 oxidizing eq producing 1 eq of N2O was established (Eq. 1):
| [1] |
Fig. 2.
(A) Stoichiometry of N2O production by cyt P460 determined with GC. Data points are averages of triplicate trials with 5 μM ferric cyt P460 in anaerobic 50 mM Hepes, pH 8.0, at 25 °C overnight. Error bars represent 1 SD of three trials. For the red triangles, the concentration of PMS is held at 1 mM, whereas the NH2OH concentration is varied; for the blue circles, the NH2OH concentration is held at 1 mM, whereas PMS concentration varies. (B) Steady-state NH2OH oxidase activity plot for cyt P460. The assay conditions were 1 μM cyt P460, 6 μM PMS, and 100 μM DCPIP with various NH2OH concentrations in anaerobic 50 mM Hepes, pH 8.0, at 25 °C. Each data point is the average of three trials, with error bars representing one SD.
Steady-State Kinetics.
Steady-state activity assays of cyt P460 were performed under anaerobic conditions using a 2,6-dichlorophenolindophenol (DCPIP)/PMS coupled assay (23) and an N2O-selective electrode. By monitoring the decay of the DCPIP absorbance at 605 nm (A605), it was determined that 2 eq of DCPIP was reduced per 1 eq of N2O produced under steady-state conditions (SI Appendix, Fig. S2). Because DCPIP is a two-electron oxidant, this stoichiometry is consistent with Eq. 1. The DCPIP/PMS assay therefore provides a convenient means of measuring steady-state N2O production by cyt P460.
Steady-state activities of cyt P460 have been reported previously (23, 26), but to our knowledge, no studies have presented steady-state activity plots. Fig. 2B shows the steady-state activity plot exhibiting nonsaturating, linear behavior from 0.05 to 20 mM NH2OH that spans turnover frequencies of 0.3–80 μM DCPIP consumed min−1⋅µM⋅enzyme−1. Although this nonsaturating behavior precludes determination of kcat or Km, the slope of the linear region suggests a kcat/Km of 5,000 M−1⋅min−1 (28). However, the characterization of pathway intermediates suggests a multistep reaction mechanism that is inconsistent with classical Michaelis–Menten kinetics (vide infra).
Characterization of Cyt P460 FeIII–NH2OH.
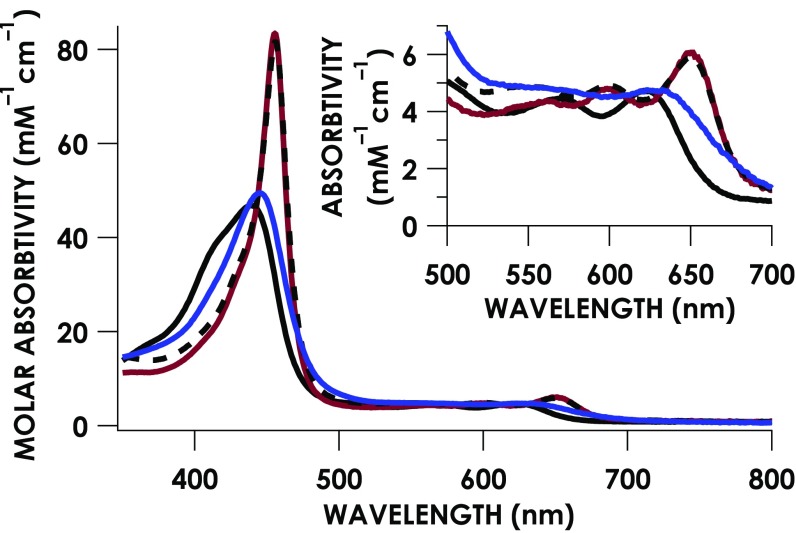
Recombinant expression and purification of cyt P460 was previously achieved by Elmore et al. (29). This method yields a British racing green protein with UV/visible absorption and electron paramagnetic resonance (EPR) spectra consistent with those reported for the enzyme isolated from N. europaea (18, 23, 30). The absorption spectrum of the as-isolated cyt P460 has a Soret band at 440 nm with a shoulder at 414 nm and Q-band maxima at 570 nm and 627 nm (Fig. 3). The corresponding EPR spectrum (Fig. 4A) is characteristic of an S = 5/2 FeIII, with g-values of 6.57, 5.09, and 1.97 (E/D = 0.03). The crystal structure of the as-isolated cyt P460 (19, 29) (Fig. 1A) shows phosphate ligated to the Fe center. These crystallization conditions used 2.4 M phosphate buffer; we expect that H2O will occupy this site under our experimental conditions, and therefore assign the as-isolated protein as an FeIII–OH2 heme center.
Fig. 3.
UV/visible absorption spectra of FeIII–OH2 cyt P460 (black), FeIII–NH2OH cyt P460 (blue), and {FeNO}6 cyt P460 generated via treatment with PROLI-NONOate (red line) or oxidation of FeIII–NH2OH (black dashed line). (Inset) Magnification of the Q-bands.
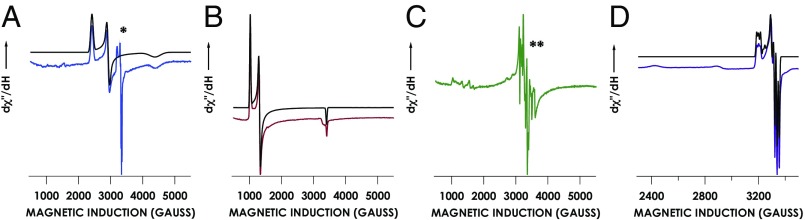
Fig. 4.
EPR spectra of species on the proposed cyt P460 NH2OH oxidase pathway. Cyt P460 at 170 μM (A) was treated with 100 mM NH2OH (B), with 2 mM NH2OH and 2 mM DCPIP (C), or with 45 mM NH2OH and 2 mM DCPIP (D) and incubated for 10 min. Black traces are spectra simulated with the parameters listed in SI Appendix, Table ST2. Spectra were collected at 10 K and 633 μW or at 20 K and 63 μW. A 5% impurity of an {FeNO}7 species is indicated by a single asterisk. An Mn2+ EPR signal is indicated by double asterisks. dχ″/dH, derivative of magnetic susceptibility vs. magnetic induction.
The addition of NH2OH to cyt P460 produces a species with a UV/visible absorption spectrum distinct from FeIII–OH2 (Fig. 3). Within the time of manual mixing, the 414-nm shoulder of the FeIII–OH2 spectrum disappears concomitant with a shift in the Soret band to 445 nm and broadening of the Q-bands. The simplest interpretation is that NH2OH substitutes for H2O at the Fe site (i.e., FeIII–NH2OH). Cyt P460 was titrated with NH2OH, and the resulting series of spectra (SI Appendix, Fig. S3A) show an isosbestic point at 438 nm, which suggests a one-step conversion from FeIII–OH2 to the putative FeIII–NH2OH. To determine the NH2OH dissociation constant [], the absorption at 414 nm (A414) was plotted against NH2OH concentration. Fitting the data to a hyperbolic binding curve resulted in a of 9 ± 1 mM (SI Appendix, Fig. S3A, Inset).
Treatment of cyt P460 with 100 mM NH2OH results in the disappearance of the S = 5/2 FeIII–OH2 signal and the appearance of two rhombic S = 1/2 EPR signals (Fig. 4B). The first signal has g-values of 2.75, 2.28, and 1.54, and is consistent with a low-spin FeIII. The EPR spectra of cyt P460 treated with increasing concentrations of NH2OH (SI Appendix, Fig. S3B) corroborate the value determined with UV-vis spectrometry. The conversion from a high-spin to low-spin FeIII is consistent with the binding of NH2OH to the Fe center; thus, we assign this S = 1/2 species as a rare example of a stable heme FeIII–NH2OH complex (31).
The second rhombic S = 1/2 signal has g-values of 2.10, 2.02, and 2.01, and 14N hyperfine coupling values of 50 MHz, 57 MHz, and 45 MHz. This second signal represents less than 5% of the total spin as determined by spin quantitation and is consistent with a five-coordinate heme FeII–NO species (32), or {FeNO}7 in Enemark–Feltham notation (33). This {FeNO}7 species can be independently generated by treating cyt P460 with the HNO donor, disodium diazen-1-ium-1,2,2 triolate (Na2N2O3, Angeli’s salt). This species is stable in the presence of NH2OH or oxidant (SI Appendix, Fig. S4). The low yield observed in the EPR spectrum and the lack of reactivity suggest that the {FeNO}7 is an off-path product and does not contribute to the productive N2O-generating cyt P460 pathway. At present, we are unsure why this oxidized species appears in the absence of O2, oxidant, or NO. One possibility is that the samples are exposed to a small amount of O2 during freezing, which would oxidize FeIII–NH2OH to {FeNO}7.
Characterization of an Intermediate in NH2OH Oxidation.
The addition of the oxidant [Ru(NH3)6]Cl3 under anaerobic conditions results in formation of a new species. Over the course of the reaction (ca. 20 min), there are two distinct phases: an accumulation phase and a decay phase. In the accumulation phase, a new species appears within 2 min. The absorption spectrum of the new species exhibits an intense and sharp Soret band centered at 455 nm and Q-bands at 554 nm, 603 nm, and 652 nm (SI Appendix, Fig. S5A). Several isosbestic points are observed during the spectral time course, indicating a single-step conversion from FeIII–NH2OH to the new species. In the decay phase, the new species is converted back to FeIII–NH2OH. The spectral time course of this phase also features several isosbestic points (SI Appendix, Fig. S5B). These data strongly suggest the accumulation and decay of an intermediate on the cyt P460 pathway, hereafter referred to as the 455-nm intermediate owing to its Soret band maximum.
In the presence of DCPIP, the formation of the 455-nm intermediate is complete at the time of mixing; the intermediate persists for 1 min, until DCPIP is completely consumed, at which point the A455 slowly decreases (SI Appendix, Fig. S6). Subsequent addition of 10 mM NH2OH hastened the decay of the 455-nm intermediate (SI Appendix, Fig. S6C). The spectral time course of the decay phase features an isosbestic point at 445 nm, consistent with the direct conversion of the 455-nm intermediate to a single species. Comparison of the final decay product spectrum to the decay product of as-isolated cyt P460 mixed with 10 mM NH2OH confirms that the decay product is FeIII–NH2OH (SI Appendix, Fig. S6B).
The persistence of the 455-nm intermediate in the presence of excess oxidant suggests that, under turnover conditions, the decay of the 455-nm intermediate is the rate-determining step. The increase in decay rate at higher NH2OH concentrations implies a bimolecular reaction between the 455-nm intermediate and NH2OH. To confirm this relationship, the formation and decay of the 455-nm intermediate at various NH2OH concentrations were monitored (SI Appendix, Fig. S8A). The sum of two exponentials (SI Appendix, Eq. S1) was fit to the A455 traces, providing observed rate constants (kobs) for both the formation [kobs(1)] and decay [kobs(2)] of the 455-nm intermediate. At higher NH2OH concentrations, kobs(1) was too fast to fit accurately, but, qualitatively, it increases with increasing NH2OH concentration. The kobs(2) parameter showed a linear dependence on NH2OH, consistent with a bimolecular reaction of NH2OH and the 455-nm intermediate. A linear fit to a plot of kobs versus NH2OH concentration provided a second-order rate constant of 0.07 mM−1⋅min−1 (SI Appendix, Fig. S8B).
The 455-nm intermediate was trapped for EPR characterization by freezing the reaction of 170 μM cyt P460 with 2 mM NH2OH and 2 mM DCPIP within 1 min. The EPR spectrum of the trapped 455-nm intermediate lacks the signals associated with FeIII–OH2 and FeIII–NH2OH, suggesting that the protein is completely converted to the 455-nm intermediate or to another EPR-silent species (Fig. 4C). The only EPR signal observed is an S = 1/2 signal with hyperfine structure that can be attributed to Mn2+ contamination in the protein sample. The lack of an Fe-based EPR signal implies that the 455-nm intermediate is either a diamagnetic or non-Kramer’s (integer spin greater than 0) species.
The decay product was prepared by treating 150 μM cyt P460 with 45 mM NH2OH and 2 mM DCPIP, followed by incubation for 10 min at room temperature. The EPR spectrum of this sample shows signals for FeIII–NH2OH and {FeNO}7 (Fig. 4D). The combined spins account for the total Fe concentration in the sample, indicating the quantitative conversion of the 455-nm intermediate to either FeIII–NH2OH (80 μM) or {FeNO}7 (70 μM), following the depletion of oxidant.
Identification of the 455-nm Intermediate as an {FeNO}6.
The need for oxidant to form the 455-nm intermediate suggests that it is an oxidized form of FeIII–NH2OH. Bari and coworkers (34) have postulated several oxidized species in their proposed mechanism of NH2OH oxidation by HAO, including an {FeNO}7, an {FeNO}6, and a ferrous-nitrous acid (FeII–ONOH) species. Other possibilities include a ferric hydroxylamine radical (FeIII–•NH2OH), which has been proposed as an intermediate in the enzymatic pathway of P450 NO reductase (35, 36) and FeII–HNO, which has been characterized in only one biological system, the myoglobin–HNO complex (37, 38). Finally, because N2O is the product of NH2OH oxidation by cyt P460 under our experimental conditions, an Fe–N2O complex is also possible. We propose that our experimental data are most consistent with assignment of the 455-nm intermediate as an {FeNO}6 species (vide infra).
The number of oxidizing equivalents required to convert FeIII–NH2OH to the 455-nm intermediate was determined by treating cyt P460 with substoichiometric NH2OH and excess cyt c (SI Appendix, Fig. S9). The reduction of cyt c was monitored by the increase in absorption at 550 nm (ε550 = 19,600 M−1⋅cm−1 (39). These experiments show that 3 electrons are required to oxidize FeIII–NH2OH to the 455-nm intermediate, suggesting an {FeNO}6 or FeII–ONOH species (33). Consistent with this observation, treatment of FeIII–OH2 with NO supplied either as the gas or via the NO donor PROLI-NONOate [1-(hydroxy-NNO-azoxy)-l-proline] produces an EPR-silent species with an absorption spectrum identical to the absorption spectrum of the 455-nm intermediate (Fig. 3). The lack of an EPR signal precludes assignment of the 455-nm intermediate as an {FeNO}7, because these species typically exhibit S = 1/2 EPR signals (32). The 455-nm intermediate generated in this way persists for ca. 1 h and is stable to excess DCPIP (SI Appendix, Fig. S10). This lack of reactivity is inconsistent with the hypothesis of Bari and coworkers (34) that the hydrolysis of an {Fe–NO}6 generates an FeII–ONOH adduct that is poised for proton-coupled, one-electron oxidation to because cyt P460 does not produce under anaerobic conditions. Taken together, these data support the assignment of the 455-nm intermediate as an {FeNO}6.
The stability of the {FeNO}6 intermediate in the absence of NH2OH and the NH2OH dependence of its decay suggests that {FeNO}6 reacts with NH2OH to form N2O. A so-called shunted {FeNO}6 species was prepared by adding 2 eq of NO supplied via PROLI-NONOate to cyt P460. The addition of 2 mM NH2OH results in the decay of {FeNO}6 to FeIII–NH2OH (SI Appendix, Fig. S11). Consumption of the shunted {FeNO}6 exhibits the same NH2OH concentration dependence, 0.07 ± .01 mM−1⋅min−1, as the {FeNO}6 that accumulates under turnover conditions.
To confirm the production of N2O in the above reaction, cyt P460 was treated with varying concentrations of NO and excess NH2OH. The amount to N2O produced was monitored by GC/mass spectrometry (MS). There is a clear 1:1 stoichiometry of N2O produced versus NO added (SI Appendix, Fig. S12A). This result is consistent with NO binding to cyt P460 to form the {FeNO}6 intermediate and subsequent reaction with NH2OH to form N2O. The same experiment was performed with NO and isotopically labeled 15NH2OH. The mass shift from 44 atomic mass units (amu) to 45 amu (SI Appendix, Fig. S12B) in the presence of cyt P460 clearly demonstrates that NO is coupled to NH2OH via cyt P460. The reaction of NO with NH2OH in the absence of cyt P460 does not result in N2O production.
Mechanism for N2O Formation from NH2OH by Cyt P460.
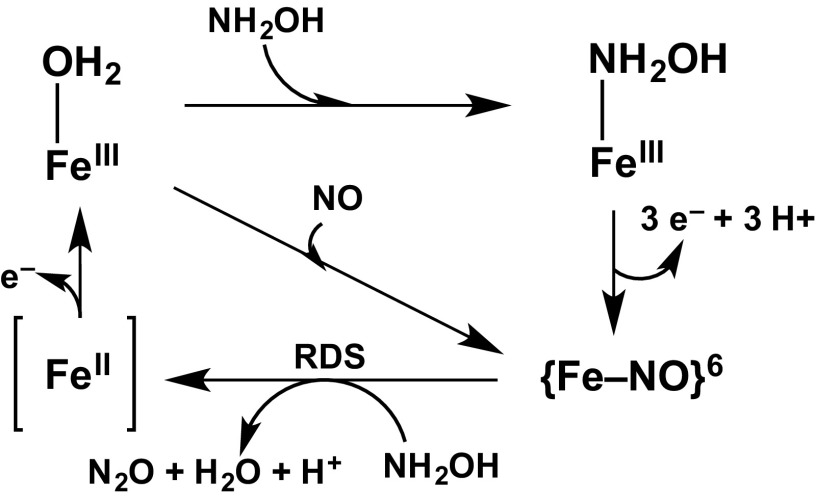
Given the above results, we propose the mechanism described in Fig. 5 for the NH2OH oxidase activity of cyt P460. The catalytic cycle initiates from the S = 5/2 FeIII–OH2, which binds NH2OH to form an S = 1/2 FeIII–NH2OH species that is stable in the absence of oxidant. In the presence of oxidant, FeIII–NH2OH is rapidly oxidized by 3 electrons to the EPR-silent {FeNO}6, which undergoes nucleophilic attack by a second equivalent of NH2OH to yield N2O and H2O. We propose that the Fe-containing product of this reaction is an FeII species that is rapidly oxidized and converted to the starting FeIII–OH2 species.
Fig. 5.
Proposed Cyt P460 NH2OH oxidase mechanism. RDS, rate-determining step.
Several other intermediates can be envisioned in the conversion of FeIII–OH2 to {FeNO}6. One-electron (K3[Fe(CN)6], CuII-azurin, and [Ru(NH3)6]Cl3) and two-electron (DCPIP and PMS) oxidants can access {FeNO}6, which suggests that rapid, subsequent one-electron oxidation steps occur in the conversion of FeIII–NH2OH to {FeNO}6. Although {FeNO}7 appeared under certain conditions (i.e., after complete consumption of oxidant), it is not a catalytically competent intermediate. The EPR spectrum of this species is consistent with a five-coordinate {FeNO}7 (32); however, a six-coordinate {FeNO}7 could be on the reaction pathway. Other possible one-electron oxidized intermediates include FeIII–•NH2OH, FeIII–•NHOH, or {FeN(H)O}8″ (40). To date, we have found no evidence to exclude any of these possibilities, and the characterization of these possible intermediates is the subject of future investigations in our laboratory.
The rate-determining step of the anaerobic oxidation of NH2OH by cyt P460 is the bimolecular reaction of NH2OH with {FeNO}6 that results in N2O formation. The intensities of the UV/visible absorption spectral features of FeIII–OH2 treated with NO are identical to the intensities observed for the {FeNO}6 intermediate formed using NH2OH and oxidant (Fig. 3). Combined with the lack of Fe-based EPR signals in these samples, these data suggest that under both conditions, all Fe sites are quantitatively converted to the {FeNO}6 species. This quantitative accumulation is consistent with the nucleophilic attack by NH2OH being the rate-determining step of the catalytic cycle. There is precedent for NH2OH reacting with [Fe(CN)5(NO)]2−, a classic example of an {FeNO}6, to form N2O with stoichiometry matching the stoichiometry above for cyt P460 (41).
We propose that NH2OH reacts with {FeNO}6 to form an FeII species, which is rapidly oxidized to return the enzyme to FeIII–OH2. This proposal is based on the precedent that [Fe(CN)5NO]2− reacts with NH2OH to form [Fe(CN)5(OH2)]3−. However, the characteristic 463-nm UV/visible absorption peak of FeII cyt P460 was never observed in our experiments as an intermediate or an end product. The FeII cyt P460 is expected to react rapidly with other species present during turnover (oxidant, NH2OH, or NO), thereby precluding its observation (vide infra). The reaction of FeII cyt P460 with NO produces the same inactive {FeNO}7 species observed when FeIII–OH2 is treated with HNO (SI Appendix, Fig. S4). FeII cyt P460 reacts with NH2OH to form FeIII–OH2 via an intermediate with a UV/visible absorption feature at 663 nm that has not been observed in any of our other experiments (SI Appendix, Fig. S13). This 663-nm intermediate forms and disappears within 3 s; assignment of this 663-nm intermediate will require rapid-mixing techniques. Critically, the reaction of FeII cyt P460 with NH2OH does not inactivate the enzyme.
Stopped-flow UV/visible absorption spectroscopy provided insight into why inactivation of the enzyme by NO is avoided during turnover (SI Appendix, Fig. S14). FeII cyt P460 is quantitatively oxidized to FeIII–OH2 by [Ru(NH3)6]Cl3 within the time of mixing (<3 ms), placing a lower limit on kobs of 1,100 s−1. The reactions with NH2OH and NO exhibit kobs of 1.3 s−1 and 2.8 s−1, respectively. These results show that oxidation of FeII cyt P460 to FeIII–OH2 is much faster than the reaction with NH2OH or NO, thereby avoiding the production of inactive {FeNO}7. After the oxidant is completely consumed, FeII cyt P460 will react with either NH2OH or NO. The reaction of FeII cyt P460 with NH2OH forms FeIII–OH2 or, in the case of high NH2OH concentration, FeIII–NH2OH, whereas the reaction of FeII cyt P460 with NO results in the formation of inactive {FeNO}7. Therefore, these reactions with FeII cyt P460 likely account for the products observed in the EPR spectrum of the {FeNO}6 intermediate decay sample (Fig. 5D).
The King–Altman method (42) was used to derive a steady-state equation based on the minimal mechanism shown in Fig. 5. In this simplified model, NH2OH binding is treated as reversible, with association (k1) and dissociation (k−1) rate constants. Both the FeIII–NH2OH oxidation (k2) and the subsequent reaction of {FeNO}6 with NH2OH (k3) are assumed to be irreversible. The resulting model (Eqs. 2–4) indicates that k2[ox] influences both kcat and Km:
| [2] |
| [3] |
| [4] |
There are two limiting regimes of the derived steady-state model in which either k−1k3 or k2[ox](k1 + k3) dominates the numerator of the Km term. In the former, Km should resemble , which was determined to be 9 mM. The steady-state activity plot lacks any clear curvature despite the inclusion of activity measurements in up to 20 mM NH2OH. This lack of curvature suggests that the second regime, which is highly dependent on k2[ox], contributes substantially under the assay conditions, thereby increasing the observed Km. Currently, we have an estimate of only k3 (0.07 mM–1⋅min–1). Given the assumptions made, we attribute the absence of saturation in the steady-state activity plot to a large Km, which suggests a large second-order rate constant for FeIII–NH2OH oxidation.
N. europaea tolerates at least 10 mM NH2OH without showing inhibited O2 uptake (43). Under the steady-state conditions studied, we estimate the rate of N2O production at 10 mM NH2OH to be 23 μM N2O min−1. The properties of the oxidant heavily influence k2 and, by extension, the rate at which N2O is produced by cyt P460. Thus, lacking the identity of the native electron transfer partner, we cannot verify that this rate is the physiological rate.
The observation that NO binding accesses a shunt in the cyt P460 catalytic pathway provides evidence for the hypothesis that cyt P460 contributes to NO detoxification in the cell (11). Furthermore, if NO binding is reversible, there may be an alternate cyt P460 NH2OH oxidation pathway that results in NO as the product. At low NH2OH concentrations, the bimolecular reaction with {FeNO}6 should be slow and NO dissociation may outpace N2O formation. This alternate NO-forming pathway could also be responsible for the observation of formation under aerobic conditions, as NO reacts with O2 in aqueous solution to form . To test this hypothesis, {FeNO}6 was generated by treating FeIII–OH2 with 1 eq of NO generated from PROLI-NONOate. Exposure of the resulting {FeNO}6 species to O2 results in the return to FeIII–OH2 and generation of (SI Appendix, Fig. S15). We therefore propose that the observed as an aerobic product is not directly formed by cyt P460, but rather is a byproduct resulting from NO dissociation from the {FeNO}6 intermediate. A detailed kinetic analysis will determine the partitioning between the - and N2O-forming pathways.
Cell-free extracts of N. europaea were previously shown to oxidize NH2OH to N2O and NO without formation of NO2− (44). Furthermore, purified HAO was shown to react with NH2OH and PMS or DCPIP under aerobic conditions to form a mixture of products, including NO, N2O, , and (15). Additionally, a stable {FeNO}6 species on the HAO P460 cofactor was observed after FeIII–OH2 was allowed to react with NO in the absence of NH2OH (25). Given this evidence, our results, and the recent report that an HAO-like protein oxidizes NH2OH to NO (27), we suggest that the biochemistry of HAO be revisited to determine if is indeed its terminal, enzymatic product.
Outlook: Environmental Consequences.
AOB are major contributors to N2O emissions from wastewater treatment plants (WWTPs), at which N. europaea is the dominant AOB species (45). There are two proposed methods for N2O emission from AOB: The first is as a product in the nitrifier denitrification pathway, and the second is as a byproduct in incomplete NH2OH oxidation. The results of our study demonstrate that the constitutively expressed cyt P460 is a direct link between NH2OH oxidation and the emission of N2O from N. europaea, thus establishing an alternative oxidative pathway to N2O. We have established through GC and kinetic analysis a strict stoichiometry of 2 eq of NH2OH and 4 oxidizing equivalents to produce 1 eq of N2O. Due to the reactivity of NH2OH with biological electrophiles, N. europaea likely has a detoxifying role at high NH2OH concentrations. Previously, cyt P460 was thought to oxidize NH2OH and NO to (46). However, we suggest herein an alternative role in NH2OH detoxification through the production of N2O (vide supra) (11).
We have demonstrated the production of N2O from NH2OH oxidation by cyt P460 under anaerobic conditions, thereby establishing a direct enzymatic link between nitrification and N2O formation via NH2OH. The identification of the source of N2O emission helps to explain the production of N2O in WWTPs under conditions of low dissolved O2 concentrations and high NH3 concentrations, conditions in which the nitrifier denitrification pathway would not dominate (47). The influx of high concentrations of NH3 in WWTPs causes an increase in NH3 oxidation rates, which, in turn, increases intracellular NH2OH concentration (4). Studies using activated sludge from WWTPs with high concentrations of NH3 have shown that transitioning from an aerobic environment to an anoxic environment increases the amount of N2O released. Isotopic labeling studies show that the N2O produced under these conditions originates from the NH2OH oxidation pathway rather than the nitrifier denitrification pathway (48). Our study pinpoints direct N2O production via an enzymatic, anaerobic NH2OH oxidation mechanism. Identifying the chemical source of this emission should aid in the design and operation of WWTPs with curtailed N2O emission.
Experimental Procedures
Materials.
Cyt P460 was prepared as previously described (29). PROLI-NONOate and Na2N2O3 (Angeli’s salt) were purchased from Cayman Chemicals. DCPIP was purchased from Alfa Aesar, PMS from Bean Town Chemical, and NH2OH⋅HCl from TCI Chemicals.
Spectroscopy.
X-band (9.40-GHz) EPR spectra were obtained using a Bruker Elexsys-II spectrometer equipped with a liquid He cryostat maintained at 10 or 20 K. Temperatures and microwave powers are listed in the figure legends. UV/visible absorption spectra were obtained using a Cary 60 UV/visible absorption spectrometer.
N2O Quantification.
All reactions were prepared and sealed in 5-mL headspace GC vials (Wheaton). The final N2O concentration was analyzed with GC (Agilent), GC/MS (GC-MATE II; JEOL), or an N2O microsensor housed within a septum-piercing needle (Unisense). For GC experiments, the headspace was measured with GC analysis using a Supel-Q PLOT (30 mm × 0.32 mm) or an RT Q-bond column. For N2O microsensor measurement, the needle probe was inserted through the septum and into the solution. Calibration standards for all experiments were made either by diluting an N2O-saturated solution into water or by decomposing Na2N2O3 in Hepes buffer, pH 8.0, in a sealed headspace vial.
Supplementary Material
Acknowledgments
We thank Boris Dzikovski for assistance with EPR data collection and Ivan Keresztes for assistance with GC/MS. We thank Cedric Mason for assistance with GC analysis in the Soil and Water Group’s laboratory (Department of Biological and Environmental Engineering, Cornell University). We thank Samantha N. MacMillan for thoughtful discussion and a careful read of this manuscript. K.M.L. thanks the Cornell University College of Arts and Sciences for startup funding and the Department of Energy Office of Science for support in the form of an Early Career Award (DE-SC0013997). EPR data were collected at the National Biomedical Center for Advanced ESR Technology, which is supported by the National Institute of General Medical Sciences of the NIH under Award P41GM103521.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 14474.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611051113/-/DCSupplemental.
References
- 1.Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326(5949):123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- 2.Goreau TJ, et al. Production of NO2– and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl Environ Microbiol. 1980;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inamori Y, Wu X-L, Mizuochi M. N2O producing capability of Nitrosomonas europaea, Nitrobacter winogradskyi, and Alcaligenes faecalis. Water Sci Technol. 1997;36(10):65–72. [Google Scholar]
- 4.Ni B-J, Peng L, Law Y, Guo J, Yuan Z. Modeling of nitrous oxide production by autotrophic ammonia-oxidizing bacteria with multiple production pathways. Environ Sci Technol. 2014;48(7):3916–3924. doi: 10.1021/es405592h. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Garcia O, Villas-Boas SG, Swift S, Chandran K, Singhal N. Clarifying the regulation of NO/N2O production in Nitrosomonas europaea during anoxic-oxic transition via flux balance analysis of a metabolic network model. Water Res. 2014;60:267–277. doi: 10.1016/j.watres.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 6.Sabba F, Picioreanu C, Pérez J, Nerenberg R. Hydroxylamine diffusion can enhance N₂O emissions in nitrifying biofilms: A modeling study. Environ Sci Technol. 2015;49(3):1486–1494. doi: 10.1021/es5046919. [DOI] [PubMed] [Google Scholar]
- 7.Hooper AB, Vannelli T, Bergmann DJ, Arciero DM. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie van Leeuwenhoek. 1997;71(1-2):59–67. doi: 10.1023/a:1000133919203. [DOI] [PubMed] [Google Scholar]
- 8.Walker CB, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA. 2010;107(19):8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ensign SA, Hyman MR, Arp DJ. In vitro activation of ammonia monooxygenase from Nitrosomonas europaea by copper. J Bacteriol. 1993;175(7):1971–1980. doi: 10.1128/jb.175.7.1971-1980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vajrala N, et al. Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci USA. 2013;110(3):1006–1011. doi: 10.1073/pnas.1214272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein LY. Surveying N2O-producing pathways in bacteria. Methods Enzymol. 2011;486:131–152. doi: 10.1016/B978-0-12-381294-0.00006-7. [DOI] [PubMed] [Google Scholar]
- 12.Stein L. Heterotrophic nitrification and nitrifier denitrification. In: Ward BB, Arp DJ, Klotz MG, editors. Nitrification. ASM Press; Washington, DC: 2011. pp. 95–116. [Google Scholar]
- 13.Zhu X, Burger M, Doane TA, Horwath WR. Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci USA. 2013;110(16):6328–6333. doi: 10.1073/pnas.1219993110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith PA, Hein GE. The alleged role of nitroxyl in certain reactions of aldehydes and alkyl halides. J Am Chem Soc. 1960;82(21):5731–5740. [Google Scholar]
- 15.Hooper AB, Terry KR. Hydroxylamine oxidoreductase of Nitrosomonas. Production of nitric oxide from hydroxylamine. Biochim Biophys Acta. 1979;571(1):12–20. doi: 10.1016/0005-2744(79)90220-1. [DOI] [PubMed] [Google Scholar]
- 16.Beaumont HJ, van Schooten B, Lens SI, Westerhoff HV, van Spanning RJ. Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J Bacteriol. 2004;186(13):4417–4421. doi: 10.1128/JB.186.13.4417-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cua LS, Stein LY. Effects of nitrite on ammonia-oxidizing activity and gene regulation in three ammonia-oxidizing bacteria. FEMS Microbiol Lett. 2011;319(2):169–175. doi: 10.1111/j.1574-6968.2011.02277.x. [DOI] [PubMed] [Google Scholar]
- 18.Erickson RH, Hooper AB. Preliminary characterization of a variant co-binding heme protein from Nitrosomonas. Biochim Biophys Acta. 1972;275(2):231–244. doi: 10.1016/0005-2728(72)90044-8. [DOI] [PubMed] [Google Scholar]
- 19.Pearson AR, et al. The crystal structure of cytochrome P460 of Nitrosomonas europaea reveals a novel cytochrome fold and heme-protein cross-link. Biochemistry. 2007;46(28):8340–8349. doi: 10.1021/bi700086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman SE, Bren KL. The chemistry and biochemistry of heme c: Functional bases for covalent attachment. Nat Prod Rep. 2008;25(6):1118–1130. doi: 10.1039/b717196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safo MK, et al. Models of the cytochromes b. Low-spin bis-ligated (porphinato) iron (III) complexes with unusual molecular structures and NMR, EPR, and Mössbauer spectra. J Am Chem Soc. 1992;114(18):7066–7075. [Google Scholar]
- 22.Bergmann DJ, Hooper AB. The primary structure of cytochrome P460 of Nitrosomonas europaea: Presence of a c-heme binding motif. FEBS Lett. 1994;353(3):324–326. doi: 10.1016/0014-5793(94)01072-2. [DOI] [PubMed] [Google Scholar]
- 23.Numata M, Saito T, Yamazaki T, Fukumori Y, Yamanaka T. Cytochrome P-460 of Nitrosomonas europaea: Further purification and further characterization. J Biochem. 1990;108(6):1016–1021. doi: 10.1093/oxfordjournals.jbchem.a123300. [DOI] [PubMed] [Google Scholar]
- 24.Cabail MZ, Pacheco AA. Selective one-electron reduction of Nitrosomonas europaea hydroxylamine oxidoreductase with nitric oxide. Inorg Chem. 2003;42(2):270–272. doi: 10.1021/ic025779n. [DOI] [PubMed] [Google Scholar]
- 25.Hendrich MP, Upadhyay AK, Riga J, Arciero DM, Hooper AB. Spectroscopic characterization of the NO adduct of hydroxylamine oxidoreductase. Biochemistry. 2002;41(14):4603–4611. doi: 10.1021/bi011332z. [DOI] [PubMed] [Google Scholar]
- 26.Zahn JA, Duncan C, DiSpirito AA. Oxidation of hydroxylamine by cytochrome P-460 of the obligate methylotroph Methylococcus capsulatus Bath. J Bacteriol. 1994;176(19):5879–5887. doi: 10.1128/jb.176.19.5879-5887.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maalcke WJ, et al. Structural basis of biological NO generation by octaheme oxidoreductases. J Biol Chem. 2014;289(3):1228–1242. doi: 10.1074/jbc.M113.525147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson KA. Transient-state kinetic analysis of enzyme reaction pathways. In: Sigman DS, editor. The Enzymes. Academic; Cambridge, MA: 1992. pp. 1–61. [Google Scholar]
- 29.Elmore BO, Pearson AR, Wilmot CM, Hooper AB. Expression, purification, crystallization and preliminary X-ray diffraction of a novel Nitrosomonas europaea cytochrome, cytochrome P460. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62(Pt 4):395–398. doi: 10.1107/S1744309106008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson KK, Kent TA, Lipscomb JD, Hooper AB, Münck E. Mössbauer, EPR, and optical studies of the P-460 center of hydroxylamine oxidoreductase from Nitrosomonas. A ferrous heme with an unusually large quadrupole splitting. J Biol Chem. 1984;259(11):6833–6840. [PubMed] [Google Scholar]
- 31.McQuarters AB, Goodrich LE, Goodrich CM, Lehnert N. Disproportionation of O-benzylhydroxylamine catalyzed by a ferric bis-picket fence porphyrin complex. Z Anorg Allg Chem. 2013;639(8-9):1520–1526. [Google Scholar]
- 32.Goodrich LE, Paulat F, Praneeth VK, Lehnert N. Electronic structure of heme-nitrosyls and its significance for nitric oxide reactivity, sensing, transport, and toxicity in biological systems. Inorg Chem. 2010;49(14):6293–6316. doi: 10.1021/ic902304a. [DOI] [PubMed] [Google Scholar]
- 33.Enemark J, Feltham R. Principles of structure, bonding, and reactivity for metal nitrosyl complexes. Coord Chem Rev. 1974;13(4):339–406. [Google Scholar]
- 34.Fernández ML, Estrin DA, Bari SE. Theoretical insight into the hydroxylamine oxidoreductase mechanism. J Inorg Biochem. 2008;102(7):1523–1530. doi: 10.1016/j.jinorgbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Daiber A, et al. Isotope effects and intermediates in the reduction of NO by P450(NOR) J Inorg Biochem. 2002;88(3-4):343–352. doi: 10.1016/s0162-0134(01)00386-5. [DOI] [PubMed] [Google Scholar]
- 36.Lehnert N, Praneeth VK, Paulat F. Electronic structure of iron(II)-porphyrin nitroxyl complexes: Molecular mechanism of fungal nitric oxide reductase (P450nor) J Comput Chem. 2006;27(12):1338–1351. doi: 10.1002/jcc.20400. [DOI] [PubMed] [Google Scholar]
- 37.Kumar MR, Fukuto JM, Miranda KM, Farmer PJ. Reactions of HNO with heme proteins: New routes to HNO-heme complexes and insight into physiological effects. Inorg Chem. 2010;49(14):6283–6292. doi: 10.1021/ic902319d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin R, Farmer PJ. The HNO adduct of myoglobin: Synthesis and characterization. J Am Chem Soc. 2000;122(10):2393–2394. [Google Scholar]
- 39.Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965;240(11):4509–4514. [PubMed] [Google Scholar]
- 40.McQuarters AB, Wirgau NE, Lehnert N. Model complexes of key intermediates in fungal cytochrome P450 nitric oxide reductase (P450nor) Curr Opin Chem Biol. 2014;19:82–89. doi: 10.1016/j.cbpa.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe SK, Andrade C, Swinehart J. Kinetic studies of the pentacyanonitrosylferrate (2-) azide and hydroxylamine reactions. Inorg Chem. 1974;13(11):2567–2572. [Google Scholar]
- 42.King EL, Altman C. A schematic method of deriving the rate laws for enzyme-catalyzed reactions. J Phys Chem. 1956;60(10):1375–1378. [Google Scholar]
- 43.Böttcher B, Koops H-P. Growth of lithotrophic ammonia-oxidizing bacteria on hydroxylamine. FEMS Microbiol Lett. 1994;122(3):263–266. [Google Scholar]
- 44.Ritchie GA, Nicholas DJ. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J. 1972;126(5):1181–1191. doi: 10.1042/bj1261181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao J, Luo X, Wu G, Li T, Peng Y. Abundance and diversity based on amoA genes of ammonia-oxidizing archaea and bacteria in ten wastewater treatment systems. Appl Microbiol Biotechnol. 2014;98(7):3339–3354. doi: 10.1007/s00253-013-5428-2. [DOI] [PubMed] [Google Scholar]
- 46.Elmore BO, Bergmann DJ, Klotz MG, Hooper AB. Cytochromes P460 and c′-beta; A new family of high-spin cytochromes c. FEBS Lett. 2007;581(5):911–916. doi: 10.1016/j.febslet.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 47.Khalil K, Mary B, Renault P. Nitrous oxide production by nitrification and denitrification in soil aggregates as affected by O2 concentration. Soil Biol Biochem. 2004;36(4):687–699. [Google Scholar]
- 48.Law Y, Ni B-J, Lant P, Yuan Z. N2O production rate of an enriched ammonia-oxidising bacteria culture exponentially correlates to its ammonia oxidation rate. Water Res. 2012;46(10):3409–3419. doi: 10.1016/j.watres.2012.03.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.